Abstract
Introduction
Spinal cord infarction in a young, otherwise healthy individual is a rare occurrence. The anterior spinal artery and posterior spinal arteries are the primary contributors to the vascular supply of the cervical supply, and these arteries arise as descending branches of the vertebral arteries. Historically, many cases have demonstrated individual variations in the vertebral arteries, such as differences in dominancy, patency, origin, and insertion. The clinical significance of these variations remains poorly understood.
Case presentation
We present a patient who sustained a spinal cord infarction at C2–C5 resulting in incomplete quadriplegia. The mechanism of injury was unclear, although the patient reported an awkward jumping motion earlier that day that preceded the onset of upper extremity weakness. After resolution of the acute phase, he was diagnosed with “Man-in-the-Barrel” syndrome. Angiographic evaluation revealed an anomalous non-dominant right vertebral artery with several pathological features: origin at the descending aorta, insertion into the right posterior inferior cerebellar artery, and impingement along its course by an anterior thoracic osteophyte.
Discussion
The vertebral arteries play an important role in the vascular supply of the cervical spine. While vertebral artery pathology such as dissection or occlusion have been documented in rare cases to result in spinal cord infarction, this case illustrates an example of clinically significant sequelae that can occur in the setting of anomalous vertebral arteries even in the absence of occlusion or dissection. Furthermore, to our knowledge this is the first reported case of a spinal cord infarction resulting from osteophytic vertebral artery impingement.
Similar content being viewed by others
Introduction
The incidence of spinal cord infarction is estimated to be 3.1 per 100,000 person-years or about 7600 cases per year in the United States [1]. Symptom onset is typically rapid and severe, and can include burning back pain, weakness and paralysis, loss of pain and temperature sensation, loss of deep-tendon reflexes, autonomic dysfunction, and neurogenic bowel/bladder. Although often of iatrogenic cause (e.g., intra-operatively during aneurysm repairs), spinal cord infarctions can also be caused by hypotension, atherosclerosis, atherothrombotic as well as fibrocartilaginous emboli, hypercoagulability, AV malformations, vasculitis, dissection, and trauma [2]. Zalewski et al. reviewed 133 patients with spontaneous spinal cord infarctions, finding the mean age to be 60 years and the causes to be idiopathic with atherosclerotic risk factors in 68% of cases, due to fibrocartilaginous embolism in 14%, aortic dissection in 5%, hypercoagulability in 4%; vertebral artery dissection in 3%; systemic hypotension in 2%; cardioembolic in 2%, and vasculitis in 2%.
The anterior spinal artery (ASA) is the most frequently implicated in spinal cord infarction, although several variable patterns of infarctions exist. In a retrospective review of 27 spinal cord infarction patients by Novy et al., 37% of the patients presented with bilateral anterior spinal cord infarctions, 15% anterior unilateral infarcts, 15% posterior unilateral, 11% central, 7% posterior bilateral, and 7% transverse [3]. The ASA, which supplies the anterior two-thirds of the spinal cord, is rostrally normally formed by the convergence of one descending branch from the fourth segment of each of the vertebral arteries [4, 5]. Often, one of the branches tend to be dominant, whereas the other can be hypoplastic; the left vertebral artery (LVA) is dominant in 50–60% of cases [6, 7]. As the ASA progresses in the caudal direction within the cervical spine, it may receive additional contributions from the thyrocervical trunk, costocervical trunk, inferior thyroid arteries. deep cervical arteries (e.g., artery of cervical enlargement), and ascending cervical arteries, as well as further contributions from the vertebral arteries [4, 5]. It is understood, however, that there is considerable individual variation in this arterial network [4].
In this case report, we present a patient with “Man-in-the-Barrel” syndrome due to extrinsic osteophytic compression (at the upper thoracic level) of an anomalous non-dominant right vertebral artery (RVA) arising aberrantly from the descending aorta and inserting aberrantly into the left posterior inferior cerebellar artery, leading to hypoperfusion and C2–C5 cord infarction.
Case presentation
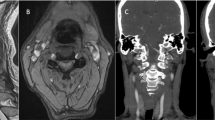
We present a 48 year old male with history of hypercholesterolemia, peripheral neuropathy, sleep apnea, and generalized anxiety disorder who experienced sudden onset neck pain when jumping on a chair at his workplace during which he reportedly twisted his neck. Symptom onset was not preceded by a large meal or oral bolus. Pain was described as a shooting sensation down his spine. This was followed by weakness in his upper and lower extremities, to the point where he could barely walk. Patient had reportedly taken benzodiazepine and opioid medications after the event. On arrival to the hospital, he appeared to be obtunded and lethargic with minimal verbal response and pinpoint pupils, and concern for acute hypoxemic respiratory failure (SpO2 79%) secondary to drug-related toxic metabolic encephalopathy. Based on laboratory studies revealing opioid and benzodiazepine intoxication, he was treated with fluids and naloxone. His mental status improved with medical management. Imaging of the brain was unremarkable. CT of the cervical spine showed non-specific cervical spondylosis without acute fracture or subluxation. CT angiogram of the neck was negative for vascular occlusion, dissection, or aneurysm. MRI of the cervical spine demonstrated T2 signal abnormality involving the anterior aspect of the spinal cord from C2 to C5 consistent with spinal cord ischemic insult within the distribution of the ASA (Fig. 1). MR angiography of the neck confirmed no dissection or stenosis involving the bilateral common or internal carotid arteries but did show a small, irregular RVA that terminated in the posterior inferior cerebellar artery (PICA) (Fig. 2). He was started on aspirin and discharged to an acute inpatient rehabilitation facility at a maximal assist level for transfers, moderate assist level for gait, and minimum to total assistance for ADLs.
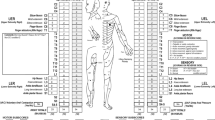
Upon initial evaluation at the rehabilitation center, he presented in no acute distress with normal vital signs. Neurological exam was conducted in reference with the ASIA/ISCoS International Standards for Neurological Classification of Spinal Cord Injury, and revealed 3/5 strength in the right elbow flexors, 1/5 strength in the left elbow flexors, 4/5 strength in the right wrist extensors, and 4/5 strength in the left wrist extensors with mild spasticity throughout the bilateral upper extremities. Remainder of major muscle groups including the lower extremities demonstrated 5/5 strength bilaterally. Sensation to pinprick and light touch were impaired in all levels below C3. Cardiac, pulmonary, and abdominal examinations was unremarkable. He exhibited neurogenic bowel and bladder. His gait was ataxic. After an inpatient rehabilitation program for 2 weeks, the patient was discharged home at a minimum assistance to modified independent level with outpatient physical and occupational therapy.
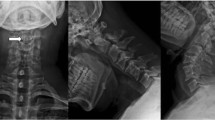
He continued to have significant neurological deficits in the upper cervical distribution when seen as an outpatient 1 month later. Further review of the initial CT angiogram revealed a contact between the RVA and an anterior T2–T3 osteophyte (Fig. 3). A spinal angiogram was then performed which revealed a dominant LVA (Fig. 4). In addition, it also demonstrated the RVA originating directly from the descending thoracic aorta, crossing midline in the thoracic region, and terminating into the right-sided posterior circulation (Fig. 5). Additional blood supply to the ASA was noted on the angiogram to be from the left thyrocervical trunk at the level of C6 to C7 and the right segmental artery at the level of T4.
The patient was seen by neurosurgery and no surgical intervention was recommended. He continued with physical and occupational therapy. Four months after the initial infarction, he had negligible improvement in his upper extremity weakness. Patient was able to ambulate independently but required assistance for bathing and dressing. His spasticity, neurogenic bladder, and neurogenic bowel remained well controlled with medications and behavioral modifications.
Discussion
This case reports a rare case of “Man-in-the-Barrel” syndrome secondary to cervical cord hypoperfusion. Man-in-the-Barrel syndrome is a known phenomenon of brachial diplegia involving proximal paresis of the upper extremities with preservation of strength in the lower extremities [8]. While typically seen in the setting of cerebral hypoperfusion at the border zones between the anterior and middle cerebral artery territories, it has also been reported to rarely occur following ASA-territory cervical spinal cord infarction stemming from vertebral artery thrombosis or dissection [9,10,11,12]. This is the first known case of cervical cord infarction and Man-in-the-Barrel syndrome to occur in the setting of an aberrant RVA being compressed by an anterior thoracic osteophyte.
The cervical spinal cord is supplied by one anterior and two posterior spinal arteries. The vertebral arteries, which normally arise from posterosuperior aspect of the respective ipsilateral subclavian arteries, travel though the transverse foramen in the cervical spine and contribute directly to the anterior and posterior spinal arteries. These two branches are often not equal in contribution, and dominancy of one vertebral artery is common [4, 6]. In historical cases, ASA syndrome secondary to vascular infarction has been demonstrated to occur via vertebral artery dissection or severe stenosis [13, 14]. This case demonstrates a unique instance in which a patient sustained a spinal cord infarction in the setting of anomalous RVA noted to be compressed by an anterior thoracic osteophyte, likely leading to decreased flow through the ASA and Man-in-the-Barrel syndrome.
In our patient, while the dominancy of the LVA was not surprising, the origin of the RVA from the descending thoracic aorta is striking. Yuan et. al. reviewed 1286 patients with aberrant vertebral arteries and found that some aberrant arteries had a single site of origin while others had dual origin [15]. Among patients with aberrant vertebral arteries of single origin, the LVA was implicated most frequently (85.6%), the RVA (11.7%) less commonly, and bilateral vertebral arteries (2.7%) least commonly, with only one patient in this entire compilation exhibiting an aberrant RVA arising directly from the descending aorta (as seen in this case) [15]. However, aberrant origin of the vertebral arteries is not usually symptomatic, and in most cases do not result in neurologic sequalae. In the same review, only 5.5% of patients with aberrant vertebral arteries exhibited syndromes attributable to the arterial variations [15].
Another less striking but likely clinically significant anatomical variation in this patient was the insertion of the RVA into the left PICA. Normally, the LVA and RVA converge at their rostral end to form the basilar artery. A branch from each of the vertebral arteries slightly caudal to this convergence give rise to the respective PICAs bilaterally. The insertion of vertebral arteries into the PICA is a well-known anatomic variant [16]. It is frequently associated with decreased caliber of the PICA-inserting vertebral artery, and even vertebral artery hypoplasia [6, 17]. In one review of patients with ischemic strokes in the vertebrobasilar artery distribution, it was found that vertebral artery insertion into the PICA was seen in ~8% of patients [18]. Furthermore, multiple case reports have documented that in patients with a hypoplastic vertebral artery that inserted into the PICA, there was an increased risk of rotational vertebral artery occlusion associated with ipsilateral head rotation, although the infarctions in these cases were primarily in the inferior cerebellum and lateral medulla [19,20,21]. Despite these associations, the clinical significance of aberrant insertion of the vertebral artery into the PICA remains unclear and disputed [6]. In the general population, it has been estimated that ~2% of otherwise healthy individuals have RVA insertion into the right PICA [16]. In our patient, however, the combination of aberrancies in both the origin and termination of the RVA may have additively contributed to the C2–C5 watershed region. In addition, this was likely additionally exacerbated by the lack of other major arterial contributions in the region. Classically, there exists a large branch from the vertebral arteries supplying the ASA around the level of C3 [4]. However, in our patient, there was no such contribution from either side, and the next significant branch was from the left thyrocervical trunk at C6-7.
Another significant contributor to the patient’s clinical presentation was the presence of anterior vertebral osteophytes at the T2–T3 level. Anterior vertebral osteophytes have been very infrequently reported in the literature. Case reports exist of anterior cervical osteophytes causing dysphagia and sleep apnea, and anterior lumbar osteophytes affecting the inferior vena cava and abdominal aorta [22]. Anterior thoracic osteophytes are most commonly observed on the vertebral bodies of T9 and T10 [23]. Per several sporadic case reports, anterior thoracic osteophytes have been associated with dysphagia in the supine position, compression of the sympathetic trunk and splanchnic nerves, trauma to the descending aorta, pseudoaneurysm formation, obstructive pneumonia, and right-sided lung fibrosis [24,25,26,27,28,29,30]. However, none of the above cases involved thoracic osteophytes as high as T2–T3 or have been associated with spinal cord infarction.
In summary, this case illustrates a rare scenario in which multiple structural/developmental anomalies predisposed towards a watershed region at C2–C5, which then was acutely infarcted upon acute rotation of the neck. It is likely that compression of the RVA led to ischemia of the central anterior horn cells in a region that lacked other major collateral contribution. This resulted in the Man-in-the-Barrel syndrome, in which the central anterior horn cells are focally affected. The lack of improvement in his symptoms correlated with the expected outcomes following spinal cord infarction. True anterior cord syndrome and posterior cerebral infarction were likely averted due to dominant circulation from LVA, as well as collateral circulation from the thyrocervical trunk and segmental arteries. Dominancy of the LVA may also have provided adequate vascular coverage throughout the patient’s life until the acute triggering events described.
To our knowledge, this is the first documented case involving osteophytic vertebral artery compression resulting in spinal cord infarction. Further research on the developmental and pathophysiologic processes underlying these anomalous anatomic variations is needed. Future initiatives to improve patient screening and early detection may be valuable to improve risk stratification and aid in primary prevention for patients predisposed to spinal cord infarction.
References
Qureshi AI, Afzal MR, Suri MFK. A Population-Based Study of the Incidence of Acute Spinal Cord Infarction. J Vasc Interv Neurol. 2017;9:44–8.
Zalewski NL, Rabinstein AA, Krecke KN, Brown RD, Wijdicks EFM, Weinshenker BG et al. Characteristics of Spontaneous Spinal Cord Infarction and Proposed Diagnostic Criteria. JAMA Neurol. 2019. https://doi.org/10.1001/jamaneurol.2018.2734.
Novy J, Carruzzo A, Maeder P, Bogousslavsky J. Spinal cord ischemia: Clinical and imaging patterns, pathogenesis, and outcomes in 27 patients. Arch Neurol. 2006. https://doi.org/10.1001/archneur.63.8.1113.
Santillan A, Nacarino V, Greenberg E, Riina HA, Gobin YP, Patsalides A. Vascular anatomy of the spinal cord. J Neurointerv Surg. 2012. https://doi.org/10.1136/neurintsurg-2011-010018.
Vargas MI, Gariani J, Sztajzel R, Barnaure-Nachbar I, Delattre BM, Lovblad KO et al. Spinal cord ischemia: practical imaging tips, pearls, and pitfalls. AJNR. Am J Neuroradiol. 2015. https://doi.org/10.3174/ajnr.A4118.
Aoyama T, Obara N. Vertebral Artery Terminating Posterior Inferior Cerebellar Artery (PICA-VA) as a Potential Risk Factor in Cervical Spine Surgery. World Neurosurg. 2020. https://doi.org/10.1016/j.wneu.2020.08.003.
Giannopoulos S, Markoula S, Kosmidou M, Pelidou SH, Kyritsis AP. Lateral medullary ischaemic events in young adults with hypoplastic vertebral artery. J Neurol Neurosurg Psychiatry. 2007. https://doi.org/10.1136/jnnp.2006.106419.
Weidauer S, Nichtweiß M, Hattingen E, Berkefeld J. Spinal cord ischemia: aetiology, clinical syndromes and imaging features. Neuroradiology. 2015. https://doi.org/10.1007/s00234-014-1464-6.
Flanagan EP, McKeon A, Weinshenker BG. Anterior spinal artery infarction causing man-in the-barrel syndrome. Neurol Clin Pract. 2014. https://doi.org/10.1212/CPJ.0000000000000032.
Rouanet C, Reges D, Rocha E, Gagliardi V, Uehara MK, Miranda MA et al. “Man in the Barrel” Syndrome with Anterior Spinal Artery Infarct due to Vertebral Artery Dissection. J Stroke Cerebrovasc Dis. 2017. https://doi.org/10.1016/j.jstrokecerebrovasdis.2016.12.016.
Berg D, Müllges W, Koltzenburg M, Bendszus M, Reiners K. Man-in-the-barrel syndrome caused by cervical spinal cord infarction. Acta Neurol Scand. 1998. https://doi.org/10.1111/j.1600-0404.1998.tb05977.x.
Antelo MJG, Facal TL, Sánchez TP, Facal MSL, Nazabal ER Man-In-The-Barrel. A Case of Cervical Spinal Cord Infarction and Review of the Literature. Open Neurol J. 2013. https://doi.org/10.2174/1874205x01307010007.
Suzuki K, Meguro K, Wada M, Nakai K, Nose T. Anterior spinal artery syndrome associated with severe stenosis of the vertebral artery. Am J Neuroradiol. 1998;19:1353–5.
Suzuki H, Kitagawa T, Gotoh M, Mitsueda-Ono T, Matsui M. Cervical cord infarction caused by dissection of the intracranial segment of the vertebral artery. Intern Med. 2018. https://doi.org/10.2169/internalmedicine.0608-17.
Yuan SM. Aberrant origin of vertebral artery and its clinical implications. Brazilian J Cardiovasc Surg. 2016. https://doi.org/10.5935/1678-9741.20150071.
Liu IW, Ho BL, Chen CF, Han K, Lin CJ, Sheng WY et al. Vertebral artery terminating in posterior inferior cerebellar artery: a normal variation with clinical significance. PLoS ONE. 2017. https://doi.org/10.1371/journal.pone.0175264.
Perren F, Poglia D, Landis T, Sztajzel R. Vertebral artery hypoplasia: a predisposing factor for posterior circulation stroke? Neurology. 2007. https://doi.org/10.1212/01.wnl.0000250258.76706.98.
Frisoni GB, Anzola GP. Vertebrobasilar ischemia after neck motion. Stroke. 1991. https://doi.org/10.1161/01.STR.22.11.1452.
Noh Y, Kwon OK, Kim HJ, Kim JS. Rotational vertebral artery syndrome due to compression of nondominant vertebral artery terminating in posterior inferior cerebellar artery. J Neurol. 2011. https://doi.org/10.1007/s00415-011-6005-1.
Yeh JF, Lin YJ, Po HL, Wang SF, Pan PY, Cheng SJ, et al. A case of bow hunter’s stroke caused by non-dominant vertebral artery. Acta Neurol Taiwan. 2005;14:69–73.
Mehalic T, Farhat SM. Vertebral artery injury from chiropractic manipulation of the neck. Surg Neurol.1974;2:125–9.
Klaassen Z, Tubbs RS, Apaydin N, Hage R, Jordan R, Loukas M. Vertebral spinal osteophytes. Anat Sci Int. 2011. https://doi.org/10.1007/s12565-010-0080-8.
O’Neill TW, McCloskey EV, Kanis JA, Bhalla AK, Reeve J, Reid DM, et al. The distribution, determinants, and clinical correlates of vertebral osteophytosis: a population based survey. J Rheumatol.1999;26:842–8.
Nathan H. Osteophytes of the spine compressing the sympathetic trunk and splanchnic nerves in the thorax. Spine (Phila Pa 1976). 1987 https://doi.org/10.1097/00007632-198707000-00003.
Otake S, Takahashi M, Ishigaki T. Focal pulmonary interstitial opacities adjacent to thoracic spine osteophytes. Am J Roentgenol. 2002. https://doi.org/10.2214/ajr.179.4.1790893.
Leon JA, Calamia KT, Leventhal JP. Chronic obstructive pneumonia caused by a vertebral body osteophyte. Mayo Clin Proc. 2000. https://doi.org/10.4065/75.2.185.
Dregelid E, Jenssen G, Jonung T, Braaten A. Pseudoaneurysm of the abdominal aorta due to a needle-like osteophyte on the first lumbar vertebra. J Vasc Surg. 2007. https://doi.org/10.1016/j.jvs.2006.12.070.
Chtata H, Koskas F, Cluzel P, Kieffer E. Traumatic pseudoaneurysm of the descending thoracic aorta inflicted by a spinal osteophyte. Ann Vasc Surg. 2005. https://doi.org/10.1007/s10016-004-0175-6.
Khairullah A, Shahrul H, Mutuyanagam SB. Diffuse Idiopathic Skeletal Hyperostosis: a Rare Cause of Dysphagia. Philipp J Otolaryngol Neck Surg. 2014. https://doi.org/10.32412/pjohns.v29i2.429.
Cai FZJ, Rischmueller M, Pile K, Brady SJ. Dysphagia associated with lower thoracic spondylosis [10]. Rheumatology. 2003. https://doi.org/10.1093/rheumatology/keg410.
Acknowledgements
We would like to acknowledge Patricia Delgado, M.D. from the University of Puerto Rico for her assistance in interpreting the radiographic studies included in this case report.
Author information
Authors and Affiliations
Contributions
AM contributed to conducting pertinent review of the literature, writing the report (especially later drafts/revisions), and compiling/including references appropriately. HM contributed to conducting a literature review, formulation of the first draft, and provided feedback for subsequent draft revisions. JD contributed by identification of the case for publication, providing mentorship during the manuscript writing process, and delivering appropriate feedback for draft revisions.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Informed consent
Informed consent was obtained from the patient. The purpose of this paper was explained, and the patient was ensured that all identifying information will be removed.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mohan, A., Mielke-Maday, H. & Delgado-Lebron, J. Cervical spinal cord infarction due to impingement of an anomalous right vertebral artery by thoracic osteophyte. Spinal Cord Ser Cases 7, 95 (2021). https://doi.org/10.1038/s41394-021-00457-8
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41394-021-00457-8