Abstract
Introduction
The occurrence of concurrent hypertrophied posterior longitudinal ligament (HPLL) and hypertrophied ligamentum flavum (HLF) in the thoracic spine is a very rare presentation. This case report describes a young female who developed thoracic myelopathy secondary to a combination of both thoracic HPLL and HLF.
Case presentation
A 30-year-old previously well female was referred for an MRI scan of the thoraco-lumbar spine. She was having lower limb weakness and difficulty in walking, which had progressed over 3 months. On examination, she was found to have spastic lower limbs with associated motor weakness. Her biochemical investigations were unremarkable. The MRI scan showed HPLL, which was uniformly hypointense on T2W images and was isointense on T1W images. The hypertrophied segment was extending from T2 level to T7 level. Similarly, the ligamentum flavum was hypertrophied from T1 level to T8 level. The thoracic spinal cord was seen compressed between the hypertrophied ligaments. The compressed cord showed central hyperintense signal pattern in T2W images. CT scan of the thoracic spine did not show any calcifications or ossifications along the ligaments. Patient underwent posterior decompressive surgery and she had an uneventful recovery.
Discussion
Although few cases of HPLL and HLF were reported in older patients in literature, both these conditions were found in this patient at a younger age. HPLL and HLF are thought to be precursors of ossification of these ligaments and these patients need long-term follow-up.
Similar content being viewed by others
Introduction
Hypertrophy of the posterior longitudinal ligament (HPLL) is defined as an abnormal thickening of the posterior longitudinal ligament (PLL) leading to the compression of the spinal canal [1]. Although the ossification of the posterior longitudinal (OPLL) is a well-documented entity causing myelopathy, documented cases of thoracic myelopathy secondary to HPLL are scanty [1]. Very few cases are reported in the literature on HPLL causing thoracic myelopathy [1, 2]. In a similar vein, although the ossification of the ligamentum flavum (OLF) is a well-documented cause of thoracic myelopathy, the hypertrophied ligamentum flavum (HLF) leading to thoracic myelopathy is a relatively rare disorder [3, 4]. Further, the reports on continuous multilevel involvement of HLF are scanty [5]. Here, we present a case of a young female who had a simultaneous continuous multilevel occurrence of HPLL and HLF in the thoracic spine leading to thoracic myelopathy.
Case presentation
A 30-year-old female patient, who was experiencing progressive lower limb weakness, was referred for an MRI scan of the spine. She was having difficulty in walking, which had progressed over the past 3 months. There was numbness in both lower limbs; but, there was no upper limb weakness or numbness. She had no previous history of trauma and had no major medical condition previously.
On examination, she was found to have spastic paraparesis with preserved sensory functions. (ASIA neurological score B). No sensory defects were identified. There were no sphincter disturbances. There were no tender areas over the spine. Her biochemical investigations including the full blood count, ESR and serum electrolytes were unremarkable.
Imaging findings
In the plain X-ray of the thoraco-lumbar spine, there was a loss of normal lumbar lordosis resulting in a straight spine. The MRI images of the spine showed thickened PLL. The PLL was isointense on T1W images (Fig. 1) and was uniformly hypointense on T2W images (Fig. 2). The hypertrophied segment was extending from T2 level to T7 level. Similarly, the ligamentum flavum was hypertrophied from T1 level to T8 level (Figs. 1 and 2). The thoracic spinal cord was seen compressed between the hypertrophied ligaments. Significant compression was noted at the level of T2 to T4. The compressed cord showed central hyperintense signal pattern in T2W images. The vertebral bodies showed a normal MRI signal pattern. The vertebral end-plates were normal. There were no paravertebral masses. The spinal cord above and below the compressed area was normal. There was no syrinx formation. Following IV gadolinium, there was no abnormal enhancement detected along the spinal cord, ligaments or in the vertebral bodies. There was no facet joint hypertrophy or evidence of facet joint degeneration. Intervertebral disc spaces were normal and there was no evidence of disc degeneration. Subsequently, CT scan of the thoracic spine was done to assess any calcifications or ossifications along the ligaments. The CT images did not show any calcifications or ossifications along the hypertrophied ligaments.
Final imaging diagnosis was HPLL and HLF compressing the thoracic spinal cord leading to myelopathy. The patient underwent decompressive surgery with thoracic laminectomy and excision of the lamina flava. Anterior decompression was not attempted on this patient. She had an uneventful recovery from the surgery. There was gradual improvement of symptoms in the first 3 months following the surgery and was discharged to the local hospital.
Discussion
HPLL was first described in 1974 by Kamikozuru et al. [2, 6]. Studies have suggested that HPLL is the precursor of OPLL, although this theory is still controversial [1, 2, 6, 7]. Among the reported cases of HPLL, the majority were reported in relation to the cervical spine [2]. According to the global literature, only a very few cases with HPLL affecting the thoracic spine were reported [1, 2, 6, 8]. Reported cases of HPLL of the thoracic spine are summarized in Table 1.
One case report described a 58-year-old female, who presented with spontaneous onset of progressive lower extremity paresthesias and progressive gait disturbance. Her MRI scan revealed hypertrophied PLL extending from T4 to T12. She had undergone ventral decompressive surgery and after 10 years, the residual PLL had shown progressive ossification [1]. Another case report was of a 61-year-old male, who presented with acute paraparesis associated with HPLL in the thoracic region. He had focal HPLL at T6 to T7 and had undergone decompressive corpectomy [2]. While these reported cases were of relatively older age group, our patient was a 30-year-old young female, who had no significant past medical or surgical condition. There were two other cases of thoracic HPLL which were reported in Japanese literature [6].
Although the ossification of the LF (OLF) is a well-documented disorder affecting the thoracic spine, multilevel contiguous involvement is a rare cause of myelopathy [5]. Furthermore, HLF is a rare cause of spine compression and few cases were found in the literature describing HLF causing thoracic myelopathy (4). Further, there are reports indicating the involvement of more than two segments in OLF resulting in poor outcomes; but, many studies have found no impact of the number of involved segments in the neurological outcome of patients with OLF [9]. However, the intramedullary signal size of the affected segment was found to be the most important predictor of surgical outcome in patients with thoracic myelopathy due to OLF [9].
The dural thickening in hypertrophic spinal pachymeningitis can mimic OPLL; however, contrast-enhanced MRI with gadolinium would demonstrate contrast enhancement in pachymeningitis differentiating it from its mimics [10].
There are few cases describing the combination of OPLL and OLF leading to cervical myelopathy [11, 12]. Another case described the concomitant occurrence of symptomatic OLF in the thoracic spine and asymptomatic OPLL of the cervical spine [13]. Concurrent occurrence of OPLL and OLF in the thoracic spine leading to thoracic myelopathy is not common and few articles are published in the [14, 15]. However, we could not find any reports in global literature where the simultaneous occurrence of HPLL and HLF in the thoracic spine.
This was a challenging case in both surgical and imaging perspectives. Since there was circumferential spinal cord compression in this patient due to the concurrent HPLL and HLF, performing both anterior and posterior decompression was challenging. Thus, only the posterior decompression was attempted. In the literature, some of the cases with circumferential spinal cord compression were managed with posterior decompression and laminectomy [12, 16]. Due to the complexed anterior anatomy, which can lead to more complications, the posterior approach is preferred over the anterior approach [16]. However, a recently published technical report has described simultaneous complete removal of OPLL and OLF through single-stage mini-thoracotomy [14]. In the imaging perspective, this case report highlights the importance of identifying HPLL and HLF in patients who present with features of myelopathy. Further, CT scanning is important in identifying calcifications or ossifications within the hypertrophied ligament. In addition, gadolinium-enhanced MRI would help in differentiating HPLL from hypertrophic spinal pachymeningitis. Since there are reports on the development of ossification and calcification within the hypertrophied segments, long-term follow-up of these patients is vital.
Data availability
All data related to this case report are included in this published paper and its supplementary information files.
References
Ikuta K, Arima J, Sasaki K, Oga M, Nakano S, Tanaka T, et al. Hypertrophy of the posterior longitudinal ligament in the thoracic spine. Spinal Cord. 2006;44:200–2. https://doi.org/10.1038/sj.sc.3101812.
Nozawa S, Shimizu K, Miyamoto K, Sakaguchi Y, Nishimoto H, Hosoe H. Sudden onset of paraparesis caused by hypertrophy of the thoracic posterior longitudinal ligament. Spinal Cord. 2003;41:53–5. https://doi.org/10.1038/sj.sc.3101396.
El Helou A, Alaywan M, Tarabay A, Nachanakian A. Ossification of ligamentum flavum, a rare cause of myelopathy: First case report of a Lebanese patient. Asian J Neurosurg. 2016;11:180 https://doi.org/10.4103/1793-5482.145067.
Bouchakour M, Stambouli A, Bentifour M, Benamara M, Beloud M. Hypertrophy of ligamentum flavum of the thoracic spine and spinal cord compression. Panarab J Neurosurg. 2011;15:85–6.
Yamada T, Torigoe I, Sakai K, Okawa A, Arai Y. Contiguous multilevel thoracic ossification of ligamentum flavum in a young adult spine. Case Rep Orthoped. 2019. https://doi.org/10.1155/2019/1640485.
Matsumoto T, Yoshida M, Yamada H, Kurimoto K, Tamaki T. Lumbar canal stenosis caused by hypertrophy of the posterior longitudinal ligament: case report. Spine. 2001;26:E576–E579. https://doi.org/10.1097/00007632-200112150-00028.
Cheung PWH, Tam V, Leung VYL, Samartzis D, Cheung KMC, Luk KDK, et al. The paradoxical relationship between ligamentum flavum hypertrophy and developmental lumbar spinal stenosis. Scoliosis Spinal Disord. 2016;11:1. https://doi.org/10.1186/s13013-016-0088-5.
Arasaki K, Anno I, Kanazawa I. Hypertrophy of the posterior longitudinal ligament: relationship with ossification of the posterior longitudinal ligament. J Neuroimaging. 1992;2:158–61. https://doi.org/10.1111/jon199223158.
Sanghvi AV, Chhabra HS, Mascarenhas AA, Mittal VK, Sangondimath GM. Thoracic myelopathy due to ossification of ligamentum flavum: a retrospective analysis of predictors of surgical outcome and factors affecting preoperative neurological status. Eur Spine J. 2011;20:205–15. https://doi.org/10.1007/s00586-010-1423-9.
Van Der Pol CB, Chakraborty S, Côté I, Humphrey-Murto S, Michaud J. Case 216: hypertrophic spinal pachymeningitis. Radiology. 2015;275:303–7. https://doi.org/10.1148/radiol.15122159.
Shah KS, Uchiyama CM. Thoracic ossification of the ligamentum flavum causing acute myelopathy in a patient with cervical ossification of the posterior longitudinal ligament: illustrative case. J Neurosurg: Case Lessons. 2021;2. https://doi.org/10.3171/CASE2178.
Kotani Y, Takahata M, Abumi K, Ito M, Sudo H, Minami A. Cervical myelopathy resulting from combined ossification of the ligamentum flavum and posterior longitudinal ligament: report of two cases and literature review. Spine J. 2013;13:e1–e6. https://doi.org/10.1016/j.spinee.2012.10.038.
Kondo S, Onari K, Watanabe KI, Hasegawa T, Toguchi A, Mihara H. Hypertrophy of the posterior longitudinal ligament is a prodromal condition to ossification: a cervical myelopathy case report. Spine 2001;26:110–4. https://doi.org/10.1097/00007632-200101010-00019.
Yoon J, Bae J, Shin SH, Bae Y, Lee SH. Novel Simultaneous Decompression Through Single-stage Mini-thoracotomy for Concurrent Ossification of the Posterior Longitudinal Ligament and Ossification of the Ligamentum Flavum at the Same Thoracic Level: A Technical Report and Literature Review. Spine 2021;46:E190–E196. https://doi.org/10.1097/BRS.0000000000003748.
Li M, Meng H, Du J, Tao H, Luo Z, Wang Z. Management of thoracic myelopathy caused by ossification of the posterior longitudinal ligament combined with ossification of the ligamentum flavum—a retrospective study. Spine J. 2012;12:1093–102. https://doi.org/10.1016/j.spinee.2012.10.022.
Li H, Wang J, Chen G, Li F, Zhu J, Chen Q. Combined upper cervical canal stenosis and cervical ossification of the posterior longitudinal ligament resulting in myelopathy: a case series and literature review. J Clin Neurosci. 2017;45:270–5. https://doi.org/10.1016/j.jocn.2017.08.003.
Acknowledgements
Authors wish to acknowledge the staff of the Neurosurgical Department, National Hospital, Kandy, Sri Lanka.
Author information
Authors and Affiliations
Contributions
MCW, LG were involved in the interpretation of the imaging findings. MCW, NDW prepared the original draft of the paper. MCW, NDW, LG read and approved the final version of the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
Informed verbal consent was obtained from the patient. The case report and the images included do not contain any personally identifiable information.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wettasinghe, M.C., Gamage, L. & Wickramasinghe, N.D. Hypertrophied posterior longitudinal ligament and ligamentum flavum causing myelopathy: a case report and literature review. Spinal Cord Ser Cases 9, 7 (2023). https://doi.org/10.1038/s41394-023-00565-7
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41394-023-00565-7

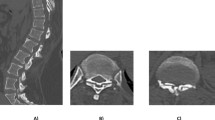


 ) and hypertrophied ligamentum flavum (
) and hypertrophied ligamentum flavum ( ).
).
 ) and hypertrophied ligamentum flavum (
) and hypertrophied ligamentum flavum ( ).
).