Abstract
Study design:
Retrospective multicenter study.
Objectives:
To analyze the predictive factors for postoperative ambulatory recovery in paretic non-ambulatory patients with metastatic spinal cord compression (MSCC).
Setting:
Japan.
Methods:
Eighty-two consecutive patients (74.4% men; mean age, 66.2 years) who could not walk before surgery due to cervical or thoracic MSCC and underwent posterior decompressive surgery between 2003 and 2014 were included. Patients were divided into two groups according to ambulatory status at 6 weeks after surgery: recovery (group R) and non-recovery (group NR). To evaluate the speed of progression of motor deficits, we assessed the period from onset of neurological symptoms to gait inability (T1).
Results:
Fifty patients (61.0%) regained the ability to walk (group R). The period of T1 demonstrated a positive correlation with probability of ambulatory recovery (P=0.00; Kendall’s tau-b=0.38), and a receiver operating characteristic curve analysis showed that the cutoff value of T1 was 5 days (area under the curve=0.72; P=0.001). In multivariate analysis, <6 days of T1 was one of the independent risk factors for failing to regain ambulatory ability (odds ratio, 8.74; P=0.00).
Conclusions:
The speed of progression of motor deficits can independently and powerfully predict the chance of postoperative ambulatory recovery as well as previously identified predictors. Since information about the speed of progression can be obtained easily by interviewing patients or family members, even if the patient is in an urgent state, our results will be helpful in clinical decision-making.
Similar content being viewed by others
Introduction
The spine is associated with ~50% of all bone metastases, making it the most frequent site of tumor invasion to bone.1 Metastatic spine disease accounts for 10–30% of new cancer diagnoses annually,2 and metastatic spinal cord compression (MSCC) affects 5–14% of all adult cancer patients during the course of their disease.3, 4, 5 Lesions may lead to myelopathy, which causes spasticity, ataxia, impaired sensation and loss-of-motor movements, and result in non-ambulatory status by compressing the spinal cord. Because survival time is strongly correlated to functional outcomes, including ambulatory ability,6, 7, 8, 9 regaining ambulatory ability is a primary goal of treatment for paretic non-ambulatory patients with MSCC.
Recently, decompressive surgery, which has been shown to be superior to radiotherapy in preserving or recovering neurological function,10, 11, 12 has become the standard treatment for MSCC. However, compared with other types of spinal surgery, treatment of spinal metastasis is associated with a greater risk of perioperative complications,13, 14, 15 which can cause shortening of a patient’s lifespan and lower quality of life.13, 16, 17 Therefore, the prediction of postoperative ambulatory status is critical when selecting the treatment modality for paretic non-ambulatory patients with MSCC. Although there have been only a small number of reports regarding the surgical outcomes for paretic non-ambulatory patients with MSCC, several independent predictive factors for regaining ambulatory ability have been reported, including preoperative general condition,18 primary tumor type,6, 18 timing of surgery6, 13 and preoperative motor power of the lower extremities.19 On the other hand, rapid progression of motor deficits has been associated significantly with poor survival and decreased ambulatory rate after radiotherapy,20, 21 and Eastley et al.22 described in their review article that recovery is unlikely if neurological symptoms progress rapidly. However, in previous studies of surgical outcomes for patients with MSCC, the speed of progression of motor deficits has not been investigated in detail.
The objective of this study was to investigate the surgical outcomes of paretic non-ambulatory patients with MSCC and to analyze the predictive factors for regaining the ability to walk, including the predictive role of the speed of motor deficit progression.
Materials and methods
Patients and data collection
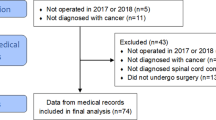
This study was reviewed and approved by the institutional review board of Niigata University. We retrospectively reviewed paretic non-ambulatory patients with MSCC who underwent palliative surgery for cervical or thoracic spinal metastasis at five institutions associated with Niigata University between 2003 and 2014. Patients who could not walk due to back or neck pain and those who had neurological deficits due to lumbar compressive lesions (cauda equina) were excluded from the study. Our indications for surgery in paretic non-ambulatory patients with MSCC included a life expectancy of at least 6 months based on an oncologist’s assessment. On the other hand, for patients who had neurological symptoms but neither gait inability nor severe spinal instability, conservative treatments, including bracing, radiation therapy, chemotherapy and hormonal therapy, were preferably performed. With regard to the surgical procedure, posterior decompression with or without stabilization using pedicle screw constructs was performed for all patients. For extradural tumors of the spinal canal, we completely resected the dorsal and lateral portion of the tumor, while partially resecting ventrally located tumors as much as possible. In principle, all patients were advised to undergo postoperative radiation therapy (30 Gy in 10 fractions), except for patients who underwent radiation therapy for metastatic spine tumor sites prior to surgery and those who suffered from postoperative complications such as wound infection.
We collected the data on patient demographics, preoperative systemic condition and neurological status, comorbidities, history of primary tumor, surgical procedures, postoperative complications, postoperative radiation therapy, neurological recovery and 6-month survival rates following the date of surgery. To evaluate the speed of progression of motor deficits, we investigated the period from the onset of neurological symptoms, such as numbness or motor weakness, to gait inability (T1). In addition, we also investigated the period from gait inability to surgery (T2). Preoperative systemic condition was assessed based on the Tokuhashi scoring system,23 which evaluates six parameters, including patient condition, location of metastasis (extraspinal bone, vertebral body and major organs), the site of primary cancer and spinal cord palsy. Neurological status was evaluated by Frankel score24 and motor power of hip flexion. Motor power of hip flexion was bilaterally evaluated using the manual muscle test (MMT), the lesser MMT was utilized for this study. Computed tomography images were used to identify pathological compression fractures with >50% collapse of affected vertebral bodies. The postoperative ambulatory status of patients was evaluated at a follow-up assessment after 6 weeks, which was the length of basic postoperative treatment and rehabilitation program in our institutions for patients with spinal metastasis. The survival rate at 6 months after surgery was investigated by reviewing medical charts or via telephone interviews with patients or their families.
The patient’s ability to walk at 6 weeks after surgery was used as the primary end point. Patients were divided into two groups: the recovery group (group R) included patients who regained the ability to walk more than 10 m at 6 weeks after surgery, even if a cane or walker was needed; the non-recovery group (group NR) included patients who were not able to walk after surgery.
Statistical analysis
Statistical analyses were performed using IBM SPSS Statistics for Windows version 21 (IBM Corp., Armonk, NY, USA). The continuous data are expressed as mean±s.d. Differences between group R and group NR were evaluated by unpaired t-test for continuous variables and by χ2-test or Fisher’s exact test for categorical variables. Analysis of the association between the evaluated target sizes was conducted by counting the correlation coefficient Kendall’s tau-b. We also plotted the receiver operating characteristic (ROC) curve to investigate cutoff values of the evaluated variables for failing to regain the ability to walk after surgery. In addition, stepwise logistic regression analysis was used to identify independent risk factors for failing to regain the ability to walk. First, a univariate analysis was performed to evaluate associations between preoperative variables and postoperative ambulatory status at 6 weeks after surgery. Factors of P<0.10 in the univariate analysis were then included in the multivariate analysis; P<0.05 was considered to be significant.
Results
Patient population and overall outcomes
The summary data of the patients are shown in Table 1 (left column). Eighty-two consecutive patients (61 men, 21 women) with a mean age of 66.2±10.6 years (range, 39–89 years) at the time of spinal surgery were included in the present study. Seventeen patients (20.7%) had at least one major comorbidity (for example, chronic obstructive pulmonary disease, diabetes mellitus and coronary artery disease). The primary tumor sites were lung in 19 patients, prostate in 19, gastrointestinal tract in 14 (stomach in 7, rectum in 4 and colon in 3), breast in 6, hematologic malignancies in 6 (malignant lymphoma in 3 and multiple myeloma in 3), liver in 5, kidney in 3, thyroid in 3 and others in 7 (heart (myxosarcoma), foot (liposarcoma), maxillary sinus, bile duct, pancreas, ureter and uterus; n=1 for each). Forty-nine of 82 patients (59.8%) underwent previous treatments for primary tumor, which included surgical resection in 28 patients (34.1%), chemotherapy or hormonal therapy in 38 (46.3%) and radiation therapy in 8 (9.8%). In 32 patients (39.0%), MSCC was diagnosed simultaneously along with a primary tumor. One patient with lung cancer underwent only follow-up examinations because pulmonary emphysema made it difficult to perform aggressive treatment. The locations of symptomatic spinal metastasis were the thoracic spine in 76 patients and cervical spine in 6 patients. The overall average Tokuhashi score was 7.1±2.7 (range, 1–12 points). Preoperative Frankel grade was A in 1 patient, B in 11 and C in 70. Fifty-two of the 82 patients (63.4%) had preoperative motor weakness (MMT<3) of hip flexion and 18 patients (22.0%) had pathological vertebral fracture with >50% collapse. With regard to the duration of neurological symptoms, T1 averaged 27.6±49.7 days (range, 0–280 days) and T2 averaged 6.8±12.2 days (range, 0–90 days). As a result, the period from the onset of neurological symptoms to surgery (T1+T2) averaged 34.4±51.5 days (range, 1–285 days).
Surgical procedures included posterior decompression with stabilization for 72 patients and without stabilization for 10 patients. Postoperative radiation therapy was administered to 46 patients (56.1%). In 20 patients (24.4%), 23 postoperative complications occurred, including wound-related problems (n=4), paralytic ileus (n=3), hematoma (n=3), neurological impairment (n=2), acute respiratory distress syndrome (n=2), colitis (n=2), urinary tract infection (n=2) and others (pulmonary embolism, pancytopenia, malposition of screws, pathological fracture of humerus, fracture of lower instrumented vertebra; n=1 for each). Six weeks after surgery, the Frankel grade improved in 52 patients (63.4%), remained unchanged in 27 (32.9%), and deteriorated in 3 (3.7%). As a result, 50 of the 82 patients (61.0%) regained the ability to walk (group R), while 32 patients remained unable to walk (group NR). Fifty-four of 82 patients were alive at the 6-month follow-up assessment (survival rate=65.9%).
Comparison between recovery and non-recovery groups
The comparison between groups R and NR is summarized in Table 1. For preoperative variables, significant differences were noted in gender, primary tumor site, Tokuhashi score, Frankel grade, preoperative motor power of hip flexion and the period of T1. In group NR, male gender (87.5%), gastrointestinal tract as the primary tumor site (31.3%), preoperative Frankel grade A or B (31.3%) and preoperative motor weakness (MMT<3) of hip flexion (78.1%) were more often observed than in group R (66.0%, 8.0%, 4.0% and 54.0%, respectively; P<0.05 for each). In group R, prostate cancer was more often observed compared with group NR (32.0 vs 9.4%, P=0.03). Tokuhashi score and the period of T1 in group R (7.9±2.6 points and 39.8±59.9 days, respectively) were significantly higher and longer than in group NR (5.8±2.4 points and 8.5±13.1 days, respectively; P<0.01 for each). The period of T2 in group R (8.6±14.8 days) tended to be longer than that of group NR (4.0±5.3 days), although the difference between the two groups was not statistically significant (P=0.095). For postoperative variables, patients in group R more frequently underwent postoperative radiation therapy (72%) and incurred less postoperative complications (14%) compared with patients in group NR (31.3 and 40.6%, respectively; P<0.01 for each). As a result, the 6-month survival rate after surgery of group R (92%) was significantly higher than that of group NR (25%, P<0.0001).
Neurological recovery dependent on the period from the onset of neurological symptoms to gait inability (T1)
To evaluate the correlation between the speed of progression of motor deficits and the chance of regaining ambulatory ability, patients were divided into four groups based on quartiles of distribution of T1, which were 3, 8 and 30 days. The duration of T1 was <3 days in 28 patients, 3–7 days in 12, 8–30 days in 16 and >30 days in 26. Analysis of associations between the duration of T1 and preoperative conditions demonstrated significant negative correlation with the incidence of motor palsy (that is, Frankel grade A or B and motor weakness of hip flexion with MMT<3) and a significant positive correlation with the incidence of pathological vertebral fractures with >50% collapse (Table 2). On the other hand, there was no significant correlation between the duration of T1 and Tokuhashi score (Table 2). In 28 patients with <3 days of T1, 11 patients (39.3%) regained the ability to walk after surgery. In 12 patients with 3–7 days of T1, 6 patients (50.0%) regained postoperative ambulatory status. In patients with 8–30 days of T1, 10 of 16 patients (62.5%) regained ambulatory ability. In 26 patients with more than 30 days of T1, 23 patients (88.5%) regained the ability to walk postoperatively. Comparison of the analyzed subgroups showed a significant correlation between the duration of T1 and regaining the ability to walk (P=0.00; Kendall’s tau-b=0.38; Figure 1).
When plotting the ROC curve of the duration of T1 for the detection of failing to regain ambulatory ability after surgery, the cutoff value was 5 days (area under the curve (AUC)=0.72, P=0.001) (Figure 2).
Preoperative risk factors for failing to regain ambulatory ability
In logistic regression analyses, Tokuhashi score and the period of T1 were dichotomized into two categories according to the cutoff values for failing to regain ambulatory ability after surgery. For Tokuhashi score, ROC analysis determined that the cutoff value for the detection of postoperative non-ambulatory status was 7.5 points (AUC=0.71, P=0.001).
In a univariate analysis, male gender, primary tumor site (prostate, gastrointestinal tract and liver), Tokuhashi score, preoperative Frankel grade and motor weakness (MMT<3) of hip flexion, and the period of T1 were associated with failing to regain ambulatory ability 6 weeks after surgery (P<0.1) (Table 3, left column). On the other hand, Tokuhashi score, which includes neurological status, did not significantly correlate with the incidence of Frankel grade A or B (P=0.20) or motor weakness (MMT<3) of hip flexion (P=0.28). Therefore, all factors with P<0.10 in the univariate analysis were then included in the multivariate analysis.
In a multivariate analysis, gastrointestinal tract as primary tumor site (odds ratio (OR), 5.47; 95% confidence interval (CI), 1.16–25.72; P=0.0032), less than an 8-point Tokuhashi score (OR, 3.78; 95% CI, 1.14–12.56; P=0.03), preoperative Frankel grade A or B (OR, 11.32; 95% CI, 1.50–85.45; P=0.019) and <6 days of T1 (OR, 8.74; 95% CI, 2.59–29.55; P=0.00) independently predicted failure to regain ambulatory ability 6 weeks after surgery (Table 3, right column).
Illustrative case
A 71-year-old male, who had received hormonal therapy for prostate cancer for 4 years, presented with difficulty in walking, which progressed for 20 days after the onset of neurological symptoms (T1=20 days). On examination, he demonstrated bilateral motor weakness (MMT of hip flexion=2) with bladder dysfunction (Frankel grade C; Tokuhashi score, 11 points). Preoperative magnetic resonance imaging showed a T12 vertebral fracture with <50% collapse and spinal cord compression (Figures 3a and b). The day after admission to our hospital, he underwent posterior decompression and instrumented fusion surgery (T2=24 days), followed by radiotherapy 2 weeks after surgery. The postoperative course was uneventful with improved neurological function, and he maintained independent ambulation at 1 year after surgery (Frankel grade D; Figures 3c and d)
A 71-year-old male with prostate cancer. (a) Preoperative magnetic resonance imaging (MRI, T2-weighted sagittal view) demonstrates a pathological fracture of the T12 vertebral body and spinal cord compression. (b) Preoperative MRI (T2-weighted axial view) shows that the T12 vertebral body, left pedicle and transverse process, and lamina were infiltrated with the tumor, and the epidural mass compressed the spinal cord. (c, d) Postoperative anteroposterior standing X-ray (c) and lateral (d) images at one year after surgery.
Discussion
Metastatic spinal cord compression, especially for paretic non-ambulatory patients, remains a challenging problem despite the development of surgical techniques and improved spinal instrumentation.10, 11, 12 The goals of surgical treatment for paretic non-ambulatory patients are to regain ambulatory ability and improve quality of life, which can lead to prolonged survival time.6, 7, 8, 13, 18, 19, 25, 26 In the present study, 61% of paretic non-ambulatory patients with MSCC regained the ability to walk after surgery, which is similar to previously reported results (50–70%).6, 12, 13, 19 Moreover, similar to a previous study,13 the 6-month survival rate after surgery of patients who regained ambulatory ability was significantly higher than that of patients who did not regain ambulatory ability (92 vs 25%). On the other hand, the relatively high complication rate associated with surgery for MSCC must be considered. In the present study, the rate of postoperative complication was 24.4%, which is similar to the findings of previous reports.13, 14, 26, 27 Thus, surgical treatment should be carefully indicated, and the preoperative prediction of postoperative ambulatory recovery is critical for paretic non-ambulatory patients with MSCC.
In this study, we analyzed risk factors associated with failing to regain the ability to walk after surgery in paretic non-ambulatory patients with MSCC using preoperative variables. We showed that male gender, gastrointestinal tract as primary tumor site, low Tokuhashi score, Frankel grade A or B, motor weakness (MMT<3) of hip flexion and a short period of T1 were more often observed in group NR, while prostate cancer as the primary tumor was more often observed in group R. Logistic regression analysis showed four preoperative variables to be significant risk factors for failing to regain the ability to walk after surgery (gastrointestinal tract as primary tumor site, Tokuhashi score <8 points, preoperative Frankel grade A or B and <6 days of T1).
With respect to the primary cancer site, Tokuhashi score and preoperative Frankel grade, our results agree with previous studies.6, 12, 18 On the other hand, Park et al.19 reported that preoperative motor strength of the lower extremities can predict postoperative ability to walk in patients with MSCC. In the present study, motor power weakness of hip flexion was more often observed in group NR, although it did not reach significance in the multivariate analysis. However, we consider motor power of hip flexion to be very important for walking because it is difficult to emulate its function with walking aids or orthoses. Therefore, we believe that preoperative motor power of hip flexion is also an important variable for predicting postoperative ambulatory status, which concurs with Park et al.’s interpretation.19
In this study, we also assessed the speed of progression of motor deficits, which was evaluated by the period from the onset of neurological symptoms to gait inability (T1). Although previous research has demonstrated that the timing of surgery is an important factor for postoperative neurological recovery in patients with MSCC,6, 28, 29, 30 those studies evaluated the period from the onset of neurological symptoms to surgery, not to gait inability. Thus, to our knowledge, no study has analyzed whether the speed of progression of motor deficits can predict neurological recovery after surgery in patients with MSCC. The present study demonstrates a significant positive correlation between the period of T1 and the probability of regaining ambulatory ability after surgery and significant negative correlations between the period of T1 and preoperative severity of motor palsy. In other words, as neurological symptoms progress rapidly, the chances of regaining the ability to walk decrease. Moreover, we demonstrated a cutoff value of 5 days, which was a powerful predictor for regaining the ability to walk in the multivariate analysis. On the other hand, the incidence of pathological vertebral fractures with >50% collapse, which is one of the factors inducing mechanical instability of spinal column, was positively correlated with the duration of T1. Therefore, the dynamics underlying the development of motor deficits, at least in our cohort, may reflect the velocity of tumor growth, and it may be one of the reasons for the lower 6-month survival rate noted in group NR.
In contrast to the results of previous studies,6, 13, 19, 28, 29, 30 the timing of surgery was not correlated with postoperative ambulatory recovery in the present study. The reason for this discrepancy may be due to the different distribution of the timing of surgery in our retrospective cohort from those in previous reports. More than 60% of patients underwent surgery within 48 h after the onset of symptoms in two studies19, 28 and within 15 days in one study.29 With respect to the period from gait inability to surgery (T2), more than 70% of patients underwent surgery within 3 days.13 In our study, the duration of neurological symptoms before surgery averaged 34.4 days (range, 1–285 days;<48 h in 6.1% of patients) and the period of T2 averaged 6.8 days (range, 0–90 days;<48 h in 45.1%). These discrepancies with previous studies may be due to bias associated with the retrospective, multicenter design of the present study. Although we believe that time is a function in neurological injury and surgical intervention should be performed as soon as possible, further prospective studies are needed to clarify the correlation between timing of surgery and neurological outcomes.
There are some limitations in this study. First, our study was a retrospective, multicenter investigation. Although all spine surgeries were performed by board-certified spine surgeons approved by the Japanese Board of Spine Surgery, there may have been differences in patient selection, surgical skill and the timing of surgery according to the surgeon, the institution, or other unforeseen variables. Second, we could not evaluate the long-term outcomes of ambulatory status after surgery because of the insufficient data about date of death and neurological status after leaving the treatment institutions. Recovery of motor function at an early time point after surgery has been reported to be consistent for long-term follow-up.19 Moreover, by evaluating neurological recovery at an early time point after surgery, we can evaluate the effects of surgery for motor deficits, excluding the development of other cancer-related symptoms. However, to our knowledge, there have been only a few retrospective, small sample size studies of long-term surgical outcomes for paretic non-ambulatory patients with MSCC.13, 19 Further prospective, high-volume studies are therefore needed to provide evidence about long-term outcomes.
Despite these limitations, our findings are meaningful in that they provide evidence of the probability of regaining postoperative ambulatory ability according to the speed of progression of motor deficits. This information will be useful not only for physicians’ decision-making but also for helping patients and their families make informed decisions regarding future courses of treatment.
Conclusion
In addition to previously reported risk factors, including the gastrointestinal tract as a primary tumor site, Tokuhashi score <8 points and preoperative Frankel grade A or B, the speed of progression of motor deficits can also independently and powerfully predict the chance of recovery of walking ability after surgery for paretic non-ambulatory patients with MSCC. In this study, faster progression of neurological deficits was associated significantly with lower probability of regaining the ability to walk after surgery. Since information about the speed of progression of motor deficits can be easily obtained by interviewing a patient or their family members, even if the patient is in an urgent state, our results will be helpful in guiding patient choices and in clinical decision-making.
Data archiving
There were no data to deposit.
References
Aebi M . Spinal metastasis in the elderly. Eur Spine J 2003; 12 (Suppl 2): S202–S213.
White AP, Kwon BK, Lindskog DM, Friedlaender GE, Grauer JN . Metastatic disease of the spine. J Am Acad Orthop Surg 2006; 14: 587–598.
Byrne TN . Spinal cord compression from epidural metastases. N Engl J Med 1992; 327: 614–619.
Nelson KA, Walsh D, Abdullah O, McDonnell F, Homsi J, Komurcu S et al. Common complications of advanced cancer. Semin Oncol 2000; 27: 34–44.
Prasad D, Schiff D . Malignant spinal-cord compression. Lancet Oncol 2005; 6: 15–24.
Chaichana KL, Woodworth GF, Sciubba DM, McGirt MJ, Witham TJ, Bydon A et al. Predictors of ambulatory function after decompressive surgery for metastatic epidural spinal cord compression. Neurosurgery 2008; 62: 683–692.
Kim CH, Chung CK, Jahng TA, Kim HJ . Surgical outcome of spinal hepatocellular carcinoma metastases. Neurosurgery 2011; 68: 888–896.
Yang SB, Cho W, Chang UK . Analysis of prognostic factors relating to postoperative survival in spinal metastases. J Korean Neurosurg Soc 2012; 51: 127–134.
Park JH, Rhim SC, Jeon SR . Efficacy of decompression and fixation for metastatic spinal cord compression: analysis of factors prognostic for survival and postoperative ambulation. J Korean Neurosurg Soc 2011; 50: 434–440.
Kim JM, Losina E, Bono CM, Schoenfeld AJ, Collins JE, Katz JN et al. Clinical outcome of metastatic spinal cord compression treated with surgical excision +/- radiation versus radiation therapy alone: a systematic review of literature. Spine 2012; 37: 78–84.
Klimo P Jr, Thompson CJ, Kestle JR, Schmidt MH . A meta-analysis of surgery versus conventional radiotherapy for the treatment of metastatic spinal epidural disease. Neuro Oncol 2005; 7: 64–76.
Patchell RA, Tibbs PA, Regine WF, Payne R, Saris S, Kryscio RJ et al. Direct decompressive surgical resection in the treatment of spinal cord compression caused by metastatic cancer: a randomised trial. Lancet 2005; 366: 643–648.
Kim CH, Chung CK, Jahng TA, Kim HJ . Resumption of ambulatory status after surgery for nonambulatory patients with epidural spinal metastasis. Spine J 2011; 11: 1015–1023.
Lee BH, Park JO, Kim HS, Park YC, Lee HM, Moon SH . Perioperative complication and surgical outcome in patients with spine metastases: retrospective 200-case series in a single institute. Clin Neurol Neurosurg 2014; 122: 80–86.
Omeis IA, Dhir M, Sciubba DM, Gottfried ON, McGirt MJ, Attenello FJ et al. Postoperative surgical site infections in patients undergoing spinal tumor surgery: incidence and risk factors. Spine 2011; 36: 1410–1419.
Spiegel DA, Sampson JH, Richardson WJ, Friedman AH, Rossitch E, Hardaker WT Jr et al. Metastatic melanoma to the spine. Demographics, risk factors, and prognosis in 114 patients. Spine 1995; 20: 2141–2146.
Tomita K, Kawahara N, Kobayashi T, Yoshida A, Murakami H, Akamaru T . Surgical strategy for spinal metastases. Spine 2001; 26: 298–306.
Yamashita T, Aota Y, Kushida K, Murayama H, Hiruma T, Takeyama M et al. Changes in physical function after palliative surgery for metastatic spinal tumor: association of the revised Tokuhashi score with neurologic recovery. Spine 2008; 33: 2341–2346.
Park JH, Jeon SR . Pre- and postoperative lower extremity motor power and ambulatory status of patients with spinal cord compression due to a metastatic spinal tumor. Spine 2013; 38: E798–E802.
Rades D, Fehlauer F, Schulte R, Veninga T, Stalpers LJ, Basic H et al. Prognostic factors for local control and survival after radiotherapy of metastatic spinal cord compression. J Clin Oncol 2006; 24: 3388–3393.
Rades D, Heidenreich F, Karstens JH . Final results of a prospective study of the prognostic value of the time to develop motor deficits before irradiation in metastatic spinal cord compression. Int J Radiat Oncol Biol Phys 2002; 53: 975–979.
Eastley N, Newey M, Ashford RU . Skeletal metastases—the role of the orthopaedic and spinal surgeon. Surg Oncol 2012; 21: 216–222.
Tokuhashi Y, Matsuzaki H, Oda H, Oshima M, Ryu J . A revised scoring system for preoperative evaluation of metastatic spine tumor prognosis. Spine 2005; 30: 2186–2191.
Frankel HL, Hancock DO, Hyslop G, Melzak J, Michaelis LS, Ungar GH et al. The value of postural reduction in the initial management of closed injuries of the spine with paraplegia and tetraplegia. I. Paraplegia 1969; 7: 179–192.
Eleraky M, Papanastassiou I, Vrionis FD . Management of metastatic spine disease. Curr Opin Support Palliat Care 2010; 4: 182–188.
Hirabayashi H, Ebara S, Kinoshita T, Yuzawa Y, Nakamura I, Takahashi J et al. Clinical outcome and survival after palliative surgery for spinal metastases: palliative surgery in spinal metastases. Cancer 2003; 97: 476–484.
Abrahm JL, Banffy MB, Harris MB . Spinal cord compression in patients with advanced metastatic cancer: ‘all I care about is walking and living my life’. JAMA 2008; 299: 937–946.
Furstenberg CH, Wiedenhofer B, Gerner HJ, Putz C . The effect of early surgical treatment on recovery in patients with metastatic compression of the spinal cord. J Bone Joint Surg Br 2009; 91: 240–244.
Hessler C, Burkhardt T, Raimund F, Regelsberger J, Vettorazzi E, Madert J et al. Dynamics of neurological deficit after surgical decompression of symptomatic vertebral metastases. Spine 2009; 34: 566–571.
Quraishi NA, Rajagopal TS, Manoharan SR, Elsayed S, Edwards KL, Boszczyk BM . Effect of timing of surgery on neurological outcome and survival in metastatic spinal cord compression. Eur Spine J 2013; 22: 1383–1388.
Acknowledgements
No funds were received in support of this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Ohashi, M., Hirano, T., Watanabe, K. et al. Preoperative prediction for regaining ambulatory ability in paretic non-ambulatory patients with metastatic spinal cord compression. Spinal Cord 55, 447–453 (2017). https://doi.org/10.1038/sc.2016.145
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sc.2016.145