Abstract
Study design:
Prospective cohort study.
Objectives:
To characterize the cerebrospinal fluid (CSF) concentrations of glial fibrillary acidic protein, neuron specific enolase (NSE), S-100β, tau and neurofilament heavy chain (NFH) within 24 h of an acute traumatic spinal cord injury (SCI), and to correlate these concentrations with the baseline severity of neurologic impairment as graded by the American Spinal Injury Association impairment scale (AIS).
Methods:
A lumbar puncture was performed to obtain CSF from 16 acute traumatic SCI patients within 24 h post injury. Neurological examinations were performed within 24 h of injury and again at 6 or 12 months post injury. The correlations between the CSF concentrations and initial AIS were calculated by using Pearson correlation coefficients. In addition, an independent Student’s t-test was used to test for differences in CSF concentrations between patients of different AIS grades.
Results:
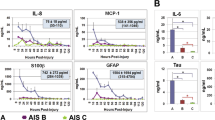
The CSF NSE concentrations were significantly correlated with the baseline neurologic impairment being either ‘motor complete’ (AIS A, B) or ‘motor incomplete’ (AIS C, D) (r=0.520, P<0.05). The mean S-100β concentration in motor complete patients was significantly higher compared with motor incomplete patients; 377.2 μg l−1 (s.d.±523 μg l−1) vs 57.1 μg l−1 (s.d.±56 μg l−1) (P<0.05), respectively. Lastly, the mean NFH concentration in motor complete patients was significantly higher compared with motor incomplete patient, 11 813 ng l−1 (s.d.±16 195 ng l−1) vs 1446.8 ng l−1 (s.d.±1533 ng l−1), (P<0.05), respectively.
Conclusion:
In this study we identified differences in the structural CSF biomarkers NSE, S-100β and NFH between motor complete and motor incomplete SCI patients. Our data showed no clear differences in any of the protein concentrations between the different AIS grades.
Similar content being viewed by others
Introduction
In traumatic spinal cord injury (SCI), much effort has been put into the evaluation of SCI severity and the prediction of neurologic recovery. Interventions intended to improve neurologic function following SCI include pharmacological,1 surgical2 and rehabilitation3 approaches. Unequivocally demonstrating the neurologic efficacy of these interventions in clinical trials has, to date, been challenging. Contributing to this difficulty has been the considerable variability in spontaneous neurologic recovery that occurs among SCI patients of the same ASIA impairment scale (AIS) grade. Measures to better stratify injury severity and precisely predict eventual neurologic recovery would be extremely valuable in the evaluation of novel pharmacologic or surgical interventions for acute SCI.4, 5
Following a traumatic SCI, the initial severity of neurologic impairment is the best predictor of long-term neurologic outcome.6 The assessment of neurologic impairment in accordance with the ISNCSCI (International Standards for Neurological Classification of Spinal Cord Injury) is considered to be most reliable and prognostic when conducted 72 h after the initial trauma.7 Before the 72 h post-injury mark, several factors such as spinal shock, medical instability or concomitant injuries affect the reliability of the neurological examination.8 Furthermore, even with a reliable baseline neurologic examination performed acutely after injury, the extent of spontaneous recovery among SCI patients with the same AIS grade is extremely variable.4 This variability in natural recovery forces investigators to enroll large numbers of patients into clinical trials of acute SCI therapies. Therefore, an accurate diagnostic–prognostic test, which more precisely predicts the neurologic outcome would greatly facilitate the conduct of such clinical trials.9
A new approach for evaluating the extent of spinal cord damage in the acute phase is the measurement of specific neural proteins within the cerebrospinal fluid (CSF).10 Trauma to the spinal cord causes an acute disruption of the spinal cord parenchyma. This is followed by a secondary axonal degeneration and further degeneration or death of nerve cells by either apoptosis or necrosis, processes that may last from days to weeks. As the spinal cord is surrounded by CSF, damage to the spinal cord releases proteins and metabolites from the nervous tissue into the CSF. This process allows for the study of ‘biomarkers’ in the CSF.10, 11, 12, 13 Although, several studies have been published concerning the use of biomarkers in CSF of patients with traumatic brain injury, only few studies exist in the field of SCI.10 The potential of this approach in traumatic SCI was recently demonstrated by using the CSF concentration of several inflammatory cytokines and structural proteins such as S100β, tau and glial fibrillary acidic protein (GFAP) in patients within 24 h post injury.9 However, several other promising markers like neuron specific enolase (NSE) and neurofilament heavy chain (NFH) have not prospectively been investigated in SCI patients within 24 h post injury.10
Therefore, the main purpose of the current study was to determine if the 24 h post-injury CSF concentrations of a number of structural markers (GFAP, NSE, S-100β, tau and NFH) correlated with the baseline AIS grade of patients with an acute traumatic SCI. We also sought to establish the relationship between these proteins and neurologic recovery.
Materials and methods
Patients
Two level 1 trauma centers (Nijmegen, the Netherlands and Vancouver, Canada) prospectively recruited patients with complete or incomplete traumatic SCI between 2007 and 2011. Patients were recruited on the basis of the following inclusion criteria: 18 years or older; blunt SCI between C2 and T12; presentation and operative decompression and/or stabilization within 24 h of injury; and the ability to undergo a valid, reliable neurological examination according to the ISNCSCI.14 Patients were excluded if they had concomitant major trauma to the chest, pelvis and/or extremities requiring immediate invasive intervention, or if they suffered from pre-existent neurodegenerative disorders. Furthermore, eye opening and verbal response according to the Glasgow Coma Scale had to be 4 and 6, respectively. All patients provided informed consent (third-party assent was not allowed).
All patients underwent a neurological assessment according to the ISNCSCI by a certified physician or study nurse having at least 1 year of experience in examining patients with SCI. They were classified as: AIS grade A (no motor or sensory function preservation in the sacral segments S4–S5), AIS grade B (sensory but not motor function preservation below the neurological level of injury (NLI) and includes the sacral segments S4–S5), AIS grade C (motor function preservation below the NLI, and more than half of the key muscles below the NLI have a muscle grade less than 3) or AIS grade D (motor function preservation below the NLI, and at least half of the key muscles below the NLI have a muscle grade of 3 or more).14
The study protocols were approved by the respective local ethics committees and were registered within the Dutch or American clinical trial registries (trialregister.nl identifier NTR1381, clinicaltrials.gov identifier NCT00135278).
Analyses
CSF samples were obtained under supervision of the spine surgeon. Using strict aseptic technique in laterally positioned patients, lumbar punctures were performed at L3–L4 or L4–L5, and a 3–5 ml sample of CSF was obtained in a polypropylene tube. In Vancouver, the lumbar puncture was followed by insertion of intrathecal catheter (PERIFIX Custom Epidural Anesthesia Kit; B. Braun Medical Inc., Bethlehem, PA, USA). Samples were drawn from this catheter using a strict sterile technique every 6–8 h. The first samples from those patients, punctured within 24 h post injury, were included in this analysis. Within 1 h of acquisition, samples were centrifuged at 3000 r.p.m. for 5 min and the supernatant was immediately stored at −80 °C until analyzed.
For the biochemical analysis, we used previously described sandwich ELISAs for following CNS-specific proteins: NFH, tau, GFAP, S-100β and NSE.15, 16, 17, 18, 19
Levels of NFHp35 were determined using a modified version of the sandwich ELISA. In summary, the microtiter plates were coated with mouse anti-phosphorylated NFHp35 antibodies (SMI35; Sternberger Monoclonals Inc., Sanbio, Uden, The Netherlands) and subsequently incubated with bovine NFHp35 standard (ICN, Burlingame, CA, USA) or CSF samples, polyclonal rabbit anti-NFHp35 antibody (Affiniti Research Products, Exeter, UK) and with polyclonal peroxidase-labeled goat anti-rabbit antibodies (Jackson, Immunoresearch, Westgrove, PA, USA). Tetramethylbenzidine was used as a substrate in the peroxidase reaction, and absorbance was read at 450 nm. Tris-buffered saline (pH 8.9) was used as a washing and dilution buffer. The detection limit of the assay was calculated as the mean+3 s.d. of the zero standard signal from 34 measurements, and was determined to be 17 ng l−1. Mean recovery of the assay was 91.2% (n=14). The intra-assay variation coefficient (VC) was 8.3% at a concentration of 71 ng l−1 (n=12), 22.5% at a concentration of 38 ng l−1 (n=12) and 2.8% at a concentration of 423 ng l−1 (n=12). The interassay VC was 18% at a concentration of 35 ng l−1 (n=18).20
Both NSE and S-100β concentrations were analyzed in an immunoluminometric assay (Byk Sangtec, Dietzenbach, Germany) by using the Liaison automated analyzer (Byk Sangtec). The assays were linear up to 200 μg l−1 (NSE) and 30 μg l−1 (S-100β). The interassay VCs were <5.3% (NSE) and <11% (S100B). CSF GFAP was measured by using a homemade sandwich ELISA21, 22 (linear up to 250 μg l−1; interassay VC <14%). CSF tau was measured by using the Innotest hTau assay (Innogenetics, Gent, Belgium; linearity up to 1200 pg l−1; interassay VC <6.0%).
The reference CSF concentration ranges were: NSE <17.5 μg l−1, Tau <300 ng l−1, GFAP <1.5 μg l−1, S-100β <3.3 μg l−1 and NFH <115 ng l−1. The reference ranges for the above structural biomarkers in CSF were determined by analysis of CSF in patients who were examined for a neurological disorder, but were diagnosed with either a systemic disease without neurological manifestations (for example, with tension-type headache or depression). As additional requirements, all routine analyses (for example, cell count, glucose, lactate, total protein, blood pigments, oligoclonal IgG bands) had to be in the normal range for each patient to be regarded as control.16, 18, 20, 21, 23
Neurological outcomes
Neurological examinations were conducted according to the ISCNSCI standards.24 All patients with an acute phase neurological examination (within the first 24 h after the injury) were included for the analysis. In addition, chronic phase (6 or 12 months) measurements had to be performed in each patient.25 On the basis of the ASIA sensory and motor scores, the level of injury and AIS grade were determined.
Statistical analysis
Statistical analyses were performed using SPSS 15.0 for windows. Data were presented as mean (s.d.) unless stated otherwise. We tested for a correlation between CSF concentrations and age as well by calculating Pearson correlation coefficients. Spearman’s rank or Pearson’s correlations test were also calculated between the CSF concentrations and initial motor complete (AIS A and B) or incomplete SCI patients (AIS C and D). An independent Student’s t-test was used to test for differences in CSF concentrations between patients of different AIS grades. The AIS grades were considered as the gold standard for SCI severity. In addition, the initial mean CSF concentrations were compared between the different AIS grades 6 or 12 months post injury.
Results
A total of 23 patients who were admitted to one of the trauma centers following blunt traumatic SCI were considered. Seven patients were excluded, three because the time of injury to CSF sampling was >24 h, two because the 6 or 12 months post-injury neurological assessments were missing and two because their CSF samples were grossly contaminated with blood. A total of 16 patients were thus included. In one of these 16 patients (case 13), there was an insufficient amount of CSF obtained to measure the NFH concentration; the measurement of the other CSF proteins in this patient were included in the overall analysis of the other CSF proteins.
Ten patients were male and the mean age of the included patients was 46 years (range, 18–84). The mechanism of injury was a fall from height in the majority of the patients. The AIS grades were A (n=7), B (n=2), C (n=4) and D (n=3). In addition, the mean time of injury to CSF sampling was 14 h (range, 3–24 h). See Table 1.
For each patient, the CSF concentrations of NSE, S-100β, GFAP, NFH and Tau are listed in Table 1. These concentrations are stratified by the baseline level of neurologic impairment (AIS A, B, C or D) in Table 2. As shown in Table 2, the concentrations of all of the proteins are generally highest in the most severely injured (AIS A), and then tend to decrease in each of the less severe AIS grades. The only exception was NFH, where the mean concentration was 11 636 ng l−1 in the AIS A and 12 431 ng l−1 in the AIS B patients (although it should be acknowledged that there were only two patients with AIS B injuries).
The mean NSE concentration in motor complete patients (AIS A and B) was significantly higher compared with motor incomplete patients (AIS C and D); 83.7 μg l−1 (s.d.±75 μg l−1) vs 25.3 μg l−1 (s.d.±17 μg l−1) (P<0.05), respectively. In addition, the mean S-100β concentration in motor complete patients was significantly higher compared with motor incomplete patients; 377.2 μg l−1 (s.d.±523 μg l−1) vs 57.1 μg l−1 (s.d.±56 μg l−1) (P<0.05), respectively. Finally, the mean NFH concentration in motor complete patients was significantly higher compared with motor incomplete patient, 11 813 ng l−1 (s.d.±16 195 ng l−1) vs 1446.8 ng l−1 (s.d.±1533 ng l−1), (P<0.05), respectively.
When comparing the mean CSF concentrations by AIS grade within 24 h post injury, the mean concentrations of all the biomarkers were lower when the AIS grade was less severe (that is, AIS C and D). Although CSF concentrations were the lowest in the AIS C and D patients, only the NSE and S-100β differed significantly (P<0.05) between AIS A and AIS C or D patients. In addition, only S-100β and NFH concentrations differed significantly (P<0.05) between AIS B and AIS C patients. Finally, the GFAP and S-100β concentrations were lower (P<0.05) in AIS D patients compared with AIS C patients (Table 2).
No significant differences in CSF concentrations were observed between males and females, nor was there a significant correlation between CSF concentrations and age. Only the NSE concentrations had a significant correlation with patients being motor complete or incomplete (r=0.520, P<0.05) within 24 h post injury. When comparing the correlation between the time of injury with CSF sampling and the biomarker concentrations in all the 16 subjects, no significant correlations were indentified. However, in the seven AIS A patients, statistically significant correlations were identified between the time post injury when the CSF samples were obtained and the CSF concentrations for NSE (r=0.897, P<0.05), S-100β (r=0.863, P<0.05) and NFH (r=0.782, P<0.05).
Finally, the concentrations of S-100β,GFAP and Tau were lower in AIS A patients who improved in their AIS grade as compared with AIS A patients who remained AIS A. Interestingly, GFAP and Tau concentrations in AIS A patients who remained AIS A at follow-up, were 9.6 and 2.5 times higher, respectively, compared with the GFAP and Tau concentrations in AIS A patients who neurologically ‘converted’ to an AIS B. See Table 3.
Discussion
In this study, when stratifying patients as motor complete (AIS A+B) versus motor incomplete (AIS C+D), there were significant differences in NSE, S100b and NFH concentrations within the CSF. With the small number of patients in this analysis, there was no significant difference in any of the proteins when comparing between the individual AIS grades.
The CSF concentrations of all the proteins examined were elevated in the SCI patients as compared with uninjured controls irrespective of the AIS severity. This suggests (not surprisingly) that trauma to the spinal cord causes a release of proteins from the cord into the CSF. Our objective was to determine if the CSF concentration of these proteins differed according to injury severity. With the small number of patients in each AIS grade (A (n=7), B (n=2), C (n=4) and D (n=3)) and the different time points of CSF collection (ranging from 3 to 24 h post injury), there were no significant differences between each AIS grade for any of the proteins tested. This was in contrast to the single-center study of Kwon et al.9 in which there were significant differences between AIS grades in the 24 h post-injury CSF concentrations for a number of different markers, including tau, S100β, GFAP, IL-6, IL-8 and MCP-1. In our current study, when stratifying patients as motor complete (AIS A+B) versus motor incomplete (AIS C+D), there were significant differences in CSF concentrations of NSE, S100β and NFH.
Aside from the small numbers of patients, a possible explanation for the variability in CSF concentrations and the inability to distinguish different injury severities was the variability with which the CSF was collected in this particularly study—ranging from 3 h to 24 h post injury, with the mean time of injury to CSF sampling of 14 h. Given the complex and dynamic pathology of SCI, it can be expected that the levels of SCI biomarkers evident within the CSF will be time dependent26 as shown previously by Kwon et al.9 For instance, S-100β and NSE reach peak levels at 6 h post injury and are not detectable after 24 h in rats.10, 27 This has also been identified in patients at risk of an ischemic SCI during endovascular stent grafting where S-100β concentrations peak at 6 h post injury.10 GFAP on the other hand has been reported to reach peak levels after 24 h in ischemic SCI.28, 29 To date, we are not aware of published studies that have reported NFH concentrations in the CSF of human SCI patients, although Casha and colleagues at the University of Calgary have measured NFH in the CSF of patients enrolled in their prospective randomized trial of minocycline for acute SCI.5, 30 A recent study, however, used 25 swines in a model that mimics blast-induced traumatic brain injury. The study identified significantly increased CSF NFH concentrations at 6 h post injury compared with pre-injury levels. Remarkably, the NFH decreased again to a level that differed nonsignificantly compared with pre-injury levels after 24 h.31 Our data also showed the influence of the time of sampling against the concentrations of NSE, GFAP and NFH in the seven AIS A patients.
In our previous review,10 we showed that structural biomarkers were not a sensitive prognostic tool according to the then available studies. However, a recent study showed the potential of CSF biomarkers.9 In the study, a biochemical model was created that utilized the levels of Interleukin-8, S-100β and GFAP at 24 h post injury to classify AIS grade and to predict segmental motor recovery in 20 cervical SCI patients. The model predicted the observed AIS grade in 89% SCI patients. In addition, segmental motor recovery in the upper extremities at 6 months post injury was predicted with either the CSF concentrations IL-8, S100β and GFAP, or the initial AIS grade. The biochemical model was comparable with (if not slightly better than) the initial AIS grade at predicting segmental motor recovery. Although these are promising results, validation of the ability for such biomarkers to distinguish injury severity requires testing in an independent cohort of patients.
Although our data showed no significant differences in S-100β, Tau, GFAP and NFH concentrations between all the different AIS grades, the mean CSF concentrations tend to suggest that the more severely a SCI patient is injured, as determined by the AIS grade, the higher the concentrations of a structural biomarker. The GFAP and Tau concentrations in Table 3 also reflect what may be a functional ‘ceiling effect’ with the AIS grading system. Conceptually, if the spinal cord is traumatically injured to a degree that produces a functionally ‘complete’ AIS A injury, doubling the mechanical severity of injury may increase the biological extent of injury, but would still result in the identical injury grading according to the AIS. This increased biological extent of injury, however, may be reflected in the CSF concentrations of structural biomarkers such as Tau and GFAP.
However, there is also a considerable variability in the concentrations among the most severely damaged spinal cords, that is, the AIS A patients. A possible explanation for this variety, may be the time that the different samples were obtained. Our data showed that this variability in timing significantly influenced the NFH, NSE and S-100β concentrations in AIS A patients.
Our results should be interpreted in the context of specific study limitations. We presented the study results of 16 SCI patients. As we used strict inclusion criteria, the interpretation of our results is limited by the small sample size patient numbers. The less severely injured SCI patients who improved in their AIS grade seemed to have lower biomarker concentrations, however, the great variability and small numbers severely limits the conclusions drawn from our analysis. In addition, our study population limits us from using a biochemical model with statistical power as proposed by a previous study.5 Also, our study protocol was not standardized for several putative confounders such as treatment regimens and blood pressure augmentation. The method of obtaining CSF differed between the two centers. As the purpose of this study was to analyze CSF samples obtained within 24 h, we believe that this has not influenced our results. However, the time between the injury and CSF sampling differed considerably among the patients in our study, ranging from 3 h to 24 h, and our data showed that this variability in timing significantly influenced the NFH, NSE and S-100β concentrations. Future studies therefore should perform CSF sampling on predetermined time intervals. Finally, although the AIS is a recognizable benchmark for the baseline neurologic assessment of the acute SCI patient, the AIS is a questionable outcome measurement, as it does not address the functional capabilities.32
As previously mentioned, the diagnostic capabilities of the currently available biomarkers will not exceed that of the initial neurological assessments, so long as they are compared with these neurological assessments as the comparative gold standard.5 Future studies are needed to determine whether structural biomarkers could be used as diagnostic markers in those SCI patients where a valid baseline neurological assessment cannot be obtained, or if they could better predict long-term outcome than this initial neurological evaluation.
Conclusion
In this study of 16 SCI patients, the structural CSF biomarkers NSE, S-100β and NFH appeared to correspond with patients having a motor complete or incomplete SCI. Our data showed no clear differences in any of the protein concentrations between the different AIS grades.
Data archiving
There were no data to deposit.
References
Bracken MB, Shepard MJ, Holford TR, Leo-Summers L, Aldrich EF, Fazl M et al. Administration of methylprednisolone for 24 or 48 hours or tirilazad mesylate for 48 hours in the treatment of acute spinal cord injury. Results of the Third National Acute Spinal Cord Injury Randomized Controlled Trial. National Acute Spinal Cord Injury Study. JAMA 1997; 277: 1597–1604.
Fehlings MG, Vaccaro A, Wilson JR, Singh A, Cadotte WD, Harrop JS et al. Early versus delayed decompression for traumatic cervical spinal cord injury: results of the Surgical Timing in Acute Spinal Cord Injury Study (STASCIS). PLoS One 2012; 7: e32037.
Steeves JD, Lammertse D, Curt A, Fawcett JW, Tuszynski MH, Ditunno JF et al. Guidelines for the conduct of clinical trials for spinal cord injury (SCI) as developed by the ICCP panel: clinical trial outcome measures. Spinal Cord 2007; 45: 206–221.
Fawcett JW, Curt A, Steeves JD, Coleman WP, Tuszynski MH, Lammertse D et al. Guidelines for the conduct of clinical trials for spinal cord injury as developed by the ICCP panel: spontaneous recovery after spinal cord injury and statistical power needed for therapeutic clinical trials. Spinal Cord 2007; 45: 190–205.
Kwon BK, Casha S, Hurlbert RJ, Yong VW . Inflammatory and structural biomarkers in acute traumatic spinal cord injury. Clin Chem Lab Med 2011; 49: 425–433.
Lammertse D, Dungan D, Dreisbach J, Falci S, Flanders A, Marino R et al. Neuroimaging in traumatic spinal cord injury: an evidence-based review for clinical practice and research. J Spinal Cord Med 2007; 30: 205–214.
Burns AS, Ditunno JF . Establishing prognosis and maximizing functional outcomes after spinal cord injury: a review of current and future directions in rehabilitation management. Spine (Phila Pa 1976) 2001; 26 (24 Suppl): S137–S145.
Burns AS, Lee BS, Ditunno JF Jr, Tessler A . Patient selection for clinical trials: the reliability of the early spinal cord injury examination. J Neurotrauma 2003; 20: 477–482.
Kwon BK, Stammers AM, Belanger LM, Bernardo A, Chan D, Bishop CM et al. Cerebrospinal fluid inflammatory cytokines and biomarkers of injury severity in acute human spinal cord injury. J Neurotrauma 2010; 27: 669–682.
Pouw MH, Hosman AJ, van Middendorp JJ, Verbeek MM, Vos PE, Van de MH . Biomarkers in spinal cord injury. Spinal Cord 2009; 47: 519–525.
Noppe M, Crols R, Andries D, Lowenthal A . Determination in human cerebrospinal fluid of glial fibrillary acidic protein, S-100 and myelin basic protein as indices of non-specific or specific central nervous tissue pathology. Clin Chim Acta 1986; 155: 143–150.
Lamers KJ, van Engelen BG, Gabreels FJ, Hommes OR, Borm GF, Wevers RA . Cerebrospinal neuron-specific enolase, S-100 and myelin basic protein in neurological disorders. Acta Neurol Scand 1995; 92: 247–251.
Mokuno K, Kiyosawa K, Sugimura K, Yasuda T, Riku S, Murayama T et al. Prognostic value of cerebrospinal fluid neuron-specific enolase and S-100b protein in Guillain-Barre syndrome. Acta Neurol Scand 1994; 89: 27–30.
American Spinal Injury Association: International Standards for Neurological Classification of Spinal Cord Injury, revised 2002. American Spinal Injury Association: Chicago, IL, USA. 2002.
Abdo WF, Bloem BR, van Geel WJ, Esselink RA, Verbeek MM . CSF neurofilament light chain and tau differentiate multiple system atrophy from Parkinson’s disease. Neurobiol Aging 2007; 28: 742–747.
Abdo WF, De JD, Hendriks JC, Horstink MW, Kremer BP, Bloem BR et al. Cerebrospinal fluid analysis differentiates multiple system atrophy from Parkinson’s disease. Mov Disord 2004; 19: 571–579.
Beems T, Simons KS, van Geel WJ, De Reus HP, Vos PE, Verbeek MM . Serum- and CSF-concentrations of brain specific proteins in hydrocephalus. Acta Neurochir (Wien) 2003; 145: 37–43.
van Geel WJ, Rosengren LE, Verbeek MM . An enzyme immunoassay to quantify neurofilament light chain in cerebrospinal fluid. J Immunol Methods 2005; 296: 179–185.
Vos PE, Lamers KJ, Hendriks JC, van Haaren M, Beems T, Zimmerman C et al. Glial and neuronal proteins in serum predict outcome after severe traumatic brain injury. Neurology 2004; 62: 1303–1310.
Abdo WF, van de Warrenburg BP, Munneke M, van Geel WJ, Bloem BR, Kremer HP et al. CSF analysis differentiates multiple-system atrophy from idiopathic late-onset cerebellar ataxia. Neurology 2006; 67: 474–479.
van Geel WJ, De Reus HP, Nijzing H, Verbeek MM, Vos PE, Lamers KJ . Measurement of glial fibrillary acidic protein in blood: an analytical method. Clin Chim Acta 2002; 326: 151–154.
Herrmann M, Vos P, Wunderlich MT, de Bruijn CH, Lamers KJ . Release of glial tissue-specific proteins after acute stroke: a comparative analysis of serum concentrations of protein S-100B and glial fibrillary acidic protein. Stroke 2000; 31: 2670–2677.
Herbert MK, Kuiperij HB, Verbeek MM . Optimisation of the quantification of glutamine synthetase and myelin basic protein in cerebrospinal fluid by a combined acidification and neutralisation protocol. J Immunol Methods 2012; 381: 1–8.
Maynard FM Jr, Bracken MB, Creasey G, Ditunno JF Jr, Donovan WH, Ducker TB et al. International Standards for Neurological and Functional Classification of Spinal Cord Injury. American Spinal Injury Association. Spinal Cord 1997; 35: 266–274.
van Middendorp JJ, Hosman AJ, Pouw MH Group E-SS Van de Meent H . Is determination between complete and incomplete traumatic spinal cord injury clinically relevant? Validation of the ASIA sacral sparing criteria in a prospective cohort of 432 patients. Spinal Cord 2009; 47: 809–816.
Lubieniecka JM, Streijger F, Lee JH, Stoynov N, Liu J, Mottus R et al. Biomarkers for severity of spinal cord injury in the cerebrospinal fluid of rats. PLoS One 2011; 6: e19247.
Cao F, Yang XF, Liu WG, Hu WW, Li G, Zheng XJ et al. Elevation of neuron-specific enolase and S-100beta protein level in experimental acute spinal cord injury. J Clin Neurosci 2008; 15: 541–544.
Matsumoto S, Matsumoto M, Yamashita A, Ohtake K, Ishida K, Morimoto Y et al. The temporal profile of the reaction of microglia, astrocytes, and macrophages in the delayed onset paraplegia after transient spinal cord ischemia in rabbits. Anesth Analg 2003; 96: 1777–1784 table of contents.
Winnerkvist A, Anderson RE, Hansson LO, Rosengren L, Estrera AE, Huynh TT et al. Multilevel somatosensory evoked potentials and cerebrospinal proteins: indicators of spinal cord injury in thoracoabdominal aortic aneurysm surgery. Eur J Cardiothorac Surg 2007; 31: 637–642.
Casha S, Zygun D, McGowan MD, Bains I, Yong VW, Hurlbert RJ . Results of a phase II placebo-controlled randomized trial of minocycline in acute spinal cord injury. Brain 2012; 135 (Pt 4): 1224–1236.
Ahmed F, Gyorgy A, Kamnaksh A, Ling G, Tong L, Parks S et al. Time dependent changes of protein biomarker levels in the cerebrospinal fluid after blast traumatic brain injury. Electrophoresis 2012; 33: 3705–3711.
van Middendorp JJ, Hosman AJ, Pouw MH, Van de MH . ASIA impairment scale conversion in traumatic SCI: is it related with the ability to walk? A descriptive comparison with functional ambulation outcome measures in 273 patients. Spinal Cord 2008; 47: 555–560.
Acknowledgements
This study was supported by the ‘Internationale Stiftung für Forschung in Paraplegie’ (IFP). BKK is the Canada Research Chair in Spinal Cord Injury and a Scholar Award from the Michael Smith Foundation for Health Research. We gratefully acknowledge funding support for this research from the Michael Smith Foundation for Health Research, the Paralyzed Veterans of America and the Vancouver Coastal Health Research Institute. We thank Herman de Reus, Anthea Stammers, Wieneke van Geel, Ria Claessens-Linskens, Marijke Beenes and Marie-Christine Bochem for technical assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Pouw, M., Kwon, B., Verbeek, M. et al. Structural biomarkers in the cerebrospinal fluid within 24 h after a traumatic spinal cord injury: a descriptive analysis of 16 subjects. Spinal Cord 52, 428–433 (2014). https://doi.org/10.1038/sc.2014.26
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sc.2014.26
Keywords
This article is cited by
-
Prognostic value of the systemic inflammatory index (SII) and systemic inflammatory response index (SIRI) in patients with traumatic spinal cord injury
European Spine Journal (2024)
-
Prognosticating acute traumatic spinal cord injury using Neurofilament (NF), Neuron Specific Enolase (NSE), Matrix Metalloproteinases (MMPs), and S-100B as biomarkers
Irish Journal of Medical Science (1971 -) (2024)
-
Glial fibrillary acidic protein is a robust biomarker in cerebrospinal fluid and peripheral blood after traumatic spinal cord injury: a prospective pilot study
Acta Neurochirurgica (2023)
-
Neuron-specific enolase level is a useful biomarker for distinguishing amyotrophic lateral sclerosis from cervical spondylotic myelopathy
Scientific Reports (2021)
-
Serum C-reactive protein is an early, simple and inexpensive prognostic marker for the progression of intramedullary lesion on magnetic resonance imaging from acute to subacute stage in patients with spinal cord injury
Spinal Cord (2021)