Abstract
Study design:
Only few studies have been published about diffusion-weighted imaging (DWI) within 24 h of traumatic spinal cord injury (tSCI).
Objectives:
The purpose of this study was to compare the imaging findings from conventional magnetic resonance imaging (MRI) and DWI in seven tSCI patients with findings in the existing literature.
Methods:
Seven patients with tSCI at neurologic levels C2–T10 were examined with conventional MRI and DWI within 24 h post-injury. DWI was obtained with a b-factor of 1000 s mm–2. American Spinal Injury Association (ASIA) scores and Spinal Cord Independence Measurement (SCIM) II item 12 after 12 months were collected. In addition, MEDLINE was searched from 1995 to 2010 to identify clinical tSCI studies reporting on MRI, DWI and apparent diffusion coefficient maps within 24 h post-injury to perform a meta-analysis. Images obtained with a b-factor of 1000 s mm–2 were compared with lower b-factors. Differences were calculated using χ2 tests.
Results:
No associations were identified between the images of the seven tSCI patients and ASIA or SCIM II scores. Eighteen SCI patients (11 from the retrieved publications) were included in the meta-analysis. The detection rates of hyperintense signals on T2-weighted and DW imaging did not show significant differences at 94 and 72%, respectively. In addition, there were no significant differences in detection rates or diffusion abnormalities between subjects in whom DW images were obtained with a maximum b-factor of 1000 or <1000 s mm–2.
Conclusion:
Our analysis suggests that T2-weighted and DW imaging have comparable detection rates for spinal cord damage in tSCI patients within 24 h post-injury.
Similar content being viewed by others
Introduction
In traumatic spinal cord injury (tSCI), much effort has been placed on evaluating SCI severity to predict recovery potential. However, an increasing number of SCI studies have begun shifting their research focus from care to cure.1, 2, 3 It is assumed that patients with more severe SCI respond differently to neuroprotective interventions than do patients with less severe SCI. An accurate prediction of the initial damage of the spinal cord that more exactly differentiates between the severity of SCI may help physicians in choosing neuroprotective interventions in the acute phase.
Conventional magnetic resonance imaging (MRI) is currently the best imaging modality for evaluating tSCI during the acute phase.4 Standard clinical MRI sequences effectively identify spinal cord compression, edema and hemorrhage in the spinal cord. In addition, the presence of large intraparenchymal hemorrhages is a well-known predictor of poor outcome following tSCI.5 Conventional MR sequences, however, do not provide enough information about the integrity of the critical, long white-matter long tracts responsible for the observed functional deficits after SCI. Previous studies have established that diffusion-weighted imaging (DWI) may have diagnostic value for SCI.6, 7, 8 DWI evaluates the free Brownian motion of water molecules in vivo. Spinal cord white-matter tracts are well organized in the craniocaudal direction, so diffusion is anisotropically oriented, with a higher apparent diffusion coefficient (ADC) along the fibers than transversely. DWI, therefore, can be used to evaluate the integrity of white-matter tracts in the spinal cord, and has the potential to provide information beyond the anatomic or spatial data provided by conventional MRI techniques. To illustrate, Shanmuganathan et al.8 identified decreased ADC values throughout the cervical spinal cord of SCI patients which were not seen on conventional MRI. The authors concluded that these abnormalities were greatest at the cord injury site and may reflect injury severity.8
Currently, the neurological examination is the most sensitive tool in predicting outcomes after the initial damage of the spinal cord in SCI patients.9 The neurological examination based on the American Spinal Injury Association (ASIA) scores is considered to be reliable and prognostic in patients who are tested 72 h after the initial trauma.10 Within 72 h post-injury, several factors, such as spinal shock, medical instability, concomitant brain injury or coma, affect the reliability of the neurological examination.11
In the therapeutic management of tSCI patients, the optimal timing of decompressive surgery remains controversial. A recent study stated that surgical decompression (within 24 h post-injury) should be considered a part of the neuroprotective management of any tSCI patient.12
The purpose of this study was (1) to compare the detection rates for spinal cord damage on conventional MR and DWI within 24 h post-injury; (2) to compare different b-factors used for DWI of the spinal cord; and (3) to compare the results with previously published data.
Materials and methods
Study population
For this study, we used data that were prospectively collected from tSCI patients. Data were collected from patients primarily referred to the Emergency Department of our level 1 trauma center between March 2008 and August 2009. Patients ⩾18 years of age were included in our diagnostic trauma protocol. All patients were examined with conventional MRI and DWI within 24 h post-injury. The ADC maps were also generated from the DWI. Neurological assessments and functional outcomes in this database were collected at five time points: during the acute phase (that is, within the first 15 days after injury), and at 1, 3, 6 and 12 months after the injury. Clinical assessments were conducted by trained neurological and rehabilitation physicians who had at least 1 year experience in examining patients with SCI.
The study protocol was approved by the local ethics committee, and written, informed consent was obtained from all subjects. See also http://www.trialregister.nl, study ID: NTR1381
Imaging
MRI studies were performed on a Siemens Avanto 1.5-T. magnet using a spine array coil (Erlangen, Germany). Sagittal T1-W SE (spin echo time) MR images (TR/TE (time of repetition/time of echo) 550/10 ms, 3 mm slice thickness), T2-W TSE (turbo spin echo time) MR images (TR/TE 4800/102 ms, 3 mm slice thickness), true inversion recovery magnitude (TIRM) images (TR/TE/time of inversion (TI) 1650/15/860 ms, 3.5 mm slice thickness) and axial T2-W TSE MR images (TR/TE 1500/123 ms, 3 mm slice thickness) were acquired in all patients. In addition, sagittal T2-weighted gradient-echo sequences were performed to identify possible hemorrhages in the spinal cord. DWI was performed in a sagittal plane in all patients using multi-shot echo planar imaging (EPI) with the following parameters: field of view (FOV) 280 × 280 mm2 or 350 × 350 mm2; slice thickness 3 mm; TR/TE 200/89; number of b-factors 2; and number of signal averages (NSA) 10. The minimum and maximum b-factors were 0 and 1000 s mm–2, respectively. Diffusion encoding gradients were played out sequentially along all three principal axes on a per-pixel basis. ADC maps were generated from the DWI on a pixel-by-pixel basis with software supplied by the manufacturer. The ADC values of the lesion in the cord were measured by visual assessment by drawing small ROIs (regions of interests) on the ADC maps with T2-weighted images as a reference. The ROI analysis is considered to be a reliable method for measurements of lesions in MRI.13 To minimize the influence of small movements during scanning of T2-weighted images and DWI, image registration was performed. We set the size and location of the ROIs so as to include only the lesion, and averaged three measurements. The images were evaluated for all subjects by an experienced neuroradiologist (AMvdV) who was blinded for the patients’ clinical status. The sagittal T2-weighted and DW images were graded as normal, hyperintense or hypointense. The sagittal ADC maps were graded as normal, decreased or increased diffusion.
Neurological outcome
Neurological examinations were conducted according to the ASIA standards.14 This requires the SCI patient to demonstrate his/her residual strength in 10 muscle groups in the arms and legs, and to report their sensation to pin-prick and light touch throughout the body, including the peri-anal region. On the basis of the ASIA sensory and motor scores, the neurological level of injury and ASIA impairment scale (AIS) were determined. For the analysis, only patients with an AIS A–D, an neurological level of injury between C2 and T10, and completely conducted examinations within 15 days post-injury (acute phase) and 12 months post-injury were included.
Functional outcome
The Spinal Cord Independence Measurement (SCIM) II is an instrument that focuses on performing everyday tasks, and captures the disability, as well as the impact of disability, on the patient's overall medical condition and comfort.15 The SCIM II consists of three main categories, namely (1) self-care, (2) respiration and sphincter management and (3) mobility.16 As independent ambulation is a high priority for recovery among SCI patients,17 the chronic phase scores from item 12 of the SCIM II (indoor mobility) were collected in each patient. This SCIM mobility item has a range of 0–8. The indoor mobility is scored as (0) requires total assistance; (1) needs an electric wheelchair or partial assistance to operate manual wheelchair; (2) moves independently in a manual wheelchair; (3) requires supervision while walking (with or without devices); (4) walks with a walking frame or crutches (swing); (5) walks with crutches or two canes (reciprocal walking); (6) walks with one cane; (7) needs leg orthosis only; and (8) walks without aid.
Retrieval of publications
A MEDLINE (PubMed interface) search was performed to compile a reference list of articles published between 1995 and March 2010. The search strategy used both key words and the following MeSH terms: spinal cord injuries; traumatic spinal cord injury; Magnetic Resonance Imaging; and Diffusion Magnetic Resonance Imaging. The abstracts and references from all identified articles were also examined for importance, relevance and overlap by two reviewers (MHP and AMvdV).
All clinical studies reporting on MRI and DWI in subjects with a tSCI were eligible for analysis. The findings of this study were compared with those in published series. Case reports were also included. Only studies or case reports in which the sagittal imaging was performed ⩽24 h post-injury were included. Studies that failed to report the interval between injury and MR imaging and the DWI technique used were excluded. The qualitative and quantitative ADC analysis of the spinal cord lesions was included. The ADCs of these studies were also graded as normal, decreased or increased diffusion.
Statistics
Descriptive statistics on age, gender and AIS were used to provide general information about the study population. As only patients with spinal cord damage were included for the meta-analysis (that is, an abnormal neurological examination being the gold standard), the detection percentage for spinal cord damage was calculated for T2-weighted images and DW images. By applying the qualitative interpretation of the ADC maps, we also evaluated any differences in possible diffusion abnormalities.
As the DW images in our study were obtained with EPI using a maximum b-factor of 1000 s mm–2, we compared our images with the images from other studies that were obtained using a lower b-factor than 1000 s mm–2. The differences in the number of patients with hypo- or hyperintensity on T2-weighted images and DW images were calculated using a χ2 or Fisher's exact test, as appropriate. The differences were considered statistically significant at P<0.05. Data were analyzed using SPSS software (version 16.0, SPSS, Chicago, IL, USA).
Results
A total of 10 patients who were admitted to our trauma center following blunt force trauma were included. All of these 10 patients were suspected of having an SCI, as assessed by the attending trauma surgeon at admission. Mechanisms of injury included motor vehicle accidents (n=4) and falls from heights (n=6). However, two patients had a motor and/or sensory deficit that was not attributable to an SCI. In addition, one polytraumatized patient (case no. 9) complained of a lower extremity sensory deficit at the scene of the accident. As the patient was sedated on arrival at our emergency department, a reliable neurological examination could not be performed and eventually this patient had no motor or sensory deficit. In these three subjects without SCI, the T2-weighted images, DW images and ADCs were all normal. A total of seven SCI subjects were thus included for the analysis, with a mean age of 62 years (range, 32–91 years). Of the seven SCI patients, the AIS were A (n=2), C (n=1) and D (n=4).
The T2-weighted images showed a hyperintensity in the cord in six of the seven tSCI patients. The DW images showed hyperintensities in five of the seven tSCI patients. In one patient, both the T2 and DW images were graded as normal. None of the images was graded as hypointense. The ADC value (mean±s.d.) in the hyperintensities of these seven patients was 536±94 × 10−6 mm2 s–1. In addition, the gradient-echo sequences showed no hemorrhages in the spinal cords of these seven tSCI patients. In case no. 8, DW images showed no abnormalities. The ADC, however, was only slightly decreased compared with the non-injured spinal cord. In addition, comparing the T2-weighted images with the b-factor 0 s mm–2 value of the DWI showed no discrepancies in the appearance of the abnormal signals.
Although the AIS improved in cases no. 6 (A–C) and 10 (D–E), no clear differences or associations were identified between the different AISs and/or item 12 of the SCIM and the findings on DWI in any of the seven tSCI patients .The only patient with a complete SCI, that is, AIS grade A, who did not improve in AIS grade also had the largest lesion size on the MR and DW images (see Figure 1). However, our data of seven patients did not show an association between lesion size and neurological or functional outcomes (Table 1).
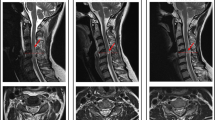
Images of case no. 7. (a) Sagittal T2-weighted imaging of an unstable fracture at the level of Th12 with a compression of the lower thoracic spinal cord. (b) There is a hyperintense lesion on the DW image. (c) The hyperintensity on the DW images is hypointense at the corresponding level on the ADC map.
MEDLINE search
Of the 32 articles from MEDLINE identified by the predefined key words, four studies were recognized as potentially relevant. Two studies were excluded, as one did not report the interval between injury and imaging and the other was not in English.18, 19 One study20 and one case report21 could be accepted for further analysis after accounting for the inclusion/exclusion criteria. In these reports, a total of 11 tSCI patients, who received conventional MRI and DW imaging within 24 h post-injury, were included (Table 2). None of the included studies reported standardized neurological or functional outcomes.
Meta-analysis
A total of 18 SCI patients (11 subjects from the retrieved publications and seven from this study) were included in the meta-analysis. Thirteen subjects were male patients and the mean age was 55 years (range, 2–91 years). The mean interval between time of injury and imaging was 10.4 h (range, 1–24 h). The included studies both used a b-factor of <1000 s mm–2, 70021 and 42420 s mm–2, respectively.
In 1/18 patients (6%), both the T2-weighted and DW images were graded as normal. In 4/18 (2%) other patients, no abnormalities on the DW images were identified, whereas hyperintensity was detected on T2-weighted images. In 13/18 patients, hyperintensity was observed on T2-weighted and DW images.
The detection rates of hyperintensities on T2-weighted imaging in subjects in whom DW imaging was performed with a maximum b-factor of 1000 s mm–2 and <1000 s mm–2 were 86 and 100%, respectively (P>0.05). The detection rates of hyperintensities on DWI in subjects with a maximum b-factor of 1000 s mm–2 and <1000 s mm–2 were 71 and 73%, respectively (P>0.05). In addition, the data showed no significant differences in decreased diffusion percentages between images obtained with a maximum b-factor of 1000 and <1000 s mm–2.
Regardless of the b-factor used, the detection rates of signal abnormalities on T2-weighted and DW imaging were 94 and 72%, respectively. This difference, however, was non-significant (Table 2).
Discussion
In this study, the imaging findings from T2-weighted imaging and DW imaging were analyzed in seven subjects with tSCI, and further compared with results previously published in the literature. Our qualitative data analysis suggest that T2-weighted and DW images have a comparable detection rate for spinal cord damage in patients with tSCI within 24 h post-injury.
Although it is hypothesized that DWI may be more sensitive in detecting signal abnormalities than conventional MRI in patients with traumatic SCI during the acute phase (that is, within 24 h post-injury), our combined data do not suggest this. No significant differences in detection rates between T2-weighted imaging and DWI in tSCI patients were identified. A maximum b-factor of 1000 s mm–2 did not lead to higher detection rates or differences in diffusion abnormalities compared with lower b-factors. In addition, a study comparing a b-factor of 1000 s mm–2 in the detection of ischemic stroke showed that a higher b-factor of 3000 s mm–2 had no impact on the diagnosis of acute infarction. However, compared with 1000 s mm–2, the b-factor of 3000 s mm–2 improved the gray–white matter differentiation on ADC maps and the visual assessment of decreased diffusion.22 Lower b-factors would require shorter scanning time, an important factor in acute SCI setting. In practice, a b-factor of 1000 mm s–2 has been accepted to be reliable in DW imaging of the spinal cord. There is no clear benefit for choosing a b-factor of 1000 mm s–2 compared with lower b-factors in the detection of traumatic SCI. However, the quality of DWI images is improved with a b-factor of 1000 mm s–2, which is important for measurements.
In 2006, the National Institute on Disability and Rehabilitation Research Spinal Cord Injury Measures Meeting made several recommendations about neuroimaging techniques that are applied in SCI patients. The commission stated that future DWI research should be focused on (1) the correlation between lesion size and clinical status and (2) the refinement of clinical and prognostic capabilities in close correlation with improvements in field strength and software.9 In our own series, six of the seven SCI patients had a hyperintensity on T2-weighted images and five of the seven SCI patients had a hyperintensity on the DW images with decreased ADC values. With these numbers, any correlation between clinical status and neuroimaging lacks statistical power. To date, only two studies have evaluated the correlation between DW images and clinical status.18, 20 Tsuchiya et al.,20 however, did not perform neurological assessments according to a standardized classification, such as the ASIA standards. Therefore, it is not clear whether the presence of hyperintensity on T2-weighted images and DW images might be associated with an incomplete or complete neurologic injury. For example, large and small regions of hyperintensity may be present in both an incomplete and a complete neurological injury.23 In addition, this study20 failed to use a standardized functional outcome scale, such as the SCIM, or functional independence measure items,24 but rather, divided the functional outcome into a poor or fair outcome. In view of these methodological limitations, one might conclude that restricted diffusion is predictive of an unfavorable prognosis. The study by Shen et al.18 conducted neurological examinations according to the ASIA standards, and these investigators showed that there was no correlation between DWI and neurological outcome in their study population of five patients.18 Remarkably, the study concluded that future DWI may provide important information complementary to conventional MRI, and allow for a better prognostic evaluation of recovery.18
The study by Marcel et al.25 showed that, in some cases, the high signal on DWI in spinal cord lesions was associated with an increased signal on ADC, which means elevated diffusion. This can be explained by the T2 shine-through effect.26 In addition, the acute phase in this meta-analysis was considered to be within 24 h post-injury. This time interval itself may be inadequate to investigate the correlation between images and neurological and functional outcomes using a qualitative interpretation. To illustrate, there is evidence that water accumulation in spinal cord parenchyma occurs as early as 2 h post-injury after SCI.27, 28 This secondary injury mechanism may therefore be the reason that we were not able to identify any qualitative differences between T2-weighted and DW images, because this early edema can also be detected on T2-weighted images. Although we were not able to identify any differences between T2-weighted images and DWI within a time interval of 24 h post-injury, a recent study showed a higher sensitivity for the detection of early pathological changes after contusive SCI using DWI, compared with conventional MRI in 40 rabbits.6 In addition, differences in ADC values between lesions in the mild, moderate and severe injury group were identified within a range of 30 min to 24 h post-injury.6
Although our study used multi-shot EPI, the other studies used single-shot EPI21 and SSFSE (single-shot fast spin echo).20 Multi-shot EPI has been proven to be less sensitive to geometric and susceptibility distortions than single-shot EPI, and provides better resolution and less blurring than single-shot EPI.29 In addition, multi-shot EPI was rated superior to SSFSE in one study.29
Some limitations of this study warrant consideration. Our small study population of 18 patients limits any valid conclusions. The qualitative interpretation of the data is also a limitation of this study. Since no standardized ADC values exist, to date, and different imaging techniques and b-factors were used in the included studies,20, 21 this qualitative data interpretation was chosen. Although we choose a qualitative interpretation, the different imaging techniques in this meta-analysis limit the clinical implications of our conclusions. With this interpretation, all ages were also included, although it is hypothesized that the ADC is higher in the aging spinal cord.30 Since ADC values can be correlated with histologic parameters, that is, axonal loss,31, 32 future studies with standard imaging techniques should focus on the correlation between standardized neurological and/or functional outcomes and quantitative data, such as the ADC or lesion size.
Two problems may exist for future MRI/DWI studies. First, due to the limited availability of MR imaging, obtaining MR/DW images from tSCI patients within 24 h post-injury is a logistic pitfall in most hospitals. Valuable time-to-intervention may be lost when waiting for these images. Second, the current technique is also limited. All data from the included images were performed on 1.5-T clinical scanners. When one considers the issue of spatial resolution and the desire to image a patient's spinal cord with a resolution that approaches in vitro cord imaging, the problem of insufficient signal becomes clear.33 It may be that the 1.5-T scanners do not offer the high signal and image quality that would allow DWI of the spinal cord to be widely and routinely implemented. As our data suggest that DWI with the current 1.5-T scanners does not clearly improve the detection rate within 24 h post-injury, future studies should implement newer acquisition techniques34 with the use of higher field strengths (3-T or higher), and should ideally be performed in large multicenter networks to investigate the prognostic capabilities of DWI in SCI.
Conclusion
Our qualitative analysis suggests that T2-weighted and DW imaging have comparable detection rates for spinal cord damage in 18 tSCI patients within 24 h post-injury. Future DWI studies in spinal cord injury should focus on the correlation between standardized neurological and functional outcomes and quantitative data such as the ADC and lesion size, to evaluate the prognostic capabilities of this technique.
Data Archiving
There was no data to deposit.
References
Knafo S, Choi D . Clinical studies in spinal cord injury: moving towards successful trials. Br J Neurosurg 2008; 22: 3–12.
Kwon BK, Okon E, Hillyer J, Mann C, Baptiste D, Weaver LC et al. A systematic review of non-invasive pharmacologic neuroprotective treatments for acute spinal cord injury. J Neurotrauma 2011; 28: 1545–1588.
Cadotte DW, Fehlings MG . Spinal cord injury: a systematic review of current treatment options. Clin Orthop Relat Res 2011; 469: 732–741.
Schaefer DM, Flanders AE, Osterholm JL, Northrup BE . Prognostic significance of magnetic resonance imaging in the acute phase of cervical spine injury. J Neurosurg 1992; 76: 218–223.
Boldin C, Raith J, Fankhauser F, Haunschmid C, Schwantzer G, Schweighofer F . Predicting neurologic recovery in cervical spinal cord injury with postoperative MR imaging. Spine (Phila Pa 1976) 2006; 31: 554–559.
Yin B, Tang Y, Ye J, Wu Y, Wang P, Huang L et al. Sensitivity and specificity of in vivo diffusion-weighted MRI in acute spinal cord injury. J Clin Neurosci 2010; 17: 1173–1179.
Schwartz ED, Hackney DB . Diffusion-weighted MRI and the evaluation of spinal cord axonal integrity following injury and treatment. Exp Neurol 2003; 184: 570–589.
Shanmuganathan K, Gullapalli RP, Zhuo J, Mirvis SE . Diffusion tensor MR imaging in cervical spine trauma. AJNR Am J Neuroradiol 2008; 29: 655–659.
Lammertse D, Dungan D, Dreisbach J, Falci S, Flanders A, Marino R et al. Neuroimaging in traumatic spinal cord injury: an evidence-based review for clinical practice and research. J Spinal Cord Med 2007; 30: 205–214.
Burns AS, Ditunno JF . Establishing prognosis and maximizing functional outcomes after spinal cord injury: a review of current and future directions in rehabilitation management. Spine (Phila Pa 1976) 2001; 26: S137–S145.
Burns AS, Lee BS, Ditunno Jr JF, Tessler A . Patient selection for clinical trials: the reliability of the early spinal cord injury examination. J Neurotrauma 2003; 20: 477–482.
Fehlings MG, Rabin D, Sears W, Cadotte DW, Aarabi B . Current practice in the timing of surgical intervention in spinal cord injury. Spine (Phila Pa 1976) 2010; 35: S166–S173.
Wetzel SG, Cha S, Johnson G, Lee P, Law M, Kasow DL et al. Relative cerebral blood volume measurements in intracranial mass lesions: interobserver and intraobserver reproducibility study. Radiology 2002; 224: 797–803.
American Spinal Injury Association. American Spinal Injury Association: International Standards for Neurological Classification of Spinal Cord Injury, revised 2002. Chicago, IL, 2002. Ref Type: Electronic Citation.
Catz A, Itzkovich M, Steinberg F, Philo O, Ring H, Ronen J et al. The Catz-Itzkovich SCIM: a revised version of the Spinal Cord Independence Measure. Disabil Rehabil 2001; 23: 263–268.
Itzkovich M, Gelernter I, Biering-Sorensen F, Weeks C, Laramee MT, Craven BC et al. The Spinal Cord Independence Measure (SCIM) version III: reliability and validity in a multi-center international study. Disabil Rehabil 2007; 29: 1926–1933.
Ditunno PL, Patrick M, Stineman M, Ditunno JF . Who wants to walk? Preferences for recovery after SCI: a longitudinal and cross-sectional study. Spinal Cord 2008; 46: 500–506.
Shen H, Tang Y, Huang L, Yang R, Wu Y, Wang P et al. Applications of diffusion-weighted MRI in thoracic spinal cord injury without radiographic abnormality. Int Orthop 2007; 31: 375–383.
Xing W, Wang X, Han Z, Liao W, Liu F . Diffusion-weighted magnetic resonance imaging for acute trauma of the spine cord. Zhong Nan Da Xue Xue Bao Yi Xue Ban 2010; 35: 760–765.
Tsuchiya K, Fujikawa A, Honya K, Tateishi H, Nitatori T . Value of diffusion-weighted MR imaging in acute cervical cord injury as a predictor of outcome. Neuroradiology 2006; 48: 803–808.
Sagiuchi T, Tachibana S, Endo M, Hayakawa K . Diffusion-weighted MRI of the cervical cord in acute spinal cord injury with type II odontoid fracture. J Comput Assist Tomogr 2002; 26: 654–656.
Cihangiroglu M, Citci B, Kilickesmez O, Firat Z, Karlỳkaya G, Ulu∂ AM et al. The utility of high b-value DWI in evaluation of ischemic stroke at 3T. Eur J Radiol 2011; 78: 75–81.
Shepard MJ, Bracken MB . Magnetic resonance imaging and neurological recovery in acute spinal cord injury: observations from the National Acute Spinal Cord Injury Study 3. Spinal Cord 1999; 37: 833–837.
Catz A, Itzkovich M, Agranov E, Ring H, Tamir A . The spinal cord independence measure (SCIM): sensitivity to functional changes in subgroups of spinal cord lesion patients. Spinal Cord 2001; 39: 97–100.
Marcel C, Kremer S, Jeantroux J, Blanc F, Dietemann JL, De Sèze J . Diffusion-weighted imaging in noncompressive myelopathies: a 33-patient prospective study. J Neurol 2010; 257: 1438–1445.
Kremer S, Oppenheim C, Schmitt E, Dietemann JL . Diffusion MRI: technique and clinical applications. J Radiol 2007; 88: 428–443.
Rowland JW, Hawryluk GW, Kwon B, Fehlings MG . Current status of acute spinal cord injury pathophysiology and emerging therapies: promise on the horizon. Neurosurg Focus 2008; 25: E2.
Saadoun S, Papadopoulos MC . Aquaporin-4 in brain and spinal cord oedema. Neuroscience 2010; 168: 1036–1046.
Bammer R, Augustin M, Prokesch RW, Stollberger R, Fazekas F . Diffusion-weighted imaging of the spinal cord: interleaved echo-planar imaging is superior to fast spin-echo. J Magn Reson Imaging 2002; 15: 364–373.
Mamata H, Jolesz FA, Maier SE . Apparent diffusion coefficient and fractional anisotropy in spinal cord: age and cervical spondylosis-related changes. J Magn Reson Imaging 2005; 22: 38–43.
Schwartz ED, Cooper ET, Chin CL, Wehrli S, Tessler A, Hackney DB . Ex vivo evaluation of ADC values within spinal cord white matter tracts. AJNR Am J Neuroradiol 2005; 26: 390–397.
Schwartz ED, Chin CL, Shumsky JS, Jawada AF, Kooper Brownb B, Wehrlic S et al. Apparent diffusion coefficients in spinal cord transplants and surrounding white matter correlate with degree of axonal dieback after injury in rats. AJNR Am J Neuroradiol 2005; 26: 7–18.
Quencer RM, Pattany PM . Diffusion-weighted imaging of the spinal cord: is there a future? AJNR Am J Neuroradiol 2000; 21: 1181–1182.
Thurnher MM, Law M . Diffusion-weighted imaging, diffusion-tensor imaging, and fiber tractography of the spinal cord. Magn Reson Imaging Clin N Am 2009; 17: 225–244.
Acknowledgements
This study was funded by the ‘Internationale Stiftung für Forschung in Paraplegie.’ We acknowledge Dr Anton Meijer for his assistance in data collection and data processing.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Pouw, M., van der Vliet, A., van Kampen, A. et al. Diffusion-weighted MR imaging within 24 h post-injury after traumatic spinal cord injury: a qualitative meta-analysis between T2-weighted imaging and diffusion-weighted MR imaging in 18 patients. Spinal Cord 50, 426–431 (2012). https://doi.org/10.1038/sc.2011.168
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sc.2011.168
Keywords
This article is cited by
-
Multishot diffusion-weighted MR imaging features in acute trauma of spinal cord
European Radiology (2014)