Abstract
Study design:
Case control.
Objective:
To clarify the predictors of low blood pressure (BP) and hyponatremia after spinal cord injury (SCI) and to discuss their pathophysiology.
Setting:
A SCI center in Japan.
Methods:
Age, gender, initial ASIA impairment scale (AIS) score, BP, blood electrolytes (sodium, K and Cl) and biochemical markers were evaluated at 1 month after injury. Risk factors of low BP and hyponatremia were analyzed using uni- and multivariate logistic regression models.
Results:
This study comprised of 172 SCI patients. Initial AIS score (Odds ratio (OR): 1.24, 95% confidence intervals (CI) 1.13–1.49, P-value <0.01) and hyponatremia (OR: 3.71, 95%CI 1.27–6.96, P<0.01) were the most important risk factors of low BP. As a second step, risk factors of hyponatremia were initial AIS score (OR: 1.36, 95%CI 1.08–2.78, P<0.01) and age (OR: 1.55, 95%CI 1.17–2.93, P<0.01).
Conclusions:
In acute and subacute period, the more severe SCI and lower AIS score patients have the more frequently low BP and/or hyponatremia do appear.
Similar content being viewed by others
Introduction
Cervical and high-thoracic spinal cord injury (SCI) is frequently associated with significant autonomic dysfunction in addition to sensory and motor deficits.1, 2, 3, 4, 5, 6 SCI often have unstable blood pressure (BP), bowel bladder dysfunction, gastrointestinal motility disorder and electrolyte disorder because normal sympathetic output is disrupted.6 Hypotension and hyponatremia associated with sympathetic dysfunction are major complications during the acute stage after traumatic cervical SCI.1, 2, 3, 4, 5, 6 These have been shown to be associated with worse outcomes such as secondary SCI, faint, pulmonary edema and rarely cardiac arrest,4, 5 so they must be treated aggressively as needed. Prediction of these complications is very important for planning rehabilitation and for proper medical care, however, there are few studies mentioning the risk factors of them. The purpose of this study is to clarify the predictors of low BP and hyponatremia after SCI.
Materials and methods
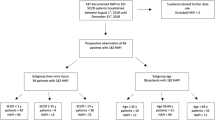
The present study includes a retrospective cohort analysis of laboratory records for patients of a single institution. This study was approved by the Institutional Review Board of Spinal Cord Injury Center Hokkaido Chuo Rosai Hospital.
All consecutive individuals with acute SCI who underwent medical treatment in our institute within 2 days after injury from January 2007 to December 2009 were selected. Patients with polytrauma, history of neurologic diseases, which has high incidence of autonomic dysfunction (that is, Parkinsonism, Shy–Drager syndrome) and history of renal diseases were excluded.
Patient charts were reviewed with particular interest in demographics (age and gender), neurologic assessment (ASIA impairment scale (AIS) score), laboratory data at 1 month after the injury, medications and preexisting medical condition. Systolic BP, blood parameters (red blood cell count, hematrocrit and hemoglobulin), electrolytes (sodium (Na), Cl and K) and biochemical markers were evaluated.
Risk factors of low BP (systolic atrial pressure <90 mm Hg, or requiring intravenous administration of crystaloids and vasopressive agents) and hyponatremia (serum Na <135 mmol l−1) were analyzed using uni- and multivariate logistic regression models. Univariate analysis was used to describe the frequency distribution of the sample. Multivariate logistic regression was used to calculate adjusted estimates of interactive association between low BP/hyponatremia and the other parameters. The estimates of associations are interpreted as odds ratios (OR) with 95% confidence intervals (CI). The P-value used for significance was set at 0.05.
Results
This study comprised of 172 cervical SCI patients (23 females and 149 males, mean age of 62.1 years) that fulfilled the inclusion criteria and exclusion criteria.
There were 82 complete and 90 incomplete quadriplegias (Table 1). The majority of patients had a moderate to severe SCI (AIS A–C: 80.8%) in this cohort of individuals with SCI.
No one received methylprednisolone. The majority of SCI patients (133 of 172, or 77.3%) underwent surgical procedures. Although there were eight individuals receiving antihypertensive medication before the injury, oral medication was stopped at hospital admission.
Among individuals with SCI, 78 subjects showed low BP and 86 showed hyponatremia. The other data collected from the records of these subjects have been summarized in Table 2.
AIS score (OR: 0.70, 95%CI 0.63–0.89, P-value <0.01) and serum Na (OR: 0.78, 95%CI 0.61–0.87, P-value <0.01) were shown to be the highly significant factors for low BP from the univariate analysis. So we entered these factors and conducted the multivariate analysis. According to the multivariate logistic regression model for low BP, AIS score (OR: 1.24, 95%CI 1.13–1.49, P-value<0.01) and hyponatremia (OR: 3.71, 95%CI 1.27–6.96, P-value<0.01) were risk factor of low BP (Tables 3 and 4). Especially, the most important risk factor of low BP was the severity of hyponatremia.
Age (OR: 0.73, 95%CI 0.58–0.88, P-value<0.01), AIS score (OR: 0.77, 95%CI 0.54–0.91, P-value<0.01), serum albumin (OR: 0.21, 95%CI 0.47–0.75), red blood cell count (OR: 0.77, 95%CI 0.55–0.89, P-value<0.01), hemoglobin (OR: 0.66, 95%CI 0.44–0.81, P-value<0.01) and hematocrit (OR: 0.77, 95%CI 0.34–0.91, P-value<0.01) were shown to be highly significant factors for hyponatremia from the univariate analysis. So we entered these factors and conducted the multivariate analysis. The multivariate analysis revealed that age (OR: 1.55, 95%CI 1.17–2.93, P-value <0.01) and AIS score (OR: 1.36, 95%CI 1.08–2.78, P-value <0.01) are potential risk factors for hyponatremia after SCI (Tables 5 and 6).
Discussion
Low BP was observed in 78 of 172 (45.3%) subjects in the present study. On a daily basis, individuals with cervical SCI frequently face the challenge of persistent low BP.7 Popa8 reported that 68% of motor-complete-cervical SCI developed arterial low BP. We selected a systolic BP below 90 mm Hg as the low BP in this study. Although the selection of 90 mm Hg is empirical, this is based on our previous experience with patients suffering orthostatic hypotension for all that systolic BP is over 85 mm Hg. A previous report also defines hypotension as a systolic BP below 90 mm Hg.1
Hyponatremia occurred in 86 of 172 (50.0%) individuals with SCI. Hyponatremia after SCI is frequently found,1, 4, 9, 10, 11 and the occurrence is 29–86% according to previous reports that defines hyponatremia as a serum Na concentration <135 mmol l−1.1, 10 Plasma Na concentration <130 mmol l−1 may trigger a variety of signs and symptoms (that is, anorexia, nausea, vomiting, disorientation and headaches)12 and is associated with a substantially increase in the mortality.13 Patients with serum Na concentration from 130–135 mmol l−1 are usually asymptomatic and do not require any treatment. However, low concentration compared with established normative serum Na values may indicate some potential physiological change.
According to the previous studies, hyponatremic individuals with SCI showed greater evidence of neurogenic hypotension than normonatremic individuals.1, 2 These alterations may occur as a consequence of loss of supraspinal control of the sympathetic nervous system. Recent studies described changes in spinal sympathetic preganglionic neurons after SCI in an attempt to better understand mechanisms underlying the impaired physiological control.14 Low BP and hyponatremia after SCI could be further complicated by morphological changes in sympathetic preganglionic neuron, that is, (1) descending vasomotor pathway15 and (2) descending renal sympathetic pathway.1
The descending vasomotor pathway in human project through the dorsal aspects of the lateral funiculus of the spinal cord. When sympathetic outflow through the descending vasomotor pathway is disrupted in the cervical or upper thoracic spinal cord, peripheral vascular resistance decreases profoundly, leading to abnormal cardiovascular control, such as low resting BP, orthostatic hypotension and autonomic dysreflexia.5 This is the primary mechanism underlying neurogenic shock and can usually be differentiated from hypovolemic shock by the relative lack of a tachycardiac response. Otherwise, the descending renal sympathetic pathway locates between 2 and 3 mm ventrolateral to the dorsolateral sulcus and renal sympathetic activity regulates the function of the tubules, the blood vessels and the juxtaglomerular globular cells in the kidney. A rise of renal sympathetic activity increases the renal tubular reabsorption throughout the nephron, decreases the renal blood flow and glomerular filtration rate, and reinforce the endogenous renin-angiotensin-aldosterone activity.1 The reason low BP and hyponatremia are strongly correlated could be that these pathways are close together at the dorsal area of lateral funiculi. Moreover, it could be mentioned that the more severe SCI and lower AIS score is the more frequently low BP and/or hyponatremia patients show due to the widespread injury reaching the dorsal area of lateral funiculi.
Hyponatremia is also frequently present during the course of an infection.16 Indeed, SCI leaves patients susceptible to the development of infection (that is, urinary tract infection, pneumonia and pressure ulcers) due to their neurogenic bladder dysfunction, respiratory complication and immobility. However, white blood cell count does not correlate with hyponatremia as well as low BP. Infection seems to contribute to the decrease of serum Na inconsiderably in our work.
There are other various potential causes of hyponatremia in SCI (that is, elevated fluid intake, antidiuretic hormone secretion and cerebral salt-wasting syndrome).1 The elderly may tend to be hyponatremic because of physiological changes of aging (that is, hormonal changes and sclerotic change of vascular system), however, little is known about the relationship between hyponatremia after SCI and aged people.
The effect of low BP is compounded by the dysfunctional hemodynamic autoregulation found in the spinal cord parenchyma immediately after a traumatic injury.17 Animal studies have shown that maintaining adequate systemic BP maintains spinal cord perfusion pressure in the physiological range and limits the amount of ischemic damage to the site of injury.18, 19, 20 As it is well known, low BP and/or hyponatremia is a common problem in both acute and chronic high-level SCI patients and sometimes requires intensive care to avoid the deterioration. Moreover, we could mention that the more severe SCI and the lower AIS score is the more frequently the patient shows low BP and/or hyponatremia. We should pay special attention in order to avoid severe complications in case of aged patients with complete quadriplegia.
References
Furlan JC, Fehlings MG . Hyponatremia in the acute stag after traumatic cervical spinal cord injury. Spine 2009; 34: 501–511.
Frisbie JH . Salt wasting, hypotension, polydipsia, and hyponatremia and the level of spinal cord injury. Spinal Cord 2007; 45: 563–568.
Palmer BF . Hyponatremia in patients with central nervous system disease: SIADH versus CSW. Trends Endcrinol Metab 2003; 14: 182–187.
Biyani A, Inman CG, el Masry WS . Hyponatremia after acute spinal injury. Injury 1993; 24: 671–673.
Teasell RW, Arnold JM, Krassioukov A, Delaney GA . Cardiovascular consequences of loss of supraspinal control of the sympathetic nervous system after spinal cord injury. Arch Phys Med Rehabil 2000; 81: 506–516.
Rodgers RB, Horn EM, Sonntag VKH . Acute treatment of patients with spinal cord injury. In: Herkowitz HN (ed). Rothman-Simeone The Spine, 6th edn. Elzevier: Philadelphia, 2011, pp 1422–1435.
Krassioukov A, Claydon VE . The clinical problems in cardiovascular control following spinal cord injury: an overview. Prog Brain Res 2006; 152: 223–229.
Popa C, Popa F, Grigorean VT, Onose G, Sandu AM, Popescu M et al. Vascular dysfunctions following spinal cord injury. J Med Life 2010; 3: 275–285.
Soni BM, Vaidyanthan S, Watt JW, Krishnan KR . A retrospective study of hyponatremia in tetraplegic/paraplegic patients with a review of the literature. Paraplegia 1994; 32: 597–607.
Peruzzi WT, Shapiro BA, Meyer Jr PR, Krumlovsky F, Seo BW . Hyponatremia in acute spinal cord injury. Crit Care Med 1994; 22: 252–258.
Claydon VE, Krassioukov AV . Orthostatic hypotension and autonomic pathways after spinal cord injury. J neurotrauma 2006; 23: 1713–1725.
Moore K, Thompson C, Trainer P . Disorders of water balance. Clin Med 2003; 3: 28–33.
Anderson RJ, Chung HM, Kluge R, Schrier RW . Hyponatremia: a prospective analysis of its epidemiology and the pathogenetic role of vasopressin. Ann Intern Med 1985; 102: 164–168.
Dibona GF . Nervous kidney – interaction between renal sympathetic nerves and the rennin-angiotensin system in the control of renal function. Hypertension 2000; 36: 1083–1088.
Furlan JC, Fehlings MG, Shannon P, Norenberg MD, Krassioukov AV . Descending vasomotor pathways in humans: correlation between axonal preservation and cardiovascular dysfunction after spinal cord injury. J neurotrauma 2003; 20: 1351–1363.
Ramesh VJ, Umamaheswara Rao GS, Kandavel T, Kumaraswamy SD, Iyyamanda UB, Chandramouli BA . Predictive model for survival among neurosurgical intensive care patients. J Neurosurg Anesthesiol 2011; 23: 183–187.
Senter HJ, Venes JL . Loss of autoregulation and posttraumatic ischemia following experimental spinal cord trauma. J Neurosurg 1979; 50: 198–206.
Tator CH, Fehlings MG . Review of the secondary injury theory of acute spinal cord trauma with emphasis on vascular mechanisms. J Neurosurg 1991; 75: 15–26.
Groah SL, Weitzenkamp D, Sett P, Soni B, Savic G . The relationship between neurological level of injury and symptomatic cardiovascular disease risk in the aging spinal injured. Spinal Cord 2001; 39: 310–317.
Frisbie JH, Steele DJ . Postual hypotension and abnormalities of salt and water metabolism in myelopathy patients. Spinal Cord 1997; 35: 303–307.
Acknowledgements
Hypotension and hyponatremia are common abnormality after SCI and are associated with integrity of descending autonomic pathways that mediate various physiological function including cardiovascular control and rennin-angiotensin-ardosterone system. Our study showed that low BP correlated with low AIS score and low Serum Na and that hyponatremia correlated with high age and low AIS score. Although the disconnection of sympathetic pathways from brainstem control following SCI may lead to a profound fall in resting arterial blood pressure and serum Na, we need to consider many other aspects.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Nakao, Y., Suda, K., Shimokawa, N. et al. Risk factor analysis for low blood pressure and hyponatremia in acutely and subacutely spinal cord injured patients. Spinal Cord 50, 285–288 (2012). https://doi.org/10.1038/sc.2011.142
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sc.2011.142
Keywords
This article is cited by
-
Mechanisms of hyponatremia and diabetes insipidus after acute spinal cord injury: a critical review
Chinese Neurosurgical Journal (2023)
-
Incidence of and factors associated with hyponatremia in traumatic cervical spinal cord injury patients
Spinal Cord Series and Cases (2022)
-
Analysis of risk factors for hyponatremia in patients with acute spinal cord injury: a retrospective single-institution study in Japan
Spinal Cord (2019)
-
A study of predictors for hyponatraemia in patients with cervical spinal cord injury
Spinal Cord (2018)
-
Re: A study of predictors for hyponatraemia in patients with cervical spinal cord injury
Spinal Cord (2018)