Abstract
Study design
Prospective observational cohort study.
Objectives
First, describe pressure injury (PI) and associated risk factors in individuals with spinal cord injury/disorder (SCI/D) during first rehabilitation. Second, evaluate a prediction model for hospital acquired PI (HAPI) development.
Setting
Acute care and rehabilitation clinic specialized in SCI/D.
Methods
Patients ≥18 years of age with SCI/D were included during first rehabilitation between 08/2018 and 12/2019. We performed a systematic literature search to identify risk factors for PI development. Patients were classified according to HAPI developed. Between group differences of patients’ characteristics and risk factors were analyzed using descriptive statistics. Logistic predictive models were performed to estimate HAPI development and receiver operator characteristic (ROC) curve was used to test the model.
Results
In total, 94 patients were included, 48 (51.1%) developed at least one HAPI and in total 93 were observed, mainly stage I and stage II HAPI according to the European Pressure Ulcer Advisory Panel. We found nine significantly associated risk factors: completeness of SCI/D, pneumonia, sedative medications, autonomic dysreflexia, Braden ≤12 points, SCIPUS ≥9 points, lower admission SCIM and lower admission FIM-cognition, longer length of stay (LOS) (p ≤ 0.0005). In a predictive model, none of the risk factors was associated with HAPI development (AUC = 0.5).
Conclusion
HAPIs in patients with SCI/D during first rehabilitation are a frequent and complex condition and associated with several risk factors. No predictive model exists but with the identified risk factors of this study, larger studies can create a tailored and flexible HAPI risk prediction model.
Similar content being viewed by others
Introduction
Patients with a spinal cord injury or disorder (SCI/D) are at risk of developing different complications, such as respiratory failure and pneumonia, autonomic dysreflexia, urinary tract infections (UTIs), bladder and bowel dysfunction, spasticity, neuropathic pain, and pressure injuries (PIs) [1]. PIs are defined as localized damage to the skin and underlying soft tissue. They occur as a result of intense and/or prolonged pressure or pressure in combination with shear and are co-affected by soft tissue conditions, skin microclimate, skin perfusion, nutrition and patients’ comorbidities [2]. Medical complications in patients with SCI/D may occur simultaneously and may influence each other and increase the risk for PI development [3,4,5]. PIs are one of the most frequent secondary medical complications in patients with SCI/D [6], and are thus clinically relevant complications in these complex patients, both in the community or inpatient setting. Especially during the initial acute care and rehabilitation after a spinal cord injury (hereafter first rehabilitation) PIs occur independent of the rehabilitation phase or the time since injury [7]. More specifically, PIs that occur during hospitalization are referred to as hospital-acquired pressure injuries (HAPIs), which are challenging for the health system as they increase length of hospital stay, treatment costs [8], and mortality [9]. According to the literature, up to 54.3% of patients with SCI/D and 61.9% of patients with cervical SCI/D are affected by HAPI(s) during their first rehabilitation [8, 9].
By 1996, more than 200 cofactors for PI and HAPI development had been considered [10]. A combination of risk factors rather than a single risk factor is considered the cause of the onset of a HAPI [11]. Several risk scales are routinely used for the prevention of HAPIs [12,13,14]. However, the Cochrane Review of 2019 indicated little or no difference in the incidence or severity of new onset HAPI with structured risk scales such as Braden or Waterlow compared to the use of clinical judgment only [15]. To reduce HAPI incidence and improve clinical management in preventing HAPI, SCI/D specific risk scales such as Spinal Cord Injury Pressure Ulcer Scale (SCIPUS) and Spinal Cord Injury Pressure Ulcer Scale-Acute (SCIPUS-A) have been developed [16]. However, the predictive value of these risk scales is still dissatisfying, and the influence of cofactors remains uncertain. No tailored and flexible HAPI risk prediction model currently exists. A scientifically based and clinically developed prediction model for HAPI development can be the starting point of a computerized decision system. This computerized decision system will use an algorithm based on the clinical data to detect, calculate, and show the potential risk of HAPI development. This could lead to the prevention of HAPI development by indicating alerts in patients at increased risk of HAPI. Accordingly, this algorithm could improve the clinical management and prevent HAPI by indicating the changing risk for HAPI in daily routine, particularly, in patients during their first rehabilitation.
The aim of this study was to elaborate risk factors and risk factor combinations for HAPI development and to test a risk predictive model in patients with newly acquired SCI/D during their first rehabilitation.
Methods
Study design and setting
This study is a prospective observational cohort study including retrospective comparative part of two groups of patients with SCI/D during their first rehabilitation at an acute and rehabilitation clinic specialized in patients with SCI/D in Switzerland. HAPI prevention and treatment follow the clinic-specific TIMES (tissue, infection, moisture, edge, surrounding skin) concept [17] and the modified Basel-Decubitus concept [18].
Cohort and observation period
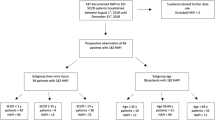
Patients with SCI/D hospitalized for their first rehabilitation between August 1st, 2018 and December 31st, 2019 were eligible. Exclusion criteria were age <18 years and documented rejection of further use of patients’ data. During this observation period, patients’ characteristics and risk factors for HAPI development were prospectively collected. At the end of the study period, patients were retrospectively classified according to the developed a HAPI: the HAPI group (patients who developed at least one HAPI) and the comparison group (CG) (patients without HAPI). Patients’ data in the HAPI group was collected until occurrence of the last HAPI. All patients admitted for first rehabilitation were included and data collection was stopped on December 31st, 2019 and censored for those still being hospitalized on December 31st, 2019 to allow for statistical analysis (Figs. 1 and 2).
Selection of risk factors
We systematically searched for articles that reported PI development-related risk factors with p ≤ 0.05 and risk scales in PubMed and MEDLINE until November 25, 2019. We identified 35 relevant publications describing risk factors in the community and inpatient settings (Supplementary material Table S1) and seven risk scales SCIPUS, SCIPUS-A, Braden, Norton, Waterlow, Gosnell, and Abruzzese [19]. Overall, we identified over 100 risk factors associated with PI development. Some factors had to be excluded due to missing standardized documentation or use in a Study’s SCI center, or due to implausibility during first rehabilitation. Furthermore, based on our clinical experience we added the following items: gastroenteritis, sleep-apnea syndrome (SAS), hypoxemia, abnormal leukocyte count, urinary management system, and muscle relaxants. Lastly, after revising available documentation of risk factors and considering factors from clinical experience, we identified 85 items. These risk factors were then applied in our inpatient setting and are displayed in Supplementary material Tables S2–S5.
Data collection
Data were extracted from the hospital medical information systems: MedFolio (nexus ag, Switzerland, Version: 2.2.0.2317), ixserv4 (ix.mid Software Technologie GmbH, Version: R20.4), WiCareDoc (WigaSoft AG, Switzerland, Version: 7.1.3), and PHÖNIX PACS (Phönix-PACS GmbH, Germany, Version: 5.4), and variables were classified as sociodemographic characteristics, non-modifiable, and modifiable risk factors. In general, sociodemographic characteristic, non-modifiable factors, and among modifiable factors all risk scales and scoring systems as well as all quantifiable variables were assessed in all patients on admission. Acute comorbidities were grouped as modifiable factors and assessed whenever they occurred in all patients. In patients who developed HAPI(s), modifiable risk factors were retrospectively analyzed three days before each HAPI occurrence. Further, all the observed variables are categorized, for general overview see Fig. 2.
Sociodemographic characteristics and non-modifiable risk factors assessed on admission: sex, age, time from SCI diagnosis until admission to the SCI center, length of hospital stay in the SCI center (LOS), PI on admission, PI in patient’s history, etiology of SCI/D, International Standards for Neurological Classification of Spinal Cord Injury (ISNCSCI) classification including American Spinal Injury Association Impairment Scale (AIS) classification [20], civil status, highest education degree, employment status, type of residence before SCI/D, health insurance, body mass index (BMI) on admission, systolic blood pressure (BP) on admission, smoking status, and alcohol consumption. BMI was categorized according to the World Health Organization (WHO) classification (underweight: <18.5, normal weight: 18.5–24.9, overweight: 25.0–29.9, obesity: ≥30) [21]. Systolic BP was categorized according to the American Heart Association classification (hypotension: <90 mmHg, normotension and prehypertension: 90–129 mmHg, hypertension: 130–179 mmHg, hypertensive crisis: ≥180 mmHg) [22]. Chronic comorbidities, although classified as non-modifiable risk factors, were assessed at discharge or on December 31, 2019 for censored patients. The following modifiable risk factors were assessed on admission: nutrition, fecal and urinary management system, fecal and urinary incontinence, mechanical ventilation, skin status and daily skin inspection, mobility, fixation, spasticity (according to Ashworth classification [23]), neuropathic pain, type of wheelchair cushion and bed surface, anticoagulants (i.e., prophylactic vs. therapeutic dosage), laboratory parameters (i.e., hemoglobin, leukocytes, C-reactive protein (CRP), blood sedimentation reaction, total protein, albumin, 25-OH-Vitamin D, creatinine, cystatin C, estimated glomerular filtration rates (GFR)), risk scales (Braden [13, 24], Waterlow [25], and SCIPUS [16]), and scoring systems such as Glasgow Coma Scale (GCS) [26], Functional Independence Measure-cognition (FIMc [27]), Multimorbidity Index (MMI [28]), and Spinal Cord Independence Measure (SCIM [29]). In the CG, the following modifiable risk factors were recorded upon occurrence: fecal and urinary incontinence, fixation, spasticity (maximal value of Ashworth classification), and neuropathic pain. Braden, Waterlow, SCIPUS, FIMc, and GCS were calculated by the clinical investigators KN and CN according to documented medical data. Renal function was classified as mild dysfunction GFR < 60, medium GFR < 30, and severe GFR < 10 ml/min using cystatine C as creatinine is unreliable in patients with paraplegia/quadriplegia due to the changed muscle mass [30]. The SCIM and MMI were assessed by the inter-professional treatment team during hospitalization.
Modifiable risk factors recorded upon occurrence: intensive care unit (ICU) stay, pneumonia, gastroenteritis, UTI, acute decompensated heart failure, vascular comorbidities (i.e., peripheral artery disease, cerebral vascular insult, arterial dissection and embolism), traumatic brain injury, poor blood glucose control (glucose level > 6 mmol/L), hyper- or hypotensive episode (BP > 160 mmHg systolic or <90 mmHg systolic), hypoxemia (oxygenation < 92%), abnormal body temperature (<35.0 °C or >37.5 °C), number, time (<2 h, 2–4 h, >4 h duration) and type (including hand, spine, plastic, urologic, visceral interventions) of surgeries, pharmaceuticals except anticoagulants (i.e., antiplatelet drugs, antibiotics, cortisone, analgesics, antipsychotics, antidepressants, sedative medications, muscle relaxants, cytostatic).
Statistical analysis
Patient characteristics and (non-)modifiable variables were analyzed using descriptive statistics and presented as mean (M) and standard deviation (SD) for normally distributed continuous variables (assessed using Q–Q plots) and count (N) and percentage (%) for categorical variables. Differences in the values and frequency of risk factors between HAPI and CG groups were investigated using the unpaired T-test and Chi-square or Fisher’s exact test, as appropriate. Bonferroni correction for multiple testing (100 tests) was applied, and thus, a p value ≤ 0.0005 was considered statistically significant. Binary logistic regression was used to determine the effect of all significant risk factors in descriptive statistic on HAPI development, and a p value ≤ 0.05 was considered statistically significant. The HAPI risk prediction equation was constructed, and a receiver operator characteristic (ROC) curve was used to predict the effect on HAPI development. The statistical analyses were performed using SPSS software (Version 25, IBM, Somers, NY, USA).
Results
Cohort and HAPI characteristics
In total, 120 patients were admitted for first rehabilitation after newly acquired SCI/D, 26 of whom refused further data use. Among the 94 included patients, 48 (51.1%) developed a HAPI (Fig. 1). Overall, median age was 57.1 ± 16.9 years (range 19 and 86 years) and did not significantly differ between the two groups (HAPI M = 57.1 ± 16.0 years, CG M = 57.3 ± 18.0 years). There were no significant sex differences between the two groups (HAPI n = 34 men, 70.8%, CG n = 30 men, 65.2%). In total, 34 (36.2%) patients had at least one PI on admission without significant difference between the two groups (p = 0.8). In the HAPI group, significantly more patients were classified as AIS A according to ISNCSCI classification (HAPI n = 24, 50.0%, CG n = 7, 15.2%, p = 0.0001), and had a traumatic etiology of SCI/D (p = 0.007). All patients’ characteristics are displayed in Table 1.
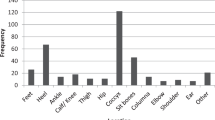
During the first rehabilitation, 48 patients (51.1%) developed at least one HAPI, and in total 93 HAPIs were observed in these 48 patients (Table 2). The most common localization was the foot (n = 25, 27%), followed by the buttocks and ischium (n = 19, 20%, n = 9, 10%), and the coccyx with sacrum (n = 16, 17%). Of all 93 HAPIs, stage I severity was the most frequent (n = 50, 53.8%), followed by stage II (n = 31, 33.3%) and stage III (n = 4, 4.3%) according to the European Pressure Ulcer Advisory Panel (EPUAP). No HAPI was classified as stage IV. However, eight recorded HAPIs (8.6%) were unstageable.
Risk factors
Five risk factors and four scoring tools were significantly different between the two groups (after Bonferroni correction p ≤ 0.0005), also six clinically relevant factors were close to the significant level; these 15 risk factors are displayed in Table 3. On admission, patients in the HAPI group compared to the CG had lower hemoglobin levels (p = 0.001), lower albumin levels (p = 0.001), and lower vitamin D levels (p = 0.006) (Table 3). During the whole observation period, patients in the HAPI group compared to the CG had a higher rate of autonomic dysreflexia (p = 0.00046), hypertensive episodes (p = 0.001), developed more frequent abnormal body temperatures (p = 0.009), and had a higher rate of surgical interventions (p = 0.001) (Table 3). In the HAPI group, the GFR was significantly lower 3 days before HAPI occurrence compared to on admission, (GFR HAPI on admission M = 78.0 ml/Min, SD = 29.74, pathologic 52.3% vs. GFR on HAPI occurrence M = 72.23 ml/Min, SD = 22.97, pathologic 80%) (Supplementary material Tables S5 and S8). Whenever a stage III HAPI occurred (n = 4, 4.3%), a pathologic GFR, increased CRP, ICU stay, and hypotensive episode were observed. A complete overview of the 85 sociodemographic characteristics and (non)-modifiable risk factors are presented in the supplementary material (Tables S2–S8).
Risk model
All five statistically significant risk factors were used as predictors of HAPI in the logistic regression analysis. In the model containing all the variables, longer LOS (p = 0.008) and pneumonia (p = 0.02) were the only significant predictors for HAPI development (without Bonferroni correction p ≤ 0.05). Moreover, LOS of more than 6 months increased the odds for HAPI development 46 times (Table 4). LOS and pneumonia were used to construct a risk prediction equation to calculate the risk for HAPI development. The area under the curve (AUC) was equal to 0.5, which did not allow discrimination in HAPI prediction (Fig. 3).
LOS = length of stay concerning SCI center only, ROC = receiver operating characteristic, Sensitivity = correctly identified patients with a HAPI, specificity = correctly identified patients without a HAPI, the blue curve = discriminative accuracy for the predictive model, area under the curve (AUC) = 0.5, the red diagonal line = results expected by chance alone.
Discussion
The most important finding of this study is that incidence of grade I and II HAPI(s) in patients with newly acquired SCI/D in first rehabilitation remained high despite the extensively integrated preventive HAPI-related therapeutic measures. The observed 15 significant and clinically relevant risk factors included completeness of spinal cord lesion as well as other (non-)modifiable SCI characteristics. The combination of these simultaneous existing risk factors supports the hypothesis of a risk constellation for HAPI development in patients with SCI/D during first rehabilitation. As a first step, these risk factor constellations can be used for quality improvement purposes by providing continuous education about the detected 15 risk factors and by increasing intuitively supported clinical management for preventive measures.
Taking the nine significantly associated risk factors: completeness of SCI/D, pneumonia, sedative medications, autonomic dysreflexia, Braden ≤ 12 points, SCIPUS ≥ 9 points, lower admission SCIM and lower admission FIM-cognition, LOS and the six clinically relevant risk factors: hypertensive episode, abnormal body temperature, surgeries, anemia, albumin and vitamin D deficiency into account, the clinical management remains challenging. Although risk scales are critically discussed in the SCI community, our results indicated an association with HAPI development related to lower Braden and higher SCIPUS scale, similar to a larger Canadian study from 2019 with 754 SCI patients in an inpatient rehabilitation setting [31]. Further, SCIPUS was developed especially for SCI/D individuals; however, in our study, Braden provided greater insight into the prediction of HAPI development, even though autonomic dysreflexia itself, which is a part of SCIPUS measure, proved in our study to have a strong association with HAPI development. These results indicate the limitations of well-established risk scales and the necessity of building scientifically based and clinically developed HAPI risk prediction models, which now, based on our results and support of modern information technologies, could be realized. The combination of all significant risk scales and risk factors also underlines the necessity of an inter-professional management for people with SCI during initial rehabilitation. The complex risk constellation seems to be influenced by individually local skin changes and changes of the generally health condition through new acute health issues in addition to the initial health condition. With regards to the respiratory system, we confirmed previous findings concerning traumatic SCI patients with reduced respiratory function associated with HAPIs [32,33,34]. Sleep-apnea syndrome (SAS) and hypoxemia were recorded in more than half of our HAPI group, but we did not find a significant association. Even though often present, SAS and hypoxemia may not be as important as pneumonia in the development of HAPI and may simply be a modifying risk factor, being underpowered to find a significant association. We interpret that pneumonia is a risk factors for HAPI development because of reduced perfusion and oxygenation, higher inflammation, and reduced immunity.
Another relevant risk factor appeared in our study. In seven cases (7.8%), a change of urinary management system took place just within 3 days before HAPI occurrence; however, the magnitude of this accidental result is at present unknown and needs to be reevaluated with a larger patient sample. In fact, in all stage III HAPIs, pathologic GFR, increased CRP, ICU stay, and hypotensive episode were recorded without showing statistically significant differences between the HAPI group and the CG. Combining the above findings, different significant risk factors and clinically relevant modifiable factors seem to influence HAPI development, which confirms that an HAPI is associated with different patient and SCI/D characteristics but also with specific skin conditions represented in risk models or other secondary health conditions, such as pneumonia, autonomic dysreflexia, or renal failure. The LOS might be on one hand a risk factor and on the other hand a consequence of a HAPI. Patients with an expected longer LOS will be at increased risk for HAPI development and on the other hand patients who develop a HAPI might be more severely ill and require a longer hospital stay, also because of a HAPI. The meaning of LOS in the overall complex risk model could therefore be tested in both ways.
In contrast to the study of PI development during intensive care for critically ill patients with cancer [35], our HAPI risk prediction model based on the significant factors LOS and pneumonia did not improve HAPI prediction in patients with newly acquired SCI/D. Therefore, to create a relevant tailored risk model, first a theoretical model such as the Directed Acyclic Graphs (DAGitty) model [36] can be used, and the odds ratio of each relevant risk factor can be combined in a more complex risk model. A larger sample size of more than 94 patients could integrate more than the five statistically significant risk factors. Taking all 15 significant and clinically relevant risk factors into account, according to rough-and-ready rule with at least 30 observations for each of the 15 factors, an observational study with at least 450 participants and estimated 500 HAPIs should be performed to develop and test a complex risk model [37], using modern methods such as cTree [38].
Our pilot study thus serves as a feasibility study to focus on all relevant risk factors, such as pneumonia or autonomic dysreflexia during first rehabilitation, as well as patient characteristics and PI risk scales, to demonstrate the risk factor constellation and tests on a basic risk prediction model. The next step should be a prospective observation study with enough patients and HAPIs to develop a data-based tailored and flexible HAPI risk prediction model. Most of the modifiable risk factors in our study were relevant during the acute, post-acute, and rehabilitative phases. Therefore, a specific phase or subgroup-related risk model is needed to adapt the HAPI prevention measures accordingly. The number of patients should be accordingly increased to avoid small subgroup observations. To optimize data collection and reduce the documentation burden for healthcare professionals in the clinical setting, big data and clinical information technologies should provide data extraction and further use within a defined algorithm [38]. The risk prediction model can then be tested in an inpatient setting. This model can also be used for educational purposes with the ultimate goal of reducing the occurrence of HAPI in patients with SCI/D during their first rehabilitation.
Limitations
A few limitations are evident in this study. First, the observation of our cohort including documentation of PI was done prospectively, however the risk factors were extracted from medical records retrospectively. Therefore, some factors could have missed in the continuous documentation which indeed could influence the failure of building up our risk model, but unlikely our results. Second, we did not prove the influence of lifestyle on PI development in detail, as these factors were often not measured and are not routinely documented in our setting. Third, this study was conducted in a high-income country, therefore, extrapolation to low-income countries and developing countries should be done with caution and additional unique factors might be important to include in the prediction model.
Conclusion
HAPI was detected in more than half of our cohort and remained a frequent and complex condition in patients with newly acquired SCI/D during first rehabilitation. We demonstrated that 15 significant and clinically relevant modifiable risk factors are associated with HAPI. Today, no scientific HAPI risk predictive model in these complex patients has been developed; however, our findings proved the association of certain risk factor constellations. The indicated HAPI-related risk factor constellation can be used in continuous education in any health service worldwide adopted to the regional and national situation, and thus this risk factors constellation can support intuitive and experience-based clinical management for quality improvement and increase the integration of preventive measures. As nowadays no patient can be clearly identified with high certainty to be at risk for HAPI, all patients should be monitored closely and HAPI preventive measures should be considered daily. Based on our results, a larger prospective observation study with at least 450 patients and 500 HAPIs could allow the establishment of a more precise risk prediction model using new technologies.
Data availability
All data are stored with the corresponding author and can be asked directly (anke.scheel-sailer@paraplegie.ch).
References
von Groote PM, Bickenbach JE, Gutenbrunner C. The World Report on Disability–implications, perspectives and opportunities for physical and rehabilitation medicine (PRM). J Phys Med Rehabil. 2014;93:S4–11. https://doi.org/10.1097/PHM.0000000000000016
Group GD Pressure ulcers: prevention and management of pressure ulcers. 2014.
Edsberg LE, Black JM, Goldberg M, McNichol L, Moore L, Sieggreen M. Revised National Pressure Ulcer Advisory Panel Pressure Injury Staging System: Revised Pressure Injury Staging System. J Wound Ostomy Continence Nurs. 2016;43:585–97. https://doi.org/10.1097/won.0000000000000281
Ehrmann C, Mahmoudi SM, Prodinger B, Kiekens C, Ertzgaard P. Impact of spasticity on functioning in spinal cord injury: an application of graphical modelling. J Rehabilit Med. 2020;52:jrm00037.
Tschannen D, Anderson C. The pressure injury predictive model: a framework for hospital-acquired pressure injuries. J Clin Nurs. 2020;29:1398–421. https://doi.org/10.1111/jocn.15171. Epub 2020.
Henzel MK, Bogie KM, Guihan M, Ho CH Pressure ulcer management and research priorities for patients with spinal cord injury: consensus opinion from SCI QUERI Expert Panel on Pressure Ulcer Research Implementation. J RehabilitRes Dev. 2011;48:xi–xxxii.
van der Wielen H, Post MWM, Lay V, Glasche K, Scheel-Sailer A. Hospital-acquired pressure ulcers in spinal cord injured patients: time to occur, time until closure and risk factors. J Spinal Cord. 2016;54:726–31. https://doi.org/10.1038/sc.2015.239. Epub 2016.
Scheel-Sailer A, Wyss A, Boldt C, Post MW, Lay V. Prevalence, location, grade of pressure ulcers and association with specific patient characteristics in adult spinal cord injury patients during the hospital stay: a prospective cohort study. J Spinal Cord. 2013;51:828–33. https://doi.org/10.1038/sc.2013.91. Epub 2013.
Hoh DJ, Rahman M, Fargen KM, Neal D, Hoh BL. Establishing standard hospital performance measures for cervical spinal trauma: a Nationwide In-patient Sample study. Spinal Cord. 2016;54:306–13.
Byrne DW, Salzberg CA. Major risk factors for pressure ulcers in the spinal cord disabled: a literature review. J Spinal Cord. 1996;34:255–63. https://doi.org/10.1038/sc.1996.46
Marin J, Nixon J, Gorecki C. A systematic review of risk factors for the development and recurrence of pressure ulcers in people with spinal cord injuries. Spinal Cord. 2013;51:522–7.
Norton D. Calculating the risk: reflections on the Norton Scale. 1989. Adv Wound Care: J Prev Healing. 1996;9:38–43.
Bergstrom N, Braden BJ, Laguzza A, VH. TheBraden scale for predicting pressure sore risk. J Nurs Res. 1987;36:205–10.
Waterlow J. Pressure sores: a risk assessment card. Nursing. 1985;81:49–55.
Afridi A, Rathore FA. Are Risk Assessment Tools Effective for the Prevention of Pressure Ulcers Formation?: A Cochrane Review Summary With Commentary. Am J Phys Med Rehabilit. 2020;99:357–8.
Salzberg CA, Byrne DW, Cayten CG, van Niewerburgh P, Murphy JG, Viehbeck M. A new pressure ulcer risk assessment scale for individuals with spinal cord injury. Am J Phys Med Rehabil. 1996;75:96–104. https://doi.org/10.1097/00002060-199603000-00004
Scheel-Sailer A, Plattner C, Flückiger B, Ling B, Schaefer D, Baumberger M, et al. Dekubitus – ein. Update. 2016;16:489–98.
Meier C, Boes S, Gemperli A, Gmünder HP, Koligi K, Metzger S, et al. Treatment and cost of pressure injury stage III or IV in four patients with spinal cord injury: the Basel Decubitus Concept. Spinal Cord Ser Cases. 2019;5:30–30.
Mortenson WB, Miller WC. A review of scales for assessing the risk of developing a pressure ulcer in individuals with SCI. Spinal Cord. 2008;46:168–75. https://doi.org/10.1038/sj.sc.3102129. Epub 2007.
Betz R, Biering-Sørensen F, Burns SP, Donovan W, Graves DE, Guest J, et al. The 2019 revision of the International Standards for Neurological Classification of Spinal Cord Injury (ISNCSCI)—What’s new? J Spinal Cord. 2019;57:815–17. https://doi.org/10.1038/s41393-019-0350-9. Epub 2019.
Organization WH Body Mass Index – BMI: WHO; 2020. http://www.euro.who.int/en/health-topics/disease-prevention/nutrition/a-healthy-lifestyle/body-massindex-bmi.
Association AH Blood Pressure Systolic 2020. https://www.heart.org/en/healthtopics/high-blood-pressure/understanding-blood-pressure-readings
Akpinar P, Atici A, Ozkan FU, Aktas I, Kulcu DG, Sarı A, et al. Reliability of the Modified Ashworth Scale and Modified Tardieu Scale in patients with spinal cord injuries. Spinal Cord. 2017;55:944–9.
Compton F, Strauss M, Hortig T, Frey J, Hoffmann F, Zidek W, et al. [Validity of the Waterlow scale for pressure ulcer risk assessment in the intensive care unit: a prospective analysis of 698 patients]. J Pflege. 2008;21:37–48. https://doi.org/10.1024/1012-5302.21.1.37
Anthony D, Parboteeah S, Saleh M, Papanikolaou P. Norton, Waterlow and Braden scores: a review of the literature and a comparison between the scores and clinical judgement. J Clin Nurs. 2008;17:646–53. https://doi.org/10.1111/j.1365-2702.2007.02029.x
Teasdale G, Maas A, Lecky F, Manley G, Stocchetti N, Murray G. The Glasgow Coma Scale at 40 years: standing the test of time. Lancet Neurol. 2014;13:844–54. https://doi.org/10.1016/S1474-4422(14)70120-6
Linacre JM, Heinemann AW, Wright BD, Granger CV, Hamilton BB. The structure and stability of the Functional Independence Measure. Arch Phys Med Rehabil. 1994;75:127–32.
Stanley J, Sarfati D. The new measuring multimorbidity index predicted mortality better than Charlson and Elixhauser indices among the general population. J Clin Epidemiol. 2017;92:99–110. https://doi.org/10.1016/j.jclinepi.2017.08.005. Epub 2017.
Itzkovich M, Gelernter I, Biering-Sorensen F, Weeks C, Laramee MT, Craven BC, et al. The Spinal Cord Independence Measure (SCIM) version III: reliability and validity in a multi-center international study. J Disabil Rehabil. 2007;29:1926–33. https://doi.org/10.1080/09638280601046302. Epub 2007.
Jenkins MA, Brown DJ, Ierino FL, Ratnaike SI. Cystatin C for estimation of glomerular filtration rate in patients with spinal cord injury. J Ann Clin Biochem. 2003;40:364–8. https://doi.org/10.1258/000456303766476995
Flett HM, Delparte JJ, Scovil CY, Higgins J, Laramée MT, Burns AS. Determining Pressure Injury Risk on Admission to Inpatient Spinal Cord Injury Rehabilitation: a Comparison of the FIM, Spinal Cord Injury Pressure Ulcer Scale, and Braden Scale. Arch Phys Med Rehabilit. 2019;100:1881–7.
Brienza D, Krishnan S, Karg P, Sowa G, Allegretti AL. Predictors of pressure ulcer incidence following traumatic spinal cord injury: a secondary analysis of a prospective longitudinal study. Spinal Cord. 2018;56:28–34.
Grigorian A, Sugimoto M, Joe V, Schubl S, Lekawa M, Dolich M, et al. Pressure Ulcer in Trauma Patients: a Higher Spinal Cord Injury Level Leads to Higher Risk. J Am Coll Clin Wound Specialists. 2017;9:24–31. e1
Gour-Provencal G, Mac-Thiong J-M, Feldman DE, Bégin J, Richard-Denis A Decreasing pressure injuries and acute care length of stay in patients with acute traumatic spinal cord injury. J Spinal Cord Med 2020;1–9. https://doi.org/10.1080/10790268.2020.1718265
Sun ZW, Guo MR, Yang LZ, Chen ZJ, Zhang ZQ. Risk Factor Analysis and Risk Prediction Model Construction of Pressure Injury in Critically Ill Patients with Cancer: a Retrospective Cohort Study in China. Med Sci Monit: Int Med J Exp Clin Res. 2020;26:e926669.
Model D DAGitty Model 2020 [http://dagitty.net/]
Peduzzi P, Concato J, Kemper E, Holford TR, Feinstein AR. A simulation study of the number of events per variable in logistic regression analysis. J Clin Epidemiol. 1996;49:1373–9. https://doi.org/10.1016/s0895-4356(96)00236-3
Fokkema M, Smits N, Zeileis A, Hothorn T, Kelderman H. Detecting treatmentsubgroup interactions in clustered data with generalized linear mixed-effects model trees. Behav Res Methods. 2018;50:2016–34.
Benchimol EI, Smeeth L, Guttmann A, Harron K, Moher D, Petersen I, et al. The REporting of studies Conducted using Observational Routinely-collected health Data (RECORD) statement. PLoS Med. 2015;12:e1001885 https://doi.org/10.1371/journal.pmed.1001885.eCollection.
Acknowledgements
We thank the “Decu Care Team” (Jessica Decker, Eva Kissling, Rita Müller, Diana Sigrist-Nix, Ivonne Zamzow) for assistance with data collection and Karin Gläsche as the main supervising and responsible nurse. A special recognition goes to Stefanie Tesini for the composition of the database.
Funding
The authors received no funding in support of this study.
Author information
Authors and Affiliations
Contributions
KN, CN and AS-S: study design, data collection, data analysis, paper preparation. JK, COS: Study design, data analysis and statistics, paper preparation. MB, DJS, RW: Study design, paper preparation.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests with respect to this research, authorship and publication of this paper. The work was a part of KN doctoral thesis (Dr med. Promotion).
Ethical approval
This study was conducted in compliance with the protocol, the current version of the Declaration of Helsinki, the ICH-GCP as well as all national legal and regulatory requirements, and excluded patients who denied their consent of further use of their data. All data were kept confidential and processed anonymously. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guidelines [39]. The Ethics Committees of Northwest and Central Switzerland approved this study involving humans on 25th November 2019 (EKNZ ID 2019-02179).
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Najmanova, K., Neuhauser, C., Krebs, J. et al. Risk factors for hospital acquired pressure injury in patients with spinal cord injury during first rehabilitation: prospective cohort study. Spinal Cord 60, 45–52 (2022). https://doi.org/10.1038/s41393-021-00681-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41393-021-00681-x
This article is cited by
-
Risk factors of major complications after flap surgery in the treatment of stage III and IV pressure injury in people with spinal cord injury/disorder: a retrospective cohort study
Spinal Cord (2024)
-
Predictors of hospital-acquired pressure injuries in patients with complete spinal cord injury: a retrospective case–control study
BMC Musculoskeletal Disorders (2023)
-
Risk constellation of hospital acquired pressure injuries in patients with a spinal cord injury/ disorder - focus on time since spinal cord injury/ disorder and patients’ age
Spinal Cord (2023)