Abstract
Objectives:
We have shown earlier that administration of the flavonoid quercetin significantly contributed to recovery of motor function after spinal cord compression injury in the adult rat. Using the same animal model, we have now designed a set of experiments to test the hypothesis that quercetin attenuates oxidative stress-related inflammatory processes early after spinal cord trauma.
Methods:
Mid-thoracic spinal cord compression injury in adult male Wistar rats was caused by extradural application and closure of a 50 g calibrated aneurysm clip for 5 s. Myeloperoxidase (MPO) levels were determined in spinal cord tissue and serum of quercetin-treated animals and controls at 6, 12, 24 and 72 h after injury. The white blood count was followed until 72 h after injury.
Results:
In quercetin-treated animals, MPO activity was significantly decreased in tissue at 12 and 24 h and in serum at 6, 12 and 24 h after injury, compared with saline controls. In quercetin-treated animals, the prevalence of ED-1 and MPO positive cells was significantly lower than in saline controls. White blood count in venous blood was significantly decreased in quercetin-treated animals at 12 and 24 h after injury.
Conclusion:
Quercetin attenuated the recruitment of neutrophils to the site of injury. The resulting lower MPO release in the injured tissue is likely to decrease the extent of secondary injury and might at least partially explain the neuroprotective effect of the flavonoid quercetin.
Similar content being viewed by others
Introduction
In the clinical setting of spinal cord injury, pathomechanisms of the secondary injury complex significantly increase the volume of the lesion and may result in complete loss of functional spinal cord tissue across the entire cross-sectional surface at the site of injury.1, 2, 3 Structures that were not directly injured by the primary mechanical impact are damaged in an unfavourable cellular environment, and damage becomes permanent in tissue structures that potentially could have recovered.4, 5 Studies in an animal model of spinal cord compression injury have shown that survival of as little as 10% of all axons in the rat spinal cord is sufficient to support significant motor function.6, 7 Therefore, protection of even a small number of primarily undamaged axons from delayed cell death may result in a considerable difference in functional outcome for the patient.
In earlier experiments, we have shown that administration of the flavonoid quercetin contributes significantly to an improved recovery of motor function in an animal model of spinal cord compression injury.8, 9 Bradley et al.10 have shown that myeloperoxidase (MPO) activity can reflect fairly well the extent of neutrophil accumulation in tissue. Pincemail et al.11 have shown that quercetin is a potent inhibitor of MPO activity in vitro in a system of activated neutrophils.
On the basis of these observations, we have designed a set of experiments to test the hypothesis that administration of quercetin attenuates inflammatory processes in the early phase after acute traumatic spinal cord injury.
Materials and methods
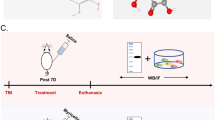
A total of 40 adult male Wistar rats (306–366 g, Charles River, Canada) were used, and all experiments were conducted in accordance with the guidelines of the Canadian Council on Animal Care. Thirty-six animals were subjected to standardized mid-thoracic spinal cord trauma (T7). The remaining four animals served as uninjured controls. We used the model of mid-thoracic spinal cord compression injury introduced by Rivlin and Tator,12 which has been well established in our laboratory for several years. In this model, an aneurysm clip with a calibrated closing force is closed around the mid-thoracic spinal cord for 5 s which, in the absence of therapeutic intervention, leaves all animals completely paraplegic. Laminectomy was performed at T6/7 level, and the spinal cord was exposed without opening the dura mater. The animals were randomly assigned to four experimental groups, being killed at 6, 12, 24 or 72 h after injury (Table 1). Half of the animals in each group received doses of 25 μmol kg−1 quercetin (quercetin dihydrate, Sigma, Oakville, Ontario, Canada) suspended in normal saline as intraperitoneal injection twice daily, starting 1 h after the injury. Animals killed at 12, 24 and 72 h after injury received their last doses about 40–50 min before they were killed. The other half of the animals received 3 ml normal saline on the same schedule as quercetin-treated animals.
MPO activity
The MPO activity was measured in both spinal cord tissue and serum of quercetin-treated animals and saline controls at 6, 12, 24 and 72 h after injury (n=4 per group). Four healthy animals served to establish normal values. The animals were killed by percardiac perfusion under inhalation anaesthesia with Halothane, using ∼300 ml ice cold physiological saline per animal. Before any saline was introduced into the vascular system, about 3 ml of blood were aspirated. Serum was collected after centrifugation for 3 min at 13 000 r.p.m. and stored in cryovials at −70 °C until biochemical assays were performed. Serial laminectomy was performed from mid-cervical to lumbar levels, while cooling with liquid nitrogen continued at regular intervals. Individual spinal cord segments were stored in cryovials at −70 °C awaiting analysis.
For the measurement of MPO activity in spinal cord tissue, a spectrophotometric method was used.13 Spinal cord segments from the site of injury (T6–8) and segments cranial (T4) and caudal (T10) to the injury site were weighed, homogenized mechanically and sonicated in 50 mM HTAB buffer (1 part tissue in 20 parts HTAB buffer, pH 6, containing 0.5% hexadecyltrimethylammonium bromide) on ice, two times for 3 s and once for 5 s. Hexadecyltrimethylammonium bromide is a detergent used to extract MPO from the neutrophil granules.14 The sonicated homogenates were centrifuged for 15 min at 13 000 g and 4 °C, after which the supernatant was transferred to a new centrifuge tube. Absorbance at 460 nm was measured after adding o-dianisidine dihydrochloride in potassium phosphate buffer (pH 6.0) with H2O2 to the supernatant, using a spectrophotometer (SPECTRA MAX 190, Molecular Devices, Sunnyvale, CA, USA). Absorbance was calculated by computer software in the Endpoint protocol. As the basis of this assay is the reaction of hydrogen peroxide and chloride to form hypochlorous acid that in turn oxidizes o-dianisidine dihydrochloride to form a coloured end product, one unit of MPO activity is defined in terms of 1 μmol oxidized o-dianisidine formed per minute at 25 °C.
For the measurement of MPO activity in serum, the same procedure was followed after dilution of the serum 1:2 with HTAB. All analyses were performed in triplicate.
Immunohistochemistry for MPO and ED-1
Two quercetin-treated animals and two saline controls were killed at 24 h, the time at which a maximum influx of white blood cells was expected. After perfusion with 4% formalin, the spinal cord segments containing the site of injury were isolated and stored in 4% formalin overnight, then transferred to a 30% sucrose solution for 48 h (cryoprotection). The samples were embedded in OCT for sagittal (longitudinal) sections, with the site of injury in the middle. Cryosections at 20-μm thickness were mounted on Superfrost plus slides (VWR). Three slides with two sections each were prepared from each of the animals. The sections were double stained with MPO antibody raised in goat (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA, 1:100 dilution; secondary AB: FITC anti-goat IgG) and monoclonal ED-1 antibody (DakoCytomation, 1:250 dilution; secondary AB: cy3 anti-mouse IgG), photographed and loaded into Image J. In each section, we measured the percentage of the total section area stained red (ED-1 positive) and green (MPO positive).
Blood cell counts
White blood cell count was performed on a full blood smear. The methanol-fixed slides were stained with Wright's (4 min) and counterstained with Giemsa (10 min). Cells were counted per field of view in 10 fields for each sample under medium magnification.
Statistical analysis
A non-parametric t-test (Mann–Whitney test) was used for analysis of MPO levels, whereas a simple unpaired t-test was used for white blood count analysis. Data between group means were considered statistically significant if P⩽0.05.
Results
An overview over the group means of MPO levels in tissue and serum of injured animals as well as group means of white blood count is given in Table 1. All values in this section are presented as mean values±s.d.
MPO activity
The MPO activity in spinal cord segments of healthy animals was not significantly different at different cord levels. Group means were 0.62 units g−1 tissue at T7 (±0.04). The mean value for serum levels of MPO in healthy animals was 0.0021 units l−1 (±0.00044). Compared with saline controls, a significant decrease of MPO activity in spinal cord tissue at the site of injury was found with quercetin treatment at 12 h (P=0.016) and at 24 h (P=0.008) after trauma. No statistically significant difference was seen at 6 h (P=0.4) and at 72 h after injury (P=0.7). Compared with the injury segment, very little MPO activity was detected in the spinal cord segments cranial (T4) or caudal (T10), three segments distant from the injury site (Figure 1). The MPO values measured in the cranial segments were generally lower than those measured in the caudal segments (Figure 2). However, the differences between cranial and caudal segments of treated and untreated animals were not statistically significant at any of the studied time points after injury.
Significantly reduced levels of MPO activity in serum were seen at 6 h (P=0.02), 12 h (P=0.04) and 24 h (P=0.04) with administration of quercetin, compared with saline controls. Although there were lower MPO activity levels at 72 h after injury, this was not statistically significant.
In healthy animals, the white blood count was 2.33/field of view (±0.58). The differences between quercetin-treated animals and saline controls were statistically significant at 12 h (P=0.0177) and 24 h (0.0341) after injury. No difference was seen at 72 h.
In the tissue of quercetin-treated animals, the prevalence of ED-1 positive cells or cell fragments was also remarkably lower than in the tissue of saline controls. In the sections obtained from the trauma site at 24 h after injury, total area measurements were 26.7% (±16.3) for ED-1 and 24.3% (±15.4) for MPO in saline controls and 14.8% of the total area (±2.8) for ED-1 positive (red) and 6.5% (±2.5) for MPO-positive (green) in quercetin-treated animals (Figure 3). The differences between treated animals and saline controls were statistically significant for ED-1 (P=0.011) and of borderline significance for MPO (P=0.051). These values are congruent with the results obtained in the biochemical assay and the cell count.
Discussion
The first days after spinal cord injury are characterized by an influx of neutrophils (peak influx at 1 day) and macrophages (peak influx at 3 days) into the tissue at the site of injury and adjacent spinal cord segments in both animal models and human patients.13, 15 Neutrophils, when stimulated, generate potent reactive oxygen species that are released into the extracellular space where they oxidize DNA, proteins and lipids, increasing the volume of secondary damage after the trauma.16, 17, 18, 19 The oxidant activity of H2O2 is significantly enhanced by the action of MPO. MPO catalyses the reaction of hydrogen peroxide and chloride anions, generating hypochlorous acid (HOCl) and chloramines. The reactivity of the latter is about two orders of magnitude higher than that of H2O2 alone.11 These oxidant species, together with hydrolytic enzymes, are released into the extracellular space. Bradley et al.10 have shown that the haemoprotein MPO activity can reflect fairly well the extent of neutrophil accumulation in tissue. MPO, stored in the granules of neutrophils, has been described as key regulator in oxidant production by cellular mediators of inflammation.20
Similar to earlier findings that peak neutrophil numbers accumulated in injured spinal cord at ∼12–24 h post-injury, we showed that the highest level of MPO in injured tissue was around 12–24 h post-injury. We were able to show in our animal model of spinal cord compression injury, that MPO levels in quercetin-treated animals were significantly lower at the 12 and 24 h time intervals at the site of injury than in saline vehicle-treated animals. Peak levels of MPO in serum from saline vehicle-treated animals occurred also in the 12–24 h post-injury time period. Although levels at 24 h appeared to be higher than at 12 h, this was not significant. Again, in the quercetin-treated animals, the MPO activity in serum was significantly lowered at the 12 and 24 h time periods as well as at the 6 h time period. The spinal cord tissue MPO activity at the sites adjacent to the injury was also lower in quercetin-treated animals, compared with saline vehicle controls; however, this did not reach the level of significance. We conclude that the MPO level changes seen in injured spinal cord tissue reflected the changes seen in serum MPO levels.
Immunocytochemistry at 24 h after injury also showed that quercetin decreased the expression of markers for neutrophils and activated macrophages in the injured tissue, compared with saline controls. This was determined by measuring the area occupied by immunofluorescent signal for both activated macrophages (ED-1-positive cells) as well as MPO-positive cells (mainly neutrophils). This decrease of immunofluorescent signal was in rough proportion to the decrease seen with MPO activity. Not surprisingly, total blood leucocytes increased after spinal cord injury with peak numbers being seen at the 12 and 24 h time periods after injury. As seen with the MPO activity, the administration of quercetin to animals with spinal cord injury significantly decreased the leucocyte count at 12 and 24 h. Given that the peripheral leucocyte count was decreased in quercetin-treated animals, our findings would support the hypothesis that quercetin decreases activation of leucocytes, thereby decreasing the numbers in blood and injured tissue. Alternatively, it could be possible that, in agreement with the earlier quoted observations made by Pincemail, the production of MPO by neutrophils in the injured tissue is decreased, resulting in an overall lowered inflammatory status and thereby a reduced peripheral leucocyte count.
The results of our experiments support our hypothesis that administration of quercetin attenuates inflammatory processes in the setting of experimental spinal cord compression injury. We have shown that twice daily administration of 25 μmol kg−1 quercetin significantly attenuated the recruitment of inflammatory cells into the injured tissue and resulted in significantly decreased MPO levels. Thus, the modulation of MPO tissue levels is one of the pathways through which the compound acts in the setting of spinal cord injury.
A statistically significant difference was seen in tissue and serum MPO levels 12 and 24 h, but not at 72 h after injury. In an earlier experimental series, we found that a significant improvement in recovery of motor function was seen when the duration of quercetin administration was increased from 24 h to 3 days.9 This suggests that much of the functional improvement gained with longer quercetin administration is caused by an attenuation of pathological processes that occur within the first 3 days after the injury. One likely mechanism of action for quercetin is the inhibition of neutrophil recruitment into the site of injury and subsequent decrease in MPO released into the traumatized tissue. The results of this study suggest strongly that administration of quercetin does indeed decrease secondary tissue damage after acute spinal cord trauma.
References
Tator CH, Rowed DW . Current concepts in the immediate management of acute spinal cord injuries. Can Med Assoc J 1979; 121: 1453–1464.
Tator CH . Update on the pathophysiology and pathology of acute spinal cord injury. Brain Pathol 1995; 5: 407–413.
Beattie MS, Bresnahan JC . Cell death, repair and recovery of function after spinal cord contusion injuries in rats. In: Kalb RG, Strittmatter SM (eds). Neurobiology of Spinal Cord Injury. Humana Press: Totowa New Jersey, 2000. pp 1–21.
Crowe MJ, Bresnahan JC, Shuman SI, Masters JN, Beattie MS . Apoptosis and delayed degeneration after spinal cord injury in rats and monkeys. Nat Med 1997; 3: 73–76.
Beattie MS, Hermann GE, Rogers RC, Bresnahan JC . Cell death in models of spinal cord injury. Prog Brain Res 2002; 137: 37–47.
Kamencic H, Griebel RW, Lyon AW, Paterson PG, Juurlink BH . Promoting glutathione synthesis after spinal cord trauma decreases secondary damage and promotes retention of function. FASEB J 2001; 15: 243–250.
Fehlings MG, Tator CH . The relationship among the severity of spinal cord injury, residual neurological function, axon counts, and counts of retrogradely labelled neurons after experimental spinal cord injury. Exp Neurol 1995; 132: 220–228.
Schültke E, Kendall E, Kamencic H, Ghong Z, Griebel RW, Juurlink BHJ . Quercetin promotes recovery from acute spinal cord injury: an MRI supported efficacious dose determination. J Neurotrauma 2003; 20: 583–591.
Schültke E, Kamencic H, Skihar VM, Griebel RW, Juurlink BHJ . Quercetin in an animal model of spinal cord compression injury: correlation of treatment duration with recovery of motor function. Spinal Cord 2009; 48: 112–117.
Bradley PP, Priebat DA, Christensen RD, Rothstein G . Measurement of cutaneous inflammation: estimation of neutrophil content with an enzyme marker. J Invest Dermatol 1982; 78: 206–209.
Pincemail J, Deby C, Thirion A, de Bruyn-Dister M, Goutier R . Human myeloperoxidase activity is inhibited in vitro by quercetin. Comparison with three related compounds. Experientia 1988; 44: 450–453.
Rivlin A, Tator CH . Effect of duration of acute spinal cord compression in a new acute injury model in the rat. Surg Neurol 1978; 10: 38–43.
Carlson SL, Parrish ME, Springer JE, Doty K, Dossett L . Acute inflammatory response in spinal cord following impact injury. Exp Neurol 1988; 15: 77–88.
Krawisz J, Sharon P, Stenson W . Quantitative assay for acute intestinal inflammation based on myeloperoxidase activity. Gastroenterology 1984; 87: 1344–1350.
Anderson DK . Chemical and cellular mediators in spinal cord injury. J Neurotrauma 1992; 9: 143–145.
Badwey JA, Karnovsky ML . Active oxygen species and the functions of phagocytic leucocytes. Annu Rev Biochem 1980; 49: 695–726.
Winterbourn CC, Garcia R, Segal AW . Production of the superoxide adduct of myeloperoxidase (compound III) by stimulated human neutrophils and its reactivity with hydrogen peroxide and chloride. Biochem J 1985; 228: 583–592.
Badwey JA, Ding J, Heyworth JA, Robinson JM . Products of inflammatory cells synergistically enhance superoxide production by phagocytic leucocytes. Adv Exp Med Biol 1991; 341: 19–33.
Hampton MB, Kettle AJ, Winterbourn CC . Inside the neutrophil phagosome: oxidants, myeloperoxidase and bacterial killing. Blood 1998; 92: 3007–3017.
Kettle AJ, Winterbourn CC . Myeloperoxidase: a key regulator of neutrophil oxidant production. Redox Rep 1997; 3: 3–15.
Acknowledgements
We thank our technical assistants Arlene Drimmie and Connie Wong (Department of Anatomy and Cell Biology) and the students Angela Damant and Viktor Skihar for help with the animal care. Lloyd Jennett from RUH is thanked for technical support, fixing our anaesthesia machine whenever it breaks down. This work was supported by the Saskatchewan Health Research Foundation and by the Christopher Reeve Paralysis Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Schültke, E., Griebel, R. & Juurlink, B. Quercetin attenuates inflammatory processes after spinal cord injury in an animal model. Spinal Cord 48, 857–861 (2010). https://doi.org/10.1038/sc.2010.45
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sc.2010.45
Keywords
This article is cited by
-
Quercetin suppresses NLRP3 inflammasome activation and attenuates histopathology in a rat model of spinal cord injury
Spinal Cord (2016)
-
Anti-inflammatory Effect of Mesenchymal Stromal Cell Transplantation and Quercetin Treatment in a Rat Model of Experimental Cerebral Ischemia
Cellular and Molecular Neurobiology (2016)