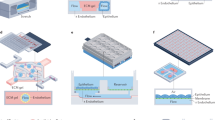
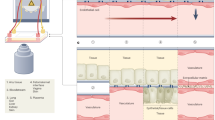
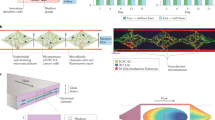
Abstract
The human microbiome orchestrates a variety of metabolic, immunological and regulatory functions in health and disease. However, experimentally dissecting host–microbiome interactions remains challenging. In this Review, we discuss how human organ-on-a-chip platforms can be designed to study fundamental microbiome mechanisms, investigate microbiome-related disease pathophysiology and provide preclinical drug screening platforms, as an alternative to conventional cell cultures or animal models. We outline key design parameters, including spatial configurations, microfluidic setups, mechanical deformation and oxygen gradients, that allow the longitudinal co-culture of aerobic and anaerobic microorganisms with human cells. Such organ-on-a-chip platforms have been explored for the preclinical modelling of microbiome-associated diseases, including infectious and reproductive organ diseases. Moreover, organ-on-a-chip platforms can be engineered to test microbiome-assisted therapeutics, for pharmacomicrobiomics and culturomics investigations, and to model microbiome-mediated multi-organ interactions. Finally, we provide a translational outlook, discussing clinical challenges, regulatory hurdles and precision interventions.
Key points
-
Human organ-on-a-chip platforms allow the investigation of host–microbiome interactions in vitro under controlled physiological and biomechanical dynamics.
-
Organ-on-a-chip models can be designed to recapitulate inter-kingdom crosstalk for preclinical disease modelling and validation of therapeutic interventions.
-
Patient-derived samples, including microbiome and cell samples, can be integrated in organ-on-a-chip platforms to model patient-specific disease milieus, reflecting the genetic and phenotypic parameters of a specific patient.
-
Human organ-on-a-chip platforms can be applied to study human-relevant drug metabolism, bioavailability and toxicity, and to investigate microbiome-associated diseases.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$99.00 per year
only $8.25 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Nicholson, J. K. et al. Host-gut microbiota metabolic interactions. Science 336, 1262–1267 (2012).
Berg, G. et al. Microbiome definition re-visited: old concepts and new challenges. Microbiome 8, 103 (2020).
Sender, R., Fuchs, S. & Milo, R. Revised estimates for the number of human and bacteria cells in the body. PLoS Biol. 14, e1002533 (2016).
Schluter, J. & Foster, K. R. The evolution of mutualism in gut microbiota via host epithelial selection. PLoS Biol. 10, e1001424 (2012).
Carding, S., Verbeke, K., Vipond, D. T., Corfe, B. M. & Owen, L. J. Dysbiosis of the gut microbiota in disease. Microb. Ecol. Health Dis. 26, 26191 (2015).
Doestzada, M. et al. Pharmacomicrobiomics: a novel route towards personalized medicine? Protein Cell 9, 432–445 (2018).
Nguyen, T. L. A., Vieira-Silva, S., Liston, A. & Raes, J. How informative is the mouse for human gut microbiota research? Dis. Model. Mech. 8, 1–16 (2015).
Seok, J. et al. Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc. Natl Acad. Sci. USA 110, 3507–3512 (2013).
Chou, D. B. et al. On-chip recapitulation of clinical bone marrow toxicities and patient-specific pathophysiology. Nat. Biomed. Eng. 4, 394–406 (2020).
Ghosh, S., Whitley, C. S., Haribabu, B. & Jala, V. R. Regulation of intestinal barrier function by microbial metabolites. Cell. Mol. Gastroenterol. Hepatol. 11, 1463–1482 (2021).
Levy, M., Kolodziejczyk, A. A., Thaiss, C. A. & Elinav, E. Dysbiosis and the immune system. Nat. Rev. Immunol. 17, 219–232 (2017).
Waclawiková, B., Codutti, A., Alim, K. & El Aidy, S. Gut microbiota-motility interregulation: insights from in vivo, ex vivo and in silico studies. Gut Microbes 14, 1997296 (2022).
Pan, R. et al. Crosstalk between the gut microbiome and colonic motility in chronic constipation: potential mechanisms and microbiota modulation. Nutrients 14, 3704 (2022).
Flint, H. J., Scott, K. P., Duncan, S. H., Louis, P. & Forano, E. Microbial degradation of complex carbohydrates in the gut. Gut Microbes 3, 289–306 (2012).
Morrison, D. J. & Preston, T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes 7, 189–200 (2016).
Wahlström, A., Sayin, S. I., Marschall, H.-U. & Bäckhed, F. Intestinal crosstalk between bile acids and microbiota and its impact on host metabolism. Cell Metab. 24, 41–50 (2016).
Haiser, H. J., Seim, K. L., Balskus, E. P. & Turnbaugh, P. J. Mechanistic insight into digoxin inactivation by Eggerthella lenta augments our understanding of its pharmacokinetics. Gut Microbes 5, 233–238 (2014).
Powell, N., Walker, M. M. & Talley, N. J. The mucosal immune system: master regulator of bidirectional gut–brain communications. Nat. Rev. Gastroenterol. Hepatol. 14, 143–159 (2017).
Tripathi, A. et al. The gut–liver axis and the intersection with the microbiome. Nat. Rev. Gastroenterol. Hepatol. 15, 397–411 (2018).
Gerbaba, T. K., Green-Harrison, L. & Buret, A. G. Modeling host-microbiome interactions in Caenorhabditis elegans. J. Nematol. 49, 348–356 (2017).
Jia, P. P. et al. Role of germ-free animal models in understanding interactions of gut microbiota to host and environmental health: a special reference to zebrafish. Environ. Pollut. 279, 116925 (2021).
Zheng, H., Powell, J. E., Steele, M. I., Dietrich, C. & Moran, N. A. Honeybee gut microbiota promotes host weight gain via bacterial metabolism and hormonal signaling. Proc. Natl Acad. Sci. USA 114, 4775–4780 (2017).
Kostic, A. D., Howitt, M. R. & Garrett, W. S. Exploring host–microbiota interactions in animal models and humans. Genes Dev. 27, 701–718 (2013).
Inoue, R. & Ushida, K. Development of the intestinal microbiota in rats and its possible interactions with the evolution of the luminal IgA in the intestine. FEMS Microbiol. Ecol. 45, 147–153 (2003).
Hildebrand, F. et al. A comparative analysis of the intestinal metagenomes present in guinea pigs (Cavia porcellus) and humans (Homo sapiens). BMC Genomics 13, 514 (2012).
Pilla, R. & Suchodolski, J. S. The role of the canine gut microbiome and metabolome in health and gastrointestinal disease. Front. Vet. Sci. 6, 498 (2020).
Patil, Y., Gooneratne, R. & Ju, X. H. Interactions between host and gut microbiota in domestic pigs: a review. Gut Microbes 11, 310–334 (2020).
Legrand, T., Wynne, J. W., Weyrich, L. S. & Oxley, A. P. A. Investigating both mucosal immunity and microbiota in response to gut enteritis in yellowtail kingfish. Microorganisms 8, 1267 (2020).
Slinger, J., Adams, M. B. & Wynne, J. W. Bacteriomic profiling of branchial lesions induced by Neoparamoeba perurans challenge reveals commensal dysbiosis and an association with Tenacibaculum dicentrarchi in AGD-affected Atlantic salmon (Salmo salar L.). Microorganisms 8, 1189 (2020).
Luo, J. J., Young, C. D., Zhou, H. M. & Wang, X. J. Mouse models for studying oral cancer: impact in the era of cancer immunotherapy. J. Dent. Res. 97, 683–690 (2018).
Catalone, B. J. et al. Mouse model of cervicovaginal toxicity and inflammation for preclinical evaluation of topical vaginal microbicides. Antimicrob. Agents Chemother. 48, 1837–1847 (2004).
Avci, P. et al. Animal models of skin disease for drug discovery. Expert Opin. Drug Discov. 8, 331–355 (2013).
Yin, H. et al. Fusobacterium nucleatum promotes liver metastasis in colorectal cancer by regulating the hepatic immune niche and altering gut microbiota. Aging 14, 1941–1958 (2022).
Ye, W. & Chen, Q. Potential applications and perspectives of humanized mouse models. Annu. Rev. Anim. Biosci. 10, 395–417 (2022).
Uzbay, T. Germ-free animal experiments in the gut microbiota studies. Curr. Opin. Pharmacol. 49, 6–10 (2019).
Staley, C. et al. Stable engraftment of human microbiota into mice with a single oral gavage following antibiotic conditioning. Microbiome 5, 87 (2017).
Fiebiger, U., Bereswill, S. & Heimesaat, M. M. Dissecting the interplay between intestinal microbiota and host immunity in health and disease: lessons learned from germfree and gnotobiotic animal models. Eur. J. Microbiol. Immunol. 6, 253–271 (2016).
Bhattarai, Y. & Kashyap, P. C. Germ-free mice model for studying host-microbial interactions. Methods Mol. Biol. 1438, 123–135 (2016).
Fontaine, C. A. et al. How free of germs is germ-free? Detection of bacterial contamination in a germ free mouse unit. Gut Microbes 6, 225–233 (2015).
Yissachar, N. et al. An intestinal organ culture system uncovers a role for the nervous system in microbe-immune crosstalk. Cell 168, 1135–1148 (2017).
Mondragón-Palomino, O. et al. Three-dimensional imaging for the quantification of spatial patterns in microbiota of the intestinal mucosa. Proc. Natl Acad. Sci. USA 119, e2118483119 (2022).
Hughes, D. L., Hughes, A., Soonawalla, Z., Mukherjee, S. & O’Neill, E. Dynamic physiological culture of ex vivo human tissue: a systematic review. Cancers 13, 2870 (2021).
Zhang, J. et al. Coculture of primary human colon monolayer with human gut bacteria. Nat. Protoc. 16, 3874–3900 (2021).
Clevers, H. Modeling development and disease with organoids. Cell 165, 1586–1597 (2016).
Günther, C., Winner, B., Neurath, M. F. & Stappenbeck, T. S. Organoids in gastrointestinal diseases: from experimental models to clinical translation. Gut 71, 1892–1908 (2022).
Kim, H. J., Huh, D., Hamilton, G. & Ingber, D. E. Human gut-on-a-chip inhabited by microbial flora that experiences intestinal peristalsis-like motions and flow. Lab Chip 12, 2165–2174 (2012).
Poletti, M., Arnauts, K., Ferrante, M. & Korcsmaros, T. Organoid-based models to study the role of host-microbiota interactions in IBD. J. Crohns Colitis 15, 1222–1235 (2021).
Hill, D. R. et al. Bacterial colonization stimulates a complex physiological response in the immature human intestinal epithelium. eLife 6, e29132 (2017).
King, S. M. et al. 3D proximal tubule tissues recapitulate key aspects of renal physiology to enable nephrotoxicity testing. Front. Physiol. 8, 123 (2017).
Costello, C. M. et al. 3-D intestinal scaffolds for evaluating the therapeutic potential of probiotics. Mol. Pharm. 11, 2030–2039 (2014).
Dos Santos, J. F. et al. Mesenchymal stem cells express epidermal markers in an in vitro reconstructed human skin model. Front. Cell Dev. Biol. 10, 1012637 (2023).
Kim, R., Wang, Y., Sims, C. E. & Allbritton, N. L. A platform for co-culture of primary human colonic epithelium with anaerobic probiotic bacteria. Front. Bioeng. Biotechnol. 10, 890396 (2022).
Chen, Y. et al. Bioengineered 3D tissue model of intestine epithelium with oxygen gradients to sustain human gut microbiome. Adv. Healthc. Mater. 11, e2200447 (2022).
Kim, H. J., Li, H., Collins, J. J. & Ingber, D. E. Contributions of microbiome and mechanical deformation to intestinal bacterial overgrowth and inflammation in a human gut-on-a-chip. Proc. Natl Acad. Sci. USA 113, E7–E15 (2016).
Shin, W. & Kim, H. J. 3D in vitro morphogenesis of human intestinal epithelium in a gut-on-a-chip or a hybrid chip with a cell culture insert. Nat. Protoc. 17, 910–939 (2022).
Shah, P. et al. A microfluidics-based in vitro model of the gastrointestinal human–microbe interface. Nat. Commun. 7, 11535 (2016).
Shin, Y. C. et al. Three-dimensional regeneration of patient-derived intestinal organoid epithelium in a physiodynamic mucosal interface-on-a-chip. Micromachines 11, 663 (2020).
Waheed, S. et al. 3D printed microfluidic devices: enablers and barriers. Lab Chip 16, 1993–2013 (2016).
Walsh, D. I., Kong, D. S., Murthy, S. K. & Carr, P. A. Enabling microfluidics: from clean rooms to makerspaces. Trends Biotechnol. 35, 383–392 (2017).
Kim, H. J. & Ingber, D. E. Gut-on-a-Chip microenvironment induces human intestinal cells to undergo villus differentiation. Integr. Biol. 5, 1130–1140 (2013).
Sunuwar, L. et al. Mechanical stimuli affect Escherichia coli heat-stable enterotoxin-cyclic GMP signaling in a human enteroid intestine-chip model. Infect. Immun. 88, e00866–19 (2020).
Shin, W., Hinojosa, C. D., Ingber, D. E. & Kim, H. J. Human intestinal morphogenesis controlled by transepithelial morphogen gradient and flow-dependent physical cues in a microengineered gut-on-a-chip. iScience 15, 391–406 (2019).
Maurer, M. et al. A three-dimensional immunocompetent intestine-on-chip model as in vitro platform for functional and microbial interaction studies. Biomaterials 220, 119396 (2019).
Shin, W., Su, Z., Yi, S. S. & Kim, H. J. Single-cell transcriptomic mapping of intestinal epithelium that undergoes 3D morphogenesis and mechanodynamic stimulation in a gut-on-a-chip. iScience 25, 105521 (2022).
Min, S. et al. Live probiotic bacteria administered in a pathomimetic Leaky Gut Chip ameliorate impaired epithelial barrier and mucosal inflammation. Sci. Rep. 12, 22641 (2022).
Shin, W. et al. A robust longitudinal co-culture of obligate anaerobic gut microbiome with human intestinal epithelium in an anoxic-oxic interface-on-a-chip. Front. Bioeng. Biotechnol. 7, 13 (2019).
Shuler, M. L., Kargi, F. & DeLisa, M. Bioprocess Engineering: Basic Concepts 3rd edn (Pearson, 2017).
Lee, K. S., Boccazzi, P., Sinskey, A. J. & Ram, R. J. Microfluidic chemostat and turbidostat with flow rate, oxygen, and temperature control for dynamic continuous culture. Lab Chip 11, 1730–1739 (2011).
Singh, S., Natalini, J. G. & Segal, L. N. Lung microbial-host interface through the lens of multi-omics. Mucosal Immunol. 15, 837–845 (2022).
Henneberg, S. et al. Antibody-guided in vivo imaging of Aspergillus fumigatus lung infections during antifungal azole treatment. Nat. Commun. 12, 1707 (2021).
Houghton, L. A., Lee, A. S., Badri, H., DeVault, K. R. & Smith, J. A. Respiratory disease and the oesophagus: reflux, reflexes and microaspiration. Nat. Rev. Gastroenterol. Hepatol. 13, 445–460 (2016).
Legner, M., McMillen, D. R. & Cvitkovitch, D. G. Role of dilution rate and nutrient availability in the formation of microbial biofilms. Front. Microbiol. 10, 916 (2019).
Mahnic, A., Auchtung, J. M., Poklar Ulrih, N., Britton, R. A. & Rupnik, M. Microbiota in vitro modulated with polyphenols shows decreased colonization resistance against Clostridioides difficile but can neutralize cytotoxicity. Sci. Rep. 10, 8358 (2020).
Gayer, C. P. & Basson, M. D. The effects of mechanical forces on intestinal physiology and pathology. Cell. Signal. 21, 1237–1244 (2009).
Zheng, L., Kelly, C. J. & Colgan, S. P. Physiologic hypoxia and oxygen homeostasis in the healthy intestine. A review in the theme: cellular responses to hypoxia. Am. J. Physiol. Cell Physiol. 309, C350–C360 (2015).
Jalili-Firoozinezhad, S. et al. A complex human gut microbiome cultured in an anaerobic intestine-on-a-chip. Nat. Biomed. Eng. 3, 520–531 (2019).
O’Toole, P. W., Marchesi, J. R. & Hill, C. Next-generation probiotics: the spectrum from probiotics to live biotherapeutics. Nat. Microbiol. 2, 17057 (2017).
Charbonneau, M. R., Isabella, V. M., Li, N. & Kurtz, C. B. Developing a new class of engineered live bacterial therapeutics to treat human diseases. Nat. Commun. 11, 1738 (2020).
Zhou, W., Chow, K.-H., Fleming, E. & Oh, J. Selective colonization ability of human fecal microbes in different mouse gut environments. ISME J. 13, 805–823 (2019).
Shin, W. & Kim, H. J. Intestinal barrier dysfunction orchestrates the onset of inflammatory host-microbiome cross-talk in a human gut inflammation-on-a-chip. Proc. Natl Acad. Sci. USA 115, E10539–E10547 (2018).
Grassart, A. et al. Bioengineered human organ-on-chip reveals intestinal microenvironment and mechanical forces impacting Shigella infection. Cell Host Microbe 26, 435–444 (2019).
Tovaglieri, A. et al. Species-specific enhancement of enterohemorrhagic E. coli pathogenesis mediated by microbiome metabolites. Microbiome 7, 43 (2019).
Boquet-Pujadas, A. et al. 4D live imaging and computational modeling of a functional gut-on-a-chip evaluate how peristalsis facilitates enteric pathogen invasion. Sci. Adv. 8, eabo5767 (2022).
Bein, A. et al. Enteric coronavirus infection and treatment modeled with an immunocompetent human intestine-on-a-chip. Front. Pharmacol. 12, 718484 (2021).
Villenave, R. et al. Human gut-on-a-chip supports polarized infection of coxsackie B1 virus in vitro. PLoS ONE 12, e0169412 (2017).
Nikolaev, M. et al. Homeostatic mini-intestines through scaffold-guided organoid morphogenesis. Nature 585, 574–578 (2020).
Lanik, W. E. et al. Microfluidic device facilitates in vitro modeling of human neonatal necrotizing enterocolitis-on-a-chip. JCI Insight 8, e146496 (2023).
Thacker, V. V. et al. A lung-on-chip model of early Mycobacterium tuberculosis infection reveals an essential role for alveolar epithelial cells in controlling bacterial growth. eLife 9, e59961 (2020).
Bai, H. et al. Mechanical control of innate immune responses against viral infection revealed in a human lung alveolus chip. Nat. Commun. 13, 1928 (2022).
Deinhardt-Emmer, S. et al. Co-infection with Staphylococcus aureus after primary influenza virus infection leads to damage of the endothelium in a human alveolus-on-a-chip model. Biofabrication 12, 025012 (2020).
Plebani, R. et al. Modeling pulmonary cystic fibrosis in a human lung airway-on-a-chip. J. Cyst. Fibros. 21, 606–615 (2022).
Si, L. et al. Clinically relevant influenza virus evolution reconstituted in a human lung airway-on-a-chip. Microbiol. Spectr. 9, e0025721 (2021).
Si, L. et al. A human-airway-on-a-chip for the rapid identification of candidate antiviral therapeutics and prophylactics. Nat. Biomed. Eng. 5, 815–829 (2021).
Buzhdygan, T. P. et al. The SARS-CoV-2 spike protein alters barrier function in 2D static and 3D microfluidic in-vitro models of the human blood-brain barrier. Neurobiol. Dis. 146, 105131 (2020).
Boghdeh, N. A. et al. Application of a human blood brain barrier organ-on-a-chip model to evaluate small molecule effectiveness against Venezuelan Equine Encephalitis virus. Viruses 14, 2799 (2022).
Kim, J. et al. Fungal brain infection modelled in a human-neurovascular-unit-on-a-chip with a functional blood–brain barrier. Nat. Biomed. Eng. 5, 830–846 (2021).
Kim, J. J. et al. A microscale, full-thickness, human skin on a chip assay simulating neutrophil responses to skin infection and antibiotic treatments. Lab Chip 19, 3094–3103 (2019).
Sun, S., Jin, L., Zheng, Y. & Zhu, J. Modeling human HSV infection via a vascularized immune-competent skin-on-chip platform. Nat. Commun. 13, 5481 (2022).
Srinivasan, A., Uppuluri, P., Lopez-Ribot, J. & Ramasubramanian, A. K. Development of a high-throughput Candida albicans biofilm chip. PLoS ONE 6, e19036 (2011).
Deguchi, S. et al. Elucidation of the liver pathophysiology of COVID-19 patients using liver-on-a-chips. PNAS Nexus 2, pgad029 (2023).
Rahimi, C. et al. Oral mucosa-on-a-chip to assess layer-specific responses to bacteria and dental materials. Biomicrofluidics 12, 054106 (2018).
Sharma, K. et al. Dynamic persistence of UPEC intracellular bacterial communities in a human bladder-chip model of urinary tract infection. eLife 10, e66481 (2021).
Mahajan, G. et al. Vaginal microbiome-host interactions modeled in a human vagina-on-a-chip. Microbiome 10, 201 (2022).
Tantengco, O. A. G. et al. Modeling ascending Ureaplasma parvum infection through the female reproductive tract using vagina‐cervix‐decidua‐organ‐on‐a‐chip and feto‐maternal interface‐organ‐on‐a‐chip. FASEB J. 36, e22551 (2022).
Zhu, Y. et al. Placental barrier-on-a-chip: modeling placental inflammatory responses to bacterial infection. ACS Biomater. Sci. Eng. 4, 3356–3363 (2018).
Goralski, T. D. et al. A novel approach to interrogating the effects of chemical warfare agent exposure using organ-on-a-chip technology and multiomic analysis. PLoS ONE 18, e0280883 (2023).
Gillooly, J. F., Hein, A. & Damiani, R. Nuclear DNA content varies with cell size across human cell types. Cold Spring Harb. Perspect. Biol. 7, a019091 (2015).
Kidder, B. L., Hu, G. & Zhao, K. ChIP-Seq: technical considerations for obtaining high-quality data. Nat. Immunol. 12, 918–922 (2011).
Tang, F. et al. RNA-Seq analysis to capture the transcriptome landscape of a single cell. Nat. Protoc. 5, 516–535 (2010).
Lombardo, J. A., Aliaghaei, M., Nguyen, Q. H., Kessenbrock, K. & Haun, J. B. Microfluidic platform accelerates tissue processing into single cells for molecular analysis and primary culture models. Nat. Commun. 12, 2858 (2021).
Kassem, S. et al. Proteomics for low cell numbers: how to optimize the sample preparation workflow for mass spectrometry analysis. J. Proteome Res. 20, 4217–4230 (2021).
Schönberger, K. et al. LC-MS-based targeted metabolomics for FACS-purified rare cells. Anal. Chem. 95, 4325–4334 (2023).
Zhou, W., Dou, M., Timilsina, S. S., Xu, F. & Li, X. Recent innovations in cost-effective polymer and paper hybrid microfluidic devices. Lab Chip 21, 2658–2683 (2021).
Ferreiro, A. L. et al. Gut microbiome composition may be an indicator of preclinical Alzheimer’s disease. Sci. Transl Med. 15, eabo2984 (2023).
Kaper, J. B., Nataro, J. P. & Mobley, H. L. Pathogenic Escherichia coli. Nat. Rev. Microbiol. 2, 123–140 (2004).
Chu, H., Chan, J. F. & Yuen, K. Y. Animal models in SARS-CoV-2 research. Nat. Methods 19, 392–394 (2022).
Hatziioannou, T. & Evans, D. T. Animal models for HIV/AIDS research. Nat. Rev. Microbiol. 10, 852–867 (2012).
Villenave, R. et al. In vitro modeling of respiratory syncytial virus infection of pediatric bronchial epithelium, the primary target of infection in vivo. Proc. Natl Acad. Sci. USA 109, 5040–5045 (2012).
Li, Y. et al. Induction and suppression of antiviral RNA interference by influenza A virus in mammalian cells. Nat. Microbiol. 2, 16250 (2016).
Pires De Souza, G. A. et al. Choosing a cellular model to study SARS-CoV-2. Front. Cell. Infect. Microbiol. 12, 1003608 (2022).
Ho, B. C. et al. Inhibition of miR-146a prevents enterovirus-induced death by restoring the production of type I interferon. Nat. Commun. 5, 3344 (2014).
Riabi, S. et al. Study of Coxsackie B viruses interactions with Coxsackie Adenovirus receptor and decay-accelerating factor using human CaCo-2 cell line. J. Biomed. Sci. 21, 50 (2014).
March, S. et al. Micropatterned coculture of primary human hepatocytes and supportive cells for the study of hepatotropic pathogens. Nat. Protoc. 10, 2027–2053 (2015).
Soorneedi, A. R. & Moore, M. D. Recent developments in norovirus interactions with bacteria. Curr. Opin. Food Sci. 48, 100926 (2022).
Ettayebi, K. et al. Replication of human noroviruses in stem cell–derived human enteroids. Science 353, 1387–1393 (2016).
Saxena, K. et al. Human intestinal enteroids: a new model to study human rotavirus infection, host restriction, and pathophysiology. J. Virol. 90, 43–56 (2016).
Qian, X., Nguyen, H. N., Jacob, F., Song, H. & Ming, G. L. Using brain organoids to understand Zika virus-induced microcephaly. Development 144, 952–957 (2017).
Thacker, V. V. et al. Rapid endotheliitis and vascular damage characterize SARS-CoV-2 infection in a human lung-on-chip model. EMBO Rep. 22, e52744 (2021).
Zhang, M. et al. Biomimetic human disease model of SARS-CoV-2-induced lung injury and immune responses on organ chip system. Adv. Sci. 8, 2002928 (2021).
Holdcroft, A. M., Ireland, D. J. & Payne, M. S. The vaginal microbiome in health and disease—what role do common intimate hygiene practices play? Microorganisms 11, 298 (2023).
Fettweis, J. M. et al. The vaginal microbiome and preterm birth. Nat. Med. 25, 1012–1021 (2019).
Lewis, F. M. T., Bernstein, K. T. & Aral, S. O. Vaginal microbiome and its relationship to behavior, sexual health, and sexually transmitted diseases. Obstet. Gynecol. 129, 643–654 (2017).
Baud, A. et al. Microbial diversity in the vaginal microbiota and its link to pregnancy outcomes. Sci. Rep. 13, 9061 (2023).
Xiao, S. et al. A microfluidic culture model of the human reproductive tract and 28-day menstrual cycle. Nat. Commun. 8, 14584 (2017).
Gnecco, J. S. et al. Hemodynamic forces enhance decidualization via endothelial-derived prostaglandin E2 and prostacyclin in a microfluidic model of the human endometrium. Hum. Reprod. 34, 702–714 (2019).
Kaur, H., Merchant, M., Haque, M. M. & Mande, S. S. Crosstalk between female gonadal hormones and vaginal microbiota across various phases of women’s gynecological lifecycle. Front. Microbiol. 11, 551 (2020).
Gardella, B. et al. The complex interplay between vaginal microbiota, HPV infection, and immunological microenvironment in cervical intraepithelial neoplasia: a literature review. Int. J. Mol. Sci. 23, 7174 (2022).
Chen, R. et al. Probiotics are a good choice for the treatment of bacterial vaginosis: a meta-analysis of randomized controlled trial. Reprod. Health 19, 137 (2022).
Lev-Sagie, A. et al. Vaginal microbiome transplantation in women with intractable bacterial vaginosis. Nat. Med. 25, 1500–1504 (2019).
De Gregorio, V. et al. Immunoresponsive microbiota-gut-on-chip reproduces barrier dysfunction, stromal reshaping and probiotics translocation under inflammation. Biomaterials 286, 121573 (2022).
Jeon, M. S. et al. Contributions of the microbiome to intestinal inflammation in a gut-on-a-chip. Nano Converg. 9, 8 (2022).
Jing, B. et al. Establishment and application of peristaltic human gut-vessel microsystem for studying host-microbial interaction. Front. Bioeng. Biotechnol. 8, 272 (2020).
Nelson, M. T. et al. Characterization of an engineered live bacterial therapeutic for the treatment of phenylketonuria in a human gut-on-a-chip. Nat. Commun. 12, 2805 (2021).
Cubillos-Ruiz, A. et al. An engineered live biotherapeutic for the prevention of antibiotic-induced dysbiosis. Nat. Biomed. Eng. 6, 910–921 (2022).
Garber, K. Drugging the gut microbiome. Nat. Biotechnol. 33, 228–231 (2015).
Waller, K. M. J., Leong, R. W. & Paramsothy, S. An update on fecal microbiota transplantation for the treatment of gastrointestinal diseases. J. Gastroenterol. Hepatol. 37, 246–255 (2022).
Suk, K. T. & Koh, H. New perspective on fecal microbiota transplantation in liver diseases. J. Gastroenterol. Hepatol. 37, 24–33 (2022).
Tan, P., Li, X., Shen, J. & Feng, Q. Fecal microbiota transplantation for the treatment of inflammatory bowel disease: an update. Front. Pharmacol. 11, 574533 (2020).
Sokol, H. et al. Fecal microbiota transplantation to maintain remission in Crohn’s disease: a pilot randomized controlled study. Microbiome 8, 12 (2020).
Kang, G. U. et al. Exploration of potential gut microbiota-derived biomarkers to predict the success of fecal microbiota transplantation in ulcerative colitis: a prospective cohort in Korea. Gut Liver 16, 775–785 (2022).
Xu, D. et al. Efficacy of fecal microbiota transplantation in irritable bowel syndrome: a systematic review and meta-analysis. Am. J. Gastroenterol. 114, 1043–1050 (2019).
Ohkusa, T., Koido, S., Nishikawa, Y. & Sato, N. Gut microbiota and chronic constipation: a review and update. Front. Med. 6, 19 (2019).
Morais, L. H., Schreiber, H. L. IV & Mazmanian, S. K. The gut microbiota–brain axis in behaviour and brain disorders. Nat. Rev. Microbiol. 19, 241–255 (2021).
Weersma, R. K., Zhernakova, A. & Fu, J. Interaction between drugs and the gut microbiome. Gut 69, 1510–1519 (2020).
Wallace, B. D. et al. Alleviating cancer drug toxicity by inhibiting a bacterial enzyme. Science 330, 831–835 (2010).
Zhang, J. et al. Microbial enzymes induce colitis by reactivating triclosan in the mouse gastrointestinal tract. Nat. Commun. 13, 136 (2022).
Zimmermann, M., Zimmermann-Kogadeeva, M., Wegmann, R. & Goodman, A. L. Mapping human microbiome drug metabolism by gut bacteria and their genes. Nature 570, 462–467 (2019).
Kieser, S., Zdobnov, E. M. & Trajkovski, M. Comprehensive mouse microbiota genome catalog reveals major difference to its human counterpart. PLoS Comput. Biol. 18, e1009947 (2022).
Sontheimer-Phelps, A. et al. Human colon-on-a-chip enables continuous in vitro analysis of colon mucus layer accumulation and physiology. Cell. Mol. Gastroenterol. Hepatol. 9, 507–526 (2020).
Lauschke, V. M. & Ingelman-Sundberg, M. The importance of patient-specific factors for hepatic drug response and toxicity. Int. J. Mol. Sci. 17, 1714 (2016).
Swanson, H. I. Drug metabolism by the host and gut microbiota: a partnership or rivalry? Drug Metab. Dispos. 43, 1499–1504 (2015).
Vázquez-Baeza, Y. et al. Impacts of the human gut microbiome on therapeutics. Annu. Rev. Pharmacol. Toxicol. 58, 253–270 (2018).
Davies, J. C. et al. Safety, pharmacokinetics, and pharmacodynamics of ivacaftor in patients aged 2–5 years with cystic fibrosis and a CFTR gating mutation (KIWI): an open-label, single-arm study. Lancet Respir. Med. 4, 107–115 (2016).
Chakrabarti, R. S. et al. Variability of cholesterol accessibility in human red blood cells measured using a bacterial cholesterol-binding toxin. eLife 6, e23355 (2017).
Forslund, S. K. et al. Combinatorial, additive and dose-dependent drug–microbiome associations. Nature 600, 500–505 (2021).
Cryan, J. F. et al. The microbiota-gut-brain axis. Physiol. Rev. 99, 1877–2013 (2019).
Awoniyi, M. et al. Protective and aggressive bacterial subsets and metabolites modify hepatobiliary inflammation and fibrosis in a murine model of PSC. Gut 72, 671–685 (2023).
Enaud, R. et al. The gut-lung axis in health and respiratory diseases: a place for inter-organ and inter-kingdom crosstalks. Front. Cell. Infect. Microbiol. 10, 9 (2020).
Ray, K. The oral–gut axis in IBD. Nat. Rev. Gastroenterol. Hepatol. 17, 532 (2020).
De Pessemier, B. et al. Gut–skin axis: current knowledge of the interrelationship between microbial dysbiosis and skin conditions. Microorganisms 9, 353 (2021).
Svegliati-Baroni, G., Patricio, B., Lioci, G., Macedo, M. P. & Gastaldelli, A. Gut-pancreas-liver axis as a target for treatment of NAFLD/NASH. Int. J. Mol. Sci. 21, 5820 (2020).
Taghinezhad-S, S. et al. Twenty years of research on HPV vaccines based on genetically modified lactic acid bacteria: an overview on the gut-vagina axis. Cell. Mol. Life Sci. 78, 1191–1206 (2021).
Schembri, M. A., Nhu, N. T. K. & Phan, M. D. Gut–bladder axis in recurrent UTI. Nat. Microbiol. 7, 601–602 (2022).
Edington, C. D. et al. Interconnected microphysiological systems for quantitative biology and pharmacology studies. Sci. Rep. 8, 4530 (2018).
Ronaldson-Bouchard, K. et al. A multi-organ chip with matured tissue niches linked by vascular flow. Nat. Biomed. Eng. 6, 351–371 (2022).
Novak, R. et al. Robotic fluidic coupling and interrogation of multiple vascularized organ chips. Nat. Biomed. Eng. 4, 407–420 (2020).
Wikswo, J. P. The relevance and potential roles of microphysiological systems in biology and medicine. Exp. Biol. Med. 239, 1061–1072 (2014).
Lagier, J. C. et al. Culture of previously uncultured members of the human gut microbiota by culturomics. Nat. Microbiol. 1, 16203 (2016).
Almeida, A. et al. A unified catalog of 204,938 reference genomes from the human gut microbiome. Nat. Biotechnol. 39, 105–114 (2021).
Morris, B. E., Henneberger, R., Huber, H. & Moissl-Eichinger, C. Microbial syntrophy: interaction for the common good. FEMS Microbiol. Rev. 37, 384–406 (2013).
Henke, M. T. et al. Ruminococcus gnavus, a member of the human gut microbiome associated with Crohn’s disease, produces an inflammatory polysaccharide. Proc. Natl Acad. Sci. USA 116, 12672–12677 (2019).
Natividad, J. M. et al. Bilophila wadsworthia aggravates high fat diet induced metabolic dysfunctions in mice. Nat. Commun. 9, 2802 (2018).
Kim, H. J., Boedicker, J. Q., Choi, J. W. & Ismagilov, R. F. Defined spatial structure stabilizes a synthetic multispecies bacterial community. Proc. Natl Acad. Sci. USA 105, 18188–18193 (2008).
Simon, J. C., Marchesi, J. R., Mougel, C. & Selosse, M. A. Host-microbiota interactions: from holobiont theory to analysis. Microbiome 7, 5 (2019).
Gutierrez Lopez, D. E., Lashinger, L. M., Weinstock, G. M. & Bray, M. S. Circadian rhythms and the gut microbiome synchronize the host’s metabolic response to diet. Cell Metab. 33, 873–887 (2021).
Sarkar, A. et al. The microbiome in psychology and cognitive neuroscience. Trends Cogn. Sci. 22, 611–636 (2018).
Tomofuji, Y. et al. Reconstruction of the personal information from human genome reads in gut metagenome sequencing data. Nat. Microbiol. 8, 1079–1094 (2023).
Woo, V. & Alenghat, T. Epigenetic regulation by gut microbiota. Gut Microbes 14, 2022407 (2022).
Stacy, A. et al. Infection trains the host for microbiota-enhanced resistance to pathogens. Cell 184, 615–627 (2021).
Davenport, E. R. et al. The human microbiome in evolution. BMC Biol. 15, 127 (2017).
Klingelhoefer, L. & Reichmann, H. Pathogenesis of Parkinson disease—the gut–brain axis and environmental factors. Nat. Rev. Neurol. 11, 625–636 (2015).
D’Alessio, S. et al. Revisiting fibrosis in inflammatory bowel disease: the gut thickens. Nat. Rev. Gastroenterol. Hepatol. 19, 169–184 (2022).
Reshetnyak, V. I., Burmistrov, A. I. & Maev, I. V. Helicobacter pylori: commensal, symbiont or pathogen? World J. Gastroenterol. 27, 545–560 (2021).
Duboux, S., Van Wijchen, M. & Kleerebezem, M. The possible link between manufacturing and probiotic efficacy; a molecular point of view on Bifidobacterium. Front. Microbiol. 12, 812536 (2021).
Johnston, C. D. & Bullman, S. The tumour-associated microbiome. Nat. Rev. Gastroenterol. Hepatol. 19, 347–348 (2022).
Neurath, M. F. Host–microbiota interactions in inflammatory bowel disease. Nat. Rev. Gastroenterol. Hepatol. 17, 76–77 (2020).
Spellberg, B. et al. The epidemic of antibiotic-resistant infections: a call to action for the medical community from the Infectious Diseases Society of America. Clin. Infect. Dis. 46, 155–164 (2008).
Grimaldi, A. et al. Improved SARS-CoV-2 sequencing surveillance allows the identification of new variants and signatures in infected patients. Genome Med. 14, 90 (2022).
Shoji, J. Y., Davis, R. P., Mummery, C. L. & Krauss, S. Global meta‐analysis of organoid and organ‐on‐chip research. Adv. Healthc. Mater. https://doi.org/10.1002/adhm.202301067 (2023).
Kawasaki, M., Goyama, T., Tachibana, Y., Nagao, I. & Ambrosini, Y. M. Farm and companion animal organoid models in translational research: a powerful tool to bridge the gap between mice and humans. Front. Med. Technol. 4, 895379 (2022).
Rodrigues, N. S. et al. Biomaterial and biofilm interactions with the pulp-dentin complex-on-a-chip. J. Dent. Res. 100, 1136–1143 (2021).
Wright, E., Neethirajan, S. & Weng, X. Microfluidic wound model for studying the behaviors of Pseudomonas aeruginosa in polymicrobial biofilms. Biotechnol. Bioeng. 112, 2351–2359 (2015).
Greenhalgh, K. et al. Integrated in vitro and in silico modeling delineates the molecular effects of a synbiotic regimen on colorectal-cancer-derived cells. Cell Rep. 27, 1621–1632 (2019).
Hu, W. et al. A cellular chip-MS system for investigation of Lactobacillus rhamnosus GG and irinotecan synergistic effects on colorectal cancer. Chin. Chem. Lett. 33, 2096–2100 (2022).
van Rijn, J. M. et al. High-definition DIC imaging uncovers transient stages of pathogen infection cycles on the surface of human adult stem cell-derived intestinal epithelium. mBio 13, e00022 (2022).
Muscogiuri, G. et al. Gut microbiota: a new path to treat obesity. Int. J. Obes. Suppl. 9, 10–19 (2019).
Lavelle, A. & Sokol, H. Gut microbiota-derived metabolites as key actors in inflammatory bowel disease. Nat. Rev. Gastroenterol. Hepatol. 17, 223–237 (2020).
Cao, Y. et al. Commensal microbiota from patients with inflammatory bowel disease produce genotoxic metabolites. Science 378, eabm3233 (2022).
Routy, B. et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science 359, 91–97 (2018).
Canyelles, M. et al. Trimethylamine N-oxide: a link among diet, gut microbiota, gene regulation of liver and intestine cholesterol homeostasis and HDL function. Int. J. Mol. Sci. 19, 3228 (2018).
Jiang, C., Li, G., Huang, P., Liu, Z. & Zhao, B. The gut microbiota and Alzheimer’s disease. J. Alzheimers Dis. 58, 1–15 (2017).
Correale, J., Hohlfeld, R. & Baranzini, S. E. The role of the gut microbiota in multiple sclerosis. Nat. Rev. Neurol. 18, 544–558 (2022).
Garcia-Gutierrez, E., Narbad, A. & Rodríguez, J. M. Autism spectrum disorder associated with gut microbiota at immune, metabolomic, and neuroactive level. Front. Neurosci. 14, 578666 (2020).
Marx, U. et al. Biology-inspired microphysiological systems to advance patient benefit and animal welfare in drug development. ALTEX 37, 365–394 (2020).
Nieskens, T. T. G. et al. Nephrotoxic antisense oligonucleotide SPC5001 induces kidney injury biomarkers in a proximal tubule-on-a-chip. Arch. Toxicol. 95, 2123–2136 (2021).
Jang, K.-J. et al. Reproducing human and cross-species drug toxicities using a Liver-Chip. Sci. Transl. Med. 11, eaax5516 (2019).
Hübner, J. et al. Simultaneous evaluation of anti-EGFR-induced tumour and adverse skin effects in a microfluidic human 3D co-culture model. Sci. Rep. 8, 15010 (2018).
Foster, A. J. et al. Integrated in vitro models for hepatic safety and metabolism: evaluation of a human Liver-Chip and liver spheroid. Arch. Toxicol. 93, 1021–1037 (2019).
McAleer, C. W. et al. On the potential of in vitro organ-chip models to define temporal pharmacokinetic-pharmacodynamic relationships. Sci. Rep. 9, 9619 (2019).
Acknowledgements
We thank H. Koh (Yonsei University Severance Hospital), D.-W. Lee (Yonsei University), H. Y. Cho (Seoul National University Hospital) and J.-M. Song (Sungshin Women’s University) for their valuable comments. This work was supported by the Bio-industrial Technology Development Program (20018770) from the Ministry of Trade, Industry & Energy (MOTIE, Korea) and the Senior Research Award from Crohn’s and Colitis Foundation of America (CCFA).
Author information
Authors and Affiliations
Contributions
All authors contributed to conceiving the idea, developing the outline, conceptualizing the figures, and writing and revising the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Bioengineering thanks Leopoldo Segal, Sabeth Verpoorte, Shaun Lee and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Emulate: https://emulatebio.com/
EU Reference Laboratory for alternatives to animal testing (EURL ECVAM): https://joint-research-centre.ec.europa.eu/eu-reference-laboratory-alternatives-animal-testing-eurl-ecvam_en
European Organ-on-chip Society (EUROoCS): https://euroocs.eu/
Human Microbiome Project Initiative (HMP): https://commonfund.nih.gov/hmp/initiatives
Human Organ and Disease Models Technologies Consortium (hDMT): https://www.hdmt.technology/
Innovative Science and Technology Approaches for New Drugs (ISTAND) Pilot Program: https://www.fda.gov/drugs/drug-development-tool-ddt-qualification-programs/innovative-science-and-technology-approaches-new-drugs-istand-pilot-program
Mimetas: https://www.mimetas.com/en/home/
National Center for Advancing Translational Sciences (NCATS) Tissue Chip Initiatives: https://ncats.nih.gov/tissuechip/projects
Organ-on-a-Chip/Tissue-on-a-Chip Engineering and Efficacy Standardization Working Group: https://www.nist.gov/pml/microsystems-and-nanotechnology-division/biophysical-and-biomedical-measurement-group/organ
The NTP Interagency Center for the Evaluation of Alternative Toxicological Methods (NICEATM): https://ntp.niehs.nih.gov/whatwestudy/niceatm
TissUse: https://www.tissuse.com/en/
US FDA Modernization Act 2.0: https://www.congress.gov/bill/117th-congress/senate-bill/5002/text
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shin, Y.C., Than, N., Min, S. et al. Modelling host–microbiome interactions in organ-on-a-chip platforms. Nat Rev Bioeng 2, 175–191 (2024). https://doi.org/10.1038/s44222-023-00130-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s44222-023-00130-9