Abstract
Schizophrenia (SCZ) is a highly heterogeneous disorder with diverse clinical manifestations and macro- and microscale biological variations, usually observed at dissociable levels. Here we propose a cross-scale, circuit-based framework to connect heterogeneous clinical symptoms, large-scale brain circuit dysfunctions, and genetic, molecular and cellular abnormalities in SCZ. Using connectomic and predictive models on three independent neuroimaging datasets (n = 1,199, including patients with SCZ and healthy controls), we first identified two macroscale dysconnectivity dimensions for corticocortical and corticostriatal circuits, each associated with specific clinical symptoms. We then associated macroscale dysconnectivity with disrupted cellular circuits using extended imaging transcriptomic and genetic analyses on multiomics data. Our findings suggest a two-dimensional cross-scale heterogeneity model of SCZ, which reveals how distinct genetic disruptions affect specific cellular-level deficits, resulting in system-level brain circuit dysconnectivity responsible for the heterogeneous symptoms in SCZ. These findings significantly improve our understanding of cross-scale heterogeneity in SCZ, advancing its pathophysiology and treatment development.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$59.00 per year
only $4.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The COBRE dataset can be accessed through the COINS data exchange portal112 (https://coins.trendscenter.org/). The UKB data can be requested through a standard protocol (https://www.ukbiobank.ac.uk/register-apply/). The Brainnetome atlas can be downloaded at https://atlas.brainnetome.org/download.html. The Neurosynth database can be downloaded at https://github.com/neurosynth/neurosynth-data. The AHBA is freely available at https://human.brain-map.org/. The Lake68 single-cell data are publicly available at the National Center for Biotechnology Information under the SuperSeries accession code GSE97942. The ABA69 single-cell data are publicly available at https://portal.brain-map.org/atlases-and-data/rnaseq. The SCZ GWAS78 data are publicly available at https://pgc.unc.edu/for-researchers/download-results/. All study data supporting the findings are provided within the paper and in its Supplementary Information. All raw data from the SCZ-I and SCZ-II datasets will be made available upon reasonable request to the corresponding authors.
Code availability
The preprocessing software for resting-state fMRI data is freely available (BRANT114 v3.35, http://brant.brainnetome.org/en/latest/). The SurfStat toolbox for surface-wide statistical comparisons is freely available at https://www.math.mcgill.ca/keith/surfstat/. Corticocortical connectivity (first functional gradient) was calculated based on open-source codes at https://github.com/NeuroanatomyAndConnectivity/gradient_analysis. The toolbox for performing spatial permutation test (spin test) is freely available at https://github.com/spin-test/spin-test. Functional decodings were performed based on the Neurosynth package openly available at https://github.com/neurosynth/neurosynth. The pyGAM package for performing symptom prediction analysis is openly available at https://github.com/dswah/pyGAM. The abagen toolbox for AHBA data processing is freely available at https://github.com/rmarkello/abagen. The pipeline for single-cell data processing is consistent with that performed by ref. 71, and the codes are openly available at https://github.com/kevmanderson/2020_PNAS_Depression. FGSEA was performed based on the fgsea package, which is openly available at https://github.com/ctlab/fgsea. The MAGMA (v 1.08)76 software and reference data are publicly available at https://ctg.cncr.nl/software/magma. The PLINK (v1.07)117 software is freely available at https://www.cog-genomics.org/plink/. GO enrichment analysis was performed using the Metascape137 platform (https://metascape.org/gp/index.html#/main/step1). The LDSC package is available at https://github.com/bulik/ldsc. All custom codes used in the analysis are publicly available at https://github.com/BingLiu-Lab/scz_cross-scale_abnormalities.
References
Owen, M. J., Sawa, A. & Mortensen, P. B. Schizophrenia. Lancet 388, 86–97 (2016).
Voineskos, A. N., Jacobs, G. R. & Ameis, S. H. Neuroimaging heterogeneity in psychosis: neurobiological underpinnings and opportunities for prognostic and therapeutic innovation. Biol. Psychiatry 88, 95–102 (2020).
Price, A. J., Jaffe, A. E. & Weinberger, D. R. Cortical cellular diversity and development in schizophrenia. Mol. Psychiatry 26, 203–217 (2021).
van den Heuvel, M. P., Scholtens, L. H., de Reus, M. A. & Kahn, R. S. Associated microscale spine density and macroscale connectivity disruptions in schizophrenia. Biol. Psychiatry 80, 293–301 (2016).
Scholtens, L. H. & van den Heuvel, M. P. Multimodal connectomics in psychiatry: bridging scales from micro to macro. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 3, 767–776 (2018).
Keshavan, M. S. et al. Neuroimaging in schizophrenia. Neuroimaging Clin. N. Am. 30, 73–83 (2020).
Gur, R. E. & Gur, R. C. Functional magnetic resonance imaging in schizophrenia. Dialogues Clin. Neurosci. 12, 333–343 (2010).
Smith, S. M. et al. Functional connectomics from resting-state fMRI. Trends Cogn. Sci. 17, 666–682 (2013).
Fornito, A., Zalesky, A., Pantelis, C. & Bullmore, E. T. Schizophrenia, neuroimaging and connectomics. Neuroimage 62, 2296–2314 (2012).
van den Heuvel, M. P. & Fornito, A. Brain networks in schizophrenia. Neuropsychol. Rev. 24, 32–48 (2014).
Li, T. et al. Brain-wide analysis of functional connectivity in first-episode and chronic stages of schizophrenia. Schizophr. Bull. 43, 436–448 (2017).
Pettersson-Yeo, W., Allen, P., Benetti, S., McGuire, P. & Mechelli, A. Dysconnectivity in schizophrenia: where are we now? Neurosci. Biobehav. Rev. 35, 1110–1124 (2011).
Lynall, M.-E. et al. Functional connectivity and brain networks in schizophrenia. J. Neurosci. 30, 9477–9487 (2010).
Woodward, N. D., Karbasforoushan, H. & Heckers, S. Thalamocortical dysconnectivity in schizophrenia. Am. J. Psychiatry 169, 1092–1099 (2012).
Fornito, A. et al. Functional dysconnectivity of corticostriatal circuitry as a risk phenotype for psychosis. JAMA Psychiatry 70, 1143–1151 (2013).
Li, A. et al. A neuroimaging biomarker for striatal dysfunction in schizophrenia. Nat. Med. 26, 558–565 (2020).
Sheffield, J. M. & Barch, D. M. Cognition and resting-state functional connectivity in schizophrenia. Neurosci. Biobehav. Rev. 61, 108–120 (2016).
Rotarska-Jagiela, A. et al. Resting-state functional network correlates of psychotic symptoms in schizophrenia. Schizophr. Res. 117, 21–30 (2010).
Shukla, D. K. et al. Aberrant frontostriatal connectivity in negative symptoms of schizophrenia. Schizophr. Bull. 45, 1051–1059 (2019).
Brady, R. O. et al. Cerebellar-prefrontal network connectivity and negative symptoms in schizophrenia. Am. J. Psychiatry 176, 512–520 (2019).
Chen, J. et al. Intrinsic connectivity patterns of task-defined brain networks allow individual prediction of cognitive symptom dimension of schizophrenia and are linked to molecular architecture. Biol. Psychiatry 89, 308–319 (2021).
Adhikari, B. M. et al. Functional network connectivity impairments and core cognitive deficits in schizophrenia. Hum. Brain Mapp. 40, 4593–4605 (2019).
Adhikari, B. M. et al. Effects of ketamine and midazolam on resting state connectivity and comparison with ENIGMA connectivity deficit patterns in schizophrenia. Hum. Brain Mapp. 41, 767–778 (2019).
Singh, T. et al. Rare coding variants in ten genes confer substantial risk for schizophrenia. Nature 604, 509–516 (2022).
Trubetskoy, V. et al. Mapping genomic loci implicates genes and synaptic biology in schizophrenia. Nature 604, 502–508 (2022).
Harrison, P. J. Postmortem studies in schizophrenia. Dialogues Clin. Neurosci. 2, 349–357 (2000).
Roeske, M. J., Konradi, C., Heckers, S. & Lewis, A. S. Hippocampal volume and hippocampal neuron density, number and size in schizophrenia: a systematic review and meta-analysis of postmortem studies. Mol. Psychiatry 26, 3524–3535 (2021).
Skene, N. G. et al. Genetic identification of brain cell types underlying schizophrenia. Nat. Genet. 50, 825–833 (2018).
Watanabe, K., Umićević Mirkov, M., de Leeuw, C. A., van den Heuvel, M. P. & Posthuma, D. Genetic mapping of cell type specificity for complex traits. Nat. Commun. 10, 3222 (2019).
Finucane, H. K. et al. Heritability enrichment of specifically expressed genes identifies disease-relevant tissues and cell types. Nat. Genet. 50, 621–629 (2018).
Calderon, D. et al. Inferring relevant cell types for complex traits by using single-cell gene expression. Am. J. Hum. Genet. 101, 686–699 (2017).
Ruzicka, W. B. et al. Single-cell dissection of schizophrenia reveals neurodevelopmental-synaptic axis and transcriptional resilience. Preprint at medRxiv https://doi.org/10.1101/2020.11.06.20225342 (2020).
Räsänen, N., Tiihonen, J., Koskuvi, M., Lehtonen, Š. & Koistinaho, J. The iPSC perspective on schizophrenia. Trends Neurosci. 45, 8–26 (2022).
Sebastian, R., Song, Y. & Pak, C. Probing the molecular and cellular pathological mechanisms of schizophrenia using human induced pluripotent stem cell models. Schizophr. Res. https://doi.org/10.1016/j.schres.2022.06.028 (2022).
Notaras, M. et al. Schizophrenia is defined by cell-specific neuropathology and multiple neurodevelopmental mechanisms in patient-derived cerebral organoids. Mol. Psychiatry 27, 1416–1434 (2022).
Margulies, D. S. et al. Situating the default-mode network along a principal gradient of macroscale cortical organization. Proc. Natl Acad. Sci. USA 113, 12574–12579 (2016).
Aine, C. J. et al. Multimodal neuroimaging in schizophrenia: description and dissemination. Neuroinformatics 15, 343–364 (2017).
Coifman, R. R. & Lafon, S. Diffusion maps. Appl. Comput. Harmon. Anal. 21, 5–30 (2006).
McCutcheon, R. A., Abi-Dargham, A. & Howes, O. D. Schizophrenia, dopamine and the striatum: from biology to symptoms. Trends Neurosci. 42, 205–220 (2019).
Shepherd, G. M. G. Corticostriatal connectivity and its role in disease. Nat. Rev. Neurosci. 14, 278–291 (2013).
Alexander-Bloch, A. F. et al. On testing for spatial correspondence between maps of human brain structure and function. Neuroimage 178, 540–551 (2018).
Power, J. D., Barnes, K. A., Snyder, A. Z., Schlaggar, B. L. & Petersen, S. E. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage 59, 2142–2154 (2012).
Murphy, K. & Fox, M. D. Towards a consensus regarding global signal regression for resting state functional connectivity MRI. Neuroimage 154, 169–173 (2017).
Hong, S.-J. et al. Atypical functional connectome hierarchy in autism. Nat. Commun. 10, 1022 (2019).
Johnson, W. E., Li, C. & Rabinovic, A. Adjusting batch effects in microarray expression data using empirical Bayes methods. Biostatistics 8, 118–127 (2007).
Friston, K. J. & Frith, C. D. Schizophrenia: a disconnection syndrome? Clin. Neurosci. 3, 89–97 (1995).
Stephan, K. E., Baldeweg, T. & Friston, K. J. Synaptic plasticity and dysconnection in schizophrenia. Biol. Psychiatry 59, 929–939 (2006).
Friston, K. J. The disconnection hypothesis. Schizophr. Res. 30, 115–125 (1998).
Friston, K., Brown, H. R., Siemerkus, J. & Stephan, K. E. The dysconnection hypothesis (2016). Schizophr. Res. 176, 83–94 (2016).
Stephan, K. E., Friston, K. J. & Frith, C. D. Dysconnection in schizophrenia: from abnormal synaptic plasticity to failures of self-monitoring. Schizophr. Bull. 35, 509–527 (2009).
Finn, E. S. et al. Functional connectome fingerprinting: identifying individuals using patterns of brain connectivity. Nat. Neurosci. 18, 1664–1671 (2015).
Worsley, K. J., Andermann, M., Koulis, T., MacDonald, D. & Evans, A. C. Detecting changes in nonisotropic images. Hum. Brain Mapp. 8, 98–101 (1999).
Eklund, A., Nichols, T. E. & Knutsson, H. Cluster failure: why fMRI inferences for spatial extent have inflated false-positive rates. Proc. Natl Acad. Sci. USA 113, 7900–7905 (2016).
Nichols, T. E. & Holmes, A. P. Nonparametric permutation tests for functional neuroimaging: a primer with examples. Hum. Brain Mapp. 15, 1–25 (2002).
Kay, S. R., Fiszbein, A. & Opler, L. A. The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophr. Bull. 13, 261–276 (1987).
Hastie, T. & Tibshirani, R. Generalized additive models. Stat. Sci. 1, 297–310 (1986).
Hastie, T. & Tibshirani, R. Generalized additive models for medical research. Stat. Methods Med. Res. 4, 187–196 (1995).
Hastie, T. & Tibshirani, R. Generalized additive models: some applications. J. Am. Stat. Assoc. 82, 371–386 (1987).
Varoquaux, G. et al. Assessing and tuning brain decoders: cross-validation, caveats, and guidelines. Neuroimage 145, 166–179 (2017).
Lindenmayer, J.-P., Bernstein-Hyman, R. & Grochowski, S. A new five factor model of schizophrenia. Psychiatr. Q. 65, 299–322 (1994).
Yarkoni, T., Poldrack, R. A., Nichols, T. E., Van Essen, D. C. & Wager, T. D. Large-scale automated synthesis of human functional neuroimaging data. Nat. Methods 8, 665–670 (2011).
Hooker, C. & Park, S. Emotion processing and its relationship to social functioning in schizophrenia patients. Psychiatry Res. 112, 41–50 (2002).
Lincoln, T. M., Mehl, S., Kesting, M.-L. & Rief, W. Negative symptoms and social cognition: identifying targets for psychological interventions. Schizophr. Bull. 37, S23–S32 (2011).
Moura, B. M. et al. A network of psychopathological, cognitive, and motor symptoms in schizophrenia spectrum disorders. Schizophr. Bull. 47, 915–926 (2021).
Guillem, F., Rinaldi, M., Pampoulova, T. & Stip, E. The complex relationships between executive functions and positive symptoms in schizophrenia. Psychol. Med. 38, 853–860 (2008).
Koshiyama, D. et al. Hierarchical pathways from sensory processing to cognitive, clinical, and functional impairments in schizophrenia. Schizophr. Bull. 47, 373–385 (2021).
Hawrylycz, M. J. et al. An anatomically comprehensive atlas of the adult human brain transcriptome. Nature 489, 391–399 (2012).
Lake, B. B. et al. Integrative single-cell analysis of transcriptional and epigenetic states in the human adult brain. Nat. Biotechnol. 36, 70–80 (2018).
Hodge, R. D. et al. Conserved cell types with divergent features in human versus mouse cortex. Nature 573, 61–68 (2019).
Korotkevich, G. et al. Fast gene set enrichment analysis. Preprint at bioRxiv https://doi.org/10.1101/060012 (2021).
Anderson, K. M. et al. Convergent molecular, cellular, and cortical neuroimaging signatures of major depressive disorder. Proc. Natl Acad. Sci. USA 117, 25138–25149 (2020).
Lake, B. B. et al. Neuronal subtypes and diversity revealed by single-nucleus RNA sequencing of the human brain. Science 352, 1586–1590 (2016).
Trifu, S. C., Kohn, B., Vlasie, A. & Patrichi, B.-E. Genetics of schizophrenia (Review). Exp. Ther. Med. 20, 3462–3468 (2020).
Gejman, P. V., Sanders, A. R. & Duan, J. The role of genetics in the etiology of schizophrenia. Psychiatr. Clin. North Am. 33, 35–66 (2010).
Krabbendam, L. & van Os, J. Schizophrenia and urbanicity: a major environmental influence—conditional on genetic risk. Schizophr. Bull. 31, 795–799 (2005).
Leeuw, C. A., de, Mooij, J. M., Heskes, T. & Posthuma, D. MAGMA: generalized gene-set analysis of GWAS data. PLoS Comput. Biol. 11, e1004219 (2015).
de Leeuw, C. A., Neale, B. M., Heskes, T. & Posthuma, D. The statistical properties of gene-set analysis. Nat. Rev. Genet. 17, 353–364 (2016).
Lam, M. et al. Comparative genetic architectures of schizophrenia in East Asian and European populations. Nat. Genet. 51, 1670–1678 (2019).
Finucane, H. K. et al. Partitioning heritability by functional annotation using genome-wide association summary statistics. Nat. Genet. 47, 1228–1235 (2015).
Kriegeskorte, N., Mur, M. & Bandettini, P. Representational similarity analysis—connecting the branches of systems neuroscience. Front. Syst. Neurosci. 2, 4 (2008).
Nath, M., Wong, T. P. & Srivastava, L. K. Neurodevelopmental insights into circuit dysconnectivity in schizophrenia. Prog. Neuropsychopharmacol. Biol. Psychiatry 104, 110047 (2021).
Heeger, D. J. & Ress, D. What does fMRI tell us about neuronal activity? Nat. Rev. Neurosci. 3, 142–151 (2002).
Dienel, S. J., Schoonover, K. E. & Lewis, D. A. Cognitive dysfunction and prefrontal cortical circuit alterations in schizophrenia: developmental trajectories. Biol. Psychiatry 92, 450–459 (2022).
Lewis, D. A. & Sweet, R. A. Schizophrenia from a neural circuitry perspective: advancing toward rational pharmacological therapies. J. Clin. Invest. 119, 706–716 (2009).
Howes, O. D. et al. Molecular imaging studies of the striatal dopaminergic system in psychosis and predictions for the prodromal phase of psychosis. Br. J. Psychiatry 191, s13–s18 (2007).
Romme, I. A. C., de Reus, M. A., Ophoff, R. A., Kahn, R. S. & van den Heuvel, M. P. Connectome disconnectivity and cortical gene expression in patients with schizophrenia. Biol. Psychiatry 81, 495–502 (2017).
Lewis, D. A. Neuroplasticity of excitatory and inhibitory cortical circuits in schizophrenia. Dialogues Clin. Neurosci. 11, 269–280 (2009).
Tkachev, D. et al. Oligodendrocyte dysfunction in schizophrenia and bipolar disorder. Lancet 362, 798–805 (2003).
Raabe, F. J. et al. Studying and modulating schizophrenia-associated dysfunctions of oligodendrocytes with patient-specific cell systems. NPJ Schizophr. 4, 23 (2018).
Spruston, N. Pyramidal neurons: dendritic structure and synaptic integration. Nat. Rev. Neurosci. 9, 206–221 (2008).
Han, W. & Šestan, N. Cortical projection neurons: sprung from the same root. Neuron 80, 1103–1105 (2013).
Michalski, J.-P. & Kothary, R. Oligodendrocytes in a nutshell. Front. Cell. Neurosci. 9, 340 (2015).
Tomassy, G. S. et al. Distinct profiles of myelin distribution along single axons of pyramidal neurons in the neocortex. Science 344, 319–324 (2014).
de Hoz, L. & Simons, M. The emerging functions of oligodendrocytes in regulating neuronal network behaviour. Bioessays 37, 60–69 (2015).
Gibson, E. M. et al. Neuronal activity promotes oligodendrogenesis and adaptive myelination in the mammalian brain. Science 344, 1252304 (2014).
Hof, P. R. et al. Loss and altered spatial distribution of oligodendrocytes in the superior frontal gyrus in schizophrenia. Biol. Psychiatry 53, 1075–1085 (2003).
Windrem, M. S. et al. Human iPSC glial mouse chimeras reveal glial contributions to schizophrenia. Cell Stem Cell 21, 195–208.e6 (2017).
Aberg, K., Saetre, P., Jareborg, N. & Jazin, E. Human QKI, a potential regulator of mRNA expression of human oligodendrocyte-related genes involved in schizophrenia. Proc. Natl Acad. Sci. USA 103, 7482–7487 (2006).
Harris, K. D. & Shepherd, G. M. G. The neocortical circuit: themes and variations. Nat. Neurosci. 18, 170–181 (2015).
Lee, J. H., Liu, Q. & Dadgar-Kiani, E. Solving brain circuit function and dysfunction with computational modeling and optogenetic fMRI. Science 378, 493–499 (2022).
Grimm, C. et al. Optogenetic activation of striatal D1R and D2R cells differentially engages downstream connected areas beyond the basal ganglia. Cell Rep. 37, 110161 (2021).
Zhang, D. et al. Spatial epigenome-transcriptome co-profiling of mammalian tissues. Nature 616, 113–122 (2023).
Huber, L. et al. Layer-dependent functional connectivity methods. Prog. Neurobiol. 207, 101835 (2021).
Carbon, M. & Correll, C. U. Thinking and acting beyond the positive: the role of the cognitive and negative symptoms in schizophrenia. CNS Spectr. 19, 35–53 (2014).
Kaar, S. J., Natesan, S., McCutcheon, R. & Howes, O. D. Antipsychotics: mechanisms underlying clinical response and side-effects and novel treatment approaches based on pathophysiology. Neuropharmacology 172, 107704 (2020).
Valiengo, L. et al. Efficacy and safety of transcranial direct current stimulation for treating negative symptoms in schizophrenia. JAMA Psychiatry 77, 121–129 (2020).
Kostova, R., Cecere, R., Thut, G. & Uhlhaas, P. J. Targeting cognition in schizophrenia through transcranial direct current stimulation: a systematic review and perspective. Schizophr. Res. 220, 300–310 (2020).
Koponen, L. M., Nieminen, J. O. & Ilmoniemi, R. J. Multi-locus transcranial magnetic stimulation-theory and implementation. Brain Stimul. 11, 849–855 (2018).
Aberra, A. S., Wang, B., Grill, W. M. & Peterchev, A. V. Simulation of transcranial magnetic stimulation in head model with morphologically-realistic cortical neurons. Brain Stimul. 13, 175–189 (2020).
Qiu, Y. et al. On-demand cell-autonomous gene therapy for brain circuit disorders. Science 378, 523–532 (2022).
Martins, D. et al. Imaging transcriptomics: convergent cellular, transcriptomic, and molecular neuroimaging signatures in the healthy adult human brain. Cell Rep. 37, 110173 (2021).
Wood, D. et al. Harnessing modern web application technology to create intuitive and efficient data visualization and sharing tools. Front. Neuroinformatics 8, 71 (2014).
Sudlow, C. et al. UK Biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 12, e1001779 (2015).
Xu, K., Liu, Y., Zhan, Y., Ren, J. & Jiang, T. BRANT: a versatile and extendable resting-state fMRI toolkit. Front. Neuroinformatics 12, 52 (2018).
Friston, K. J., Williams, S., Howard, R., Frackowiak, R. S. & Turner, R. Movement-related effects in fMRI time-series. Magn. Reson. Med. 35, 346–355 (1996).
Fischl, B., Sereno, M. I., Tootell, R. B. H. & Dale, A. M. High-resolution intersubject averaging and a coordinate system for the cortical surface. Hum. Brain Mapp. 8, 272–284 (1999).
Purcell, S. et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am. J. Hum. Genet. 81, 559–575 (2007).
Liu, B. et al. Polygenic risk for schizophrenia influences cortical gyrification in 2 independent general populations. Schizophr. Bull. 43, 673–680 (2017).
Patterson, N., Price, A. L. & Reich, D. Population structure and eigenanalysis. PLoS Genet. 2, e190 (2006).
Price, A. L. et al. Principal components analysis corrects for stratification in genome-wide association studies. Nat. Genet. 38, 904–909 (2006).
Thorisson, G. A., Smith, A. V., Krishnan, L. & Stein, L. D. The International HapMap Project web site. Genome Res. 15, 1592–1593 (2005).
Delaneau, O., Marchini, J. & Zagury, J.-F. A linear complexity phasing method for thousands of genomes. Nat. Methods 9, 179–181 (2011).
Howie, B. N., Donnelly, P. & Marchini, J. A flexible and accurate genotype imputation method for the next generation of genome-wide association studies. PLoS Genet. 5, e1000529 (2009).
Auton, A. et al. A global reference for human genetic variation. Nature 526, 68–74 (2015).
Huntenburg, J. M., Bazin, P.-L. & Margulies, D. S. Large-scale gradients in human cortical organization. Trends Cogn. Sci. 22, 21–31 (2018).
Zhao, Y. et al. The development of cortical functional hierarchy is associated with the molecular organization of prenatal/postnatal periods. Cereb. Cortex https://doi.org/10.1093/cercor/bhac340 (2022).
Langs, G., Golland, P. & Ghosh, S. S. Predicting activation across individuals with resting-state functional connectivity based multi-atlas label fusion. Med. Image Comput. Comput. Assist. Interv. 9350, 313–320 (2015).
Fan, L. et al. The human Brainnetome atlas: a new brain atlas based on connectional architecture. Cereb. Cortex 26, 3508–3526 (2016).
Bethlehem, R. A. I. et al. Brain charts for the human lifespan. Nature 604, 525–533 (2022).
Baum, G. L. et al. Development of structure–function coupling in human brain networks during youth. Proc. Natl Acad. Sci. USA 117, 771–778 (2020).
Eilers, P. H. C. & Marx, B. D. Flexible smoothing with B-splines and penalties. Stat. Sci. 11, 89–121 (1996).
Bergstra, J. & Bengio, Y. Random search for hyper-parameter optimization. J. Mach. Learn. Res. 13, 281–305 (2012).
Poldrack, R. A. et al. Discovering relations between mind, brain, and mental disorders using topic mapping. PLoS Comput. Biol. 8, e1002707 (2012).
Wang, H.-T. et al. Neurocognitive patterns dissociating semantic processing from executive control are linked to more detailed off-task mental time travel. Sci. Rep. 10, 11904 (2020).
Markello, R. D. et al. Standardizing workflows in imaging transcriptomics with the abagen toolbox. eLife 10, e72129 (2021).
Arnatkevičiūtė, A., Fulcher, B. D. & Fornito, A. A practical guide to linking brain-wide gene expression and neuroimaging data. Neuroimage 189, 353–367 (2019).
Zhou, Y. et al. Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat. Commun. 10, 1523 (2019).
Shannon, P. et al. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 13, 2498–2504 (2003).
Stuart, T. et al. Comprehensive INtegration of Single-cell Data. Cell 177, 1888–1902.e21 (2019).
Subramanian, A. et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl Acad. Sci. USA 102, 15545–15550 (2005).
Skene, N. G. & Grant, S. G. N. Identification of vulnerable cell types in major brain disorders using single cell transcriptomes and expression weighted cell type enrichment. Front. Neurosci. 10, 16 (2016).
Li, Z. et al. Genome-wide association analysis identifies 30 new susceptibility loci for schizophrenia. Nat. Genet. 49, 1576–1583 (2017).
Choi, S. W., Mak, T. S.-H. & O’Reilly, P. F. Tutorial: a guide to performing polygenic risk score analyses. Nat. Protoc. 15, 2759–2772 (2020).
Acknowledgements
This work was supported by the Startup Funds of Beijing Normal University (to B.L.), the National Key Basic Research and Development Program (973) (grant 2011CB707800 to T.J.), the National Key Research and Development Plan (grant 2016YFC0904300 to B.L.), the Natural Science Foundation of China (grant 81771451 to B.L.; grant 82171543 to A.L.), the Science and Technology Innovation 2030—Brain Science and Brain-Inspired Intelligence Project of China (grant 2021ZD0200200 to T.J.).
Author information
Authors and Affiliations
Contributions
B.L. and T.J. led the project. B.L. and M.W. were responsible for the study concept and the design of the study. H.Y. and W.Y. provided crucial advice for the study. A.L. made substantial contributions to the paper and provided critical comments. M.W. and B.L. analyzed the data, created the figures and wrote the paper. Y.L., L.F., K.H., Y.S., Y.Z., J.L., X.T. and M.S. participated in discussions of the results and the paper. P.L., J.C., Y.C., Huaning Wang, W.L., Z.L., Y.Y., H.G., L. Lv, L. Lu, J.Y., Huiling Wang, H.Z., H. Wu, Y.N. and D.Z. contributed to the data acquisition.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Mental Health thanks Marta Bosia, Katharina Schmack and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Supra-threshold permutation test based on cluster size (10,000 iterations, cluster defining threshold (cdt)=0.001).
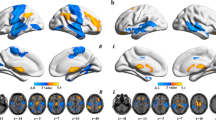
Surface-based corticocortical and corticostriatal connectivity comparisons (based on a two-sample two-sided t-test) between patients with SCZ and NC, with multiple comparisons corrected using permutation-based cluster thresholding instead of random field theory (see Fig. 2a,c).
Extended Data Fig. 2 Nested 10-fold cross-validation process.
The procedure was applied for model training and selection in the SCZ-I dataset.
Extended Data Fig. 3 Comparisons of functional connectivity between high and low PANSS dimension subgroups.
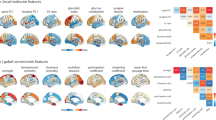
a. Based on the negative dimension of the PANSS 5-factor model, the top and bottom 30% of SCZ patients (n = 513) from the combined SCZ-I and SCZ-II datasets were selected, forming two subgroups: N-high and N-low. The two subgroups were further screened using a two-sample t-test (two-sided) to ensure no differences in the other four PANSS dimensions. b. Corticocortical connectivity was compared between the N-high and N-low subgroups using the methodology in Fig. 1, resulting in a differential map, termed N-map. The N-map demonstrated a significant positive spatial correlation with the dysconnectivity t-map from the SCZ and NC group comparison (see Fig. 1). Mean connectivity scores from the ten dysconnectivity clusters (Fig. 2a) were significantly higher in the N-high subgroup. c. Corticostriatal connectivity was compared between the N-high and N-low subgroups. The corticostriatal N-map and dysconnectivity t-map displayed a significant positive spatial correlation. The absolute connectivity values from eight clusters (Fig. 2c) significantly decreased in the N-high subgroup. d-f. A similar analysis, like panels a-c, compared functional connectivity between high and low positive subgroups. The corticocortical P-map and dysconnectivity t-map showed no correlation, with no significant differences in connectivity strength (absolute value) of the ten clusters between P-high and P-low subgroups (e). However, a significant positive correlation was found between the corticostriatal P-map and dysconnectivity t-map, while the eight clusters’ connectivity strength significantly decreased in the P-high subgroup (f). g-i. Like panels a-c and d-f, functional connectivity differences were examined between the high and low cognitive subgroups. Significant differences were found in corticocortical connectivity for C-high and C-low subgroups, while corticostriatal connectivity showed no significant differences. Spatial correlations between maps were quantified using two-sided Pearson’s r. The significance (Pspin) was determined using the spin-based permutation test. Connectivity strength differences were measured using the two-sample t-test (two-sided). The error bars represent mean values ± 95% confidence interval. The box plot presents minimum, 25th percentile, median, 75th percentile, and maximum values (excluding outliers) for the combined high and low groups.
Extended Data Fig. 4 Functional decodings of corticocortical and corticostriatal dysconnectivity.
The spatial correlations (two-sided Pearson’s r) between t-maps and 24 predefined topic maps (each is composed of related terms) from the Neurosynth database were calculated. The significance (Pperm) was estimated using permutation tests. The warm or cool color indicates a positive or negative correlation, respectively. Only significant correlations (Pperm<0.05) are shown with colors, and darker colors indicate a higher significance. The face/affective processing map (the label is marked in bold and surrounded by a red box) is significantly, positively, and consistently correlated with corticocortical dysconnectivity t-maps across three datasets (a). There were three cognitive maps (pain, cued attention, and inhibition error) that were significantly, negatively, and consistently correlated with corticostriatal dysconnectivity t-maps across three datasets (b).
Extended Data Fig. 5 Cell enrichment of individual dysconnectivity.
a, c, e, g, The line graphs illustrate individual-level enrichment scores (NESs) of Lake and ABA cells for corticocortical and corticostriatal dysconnectivity. The participants were derived from the SCZ-I, SCZ-II, and COBRE datasets (total SCZ/NC=585/614). The error bars represent mean values ± 95% confidence interval. b, d, f, h, The heatmaps show pairwise comparisons of individual NES scores within the SCZ group (n = 585), using a two-sample t-test (two-sided). Blank squares indicate no significant differences between cells.
Extended Data Fig. 6 Cell enrichment comparisons of dysconnectivity transcriptional correlates between SCZ and MDD.
a, b, Cortical renderings show corticocortical (a) and corticostriatal (b) connectivity differences (t-maps) between patients with MDD and NC in the MDD-XX dataset (MDD, n = 75; NC, n = 74). The MDD dysconnectivity t-maps were obtained by applying the same analytical method as in Fig. 1. The warm or cool color indicates connectivity is increased or decreased in MDD. c. Cell enrichment of MDD dysconnectivity transcriptional correlates was conducted as with SCZ in Fig. 3. MDD corticocortical dysconnectivity transcriptional correlated genes were most significantly positively enriched in In6 and negatively enriched in Ast. The positively and negatively enriched cells with the most significance for MDD corticostriatal dysconnectivity were Ex3 and Ast, respectively. The solid circles with warm or cool colors indicate positive or negative enrichment, determined by NES. Darker colors mean greater NES. The radius is quantified by -log10(adjusted P value) (two-sided, FDR correction). Larger circles mean more significance of enrichment. NS, non-significant. NES, normalized enrichment score.
Extended Data Fig. 7 Gene Ontology enrichment of dysconnectivity transcriptional correlates.
Metascape enrichment networks show inter-cluster and intra-cluster similarities of enriched biological process terms. Each term is characterized by a circle node, where its size represents the significance level of enrichment, and its color denotes cluster identity (nodes with the same color belong to the same cluster). For each kind of dysconnectivity, a sorted gene list (in descending order) was obtained based on averaged spatial correlations between all AHBA genes and dysconnectivity t-maps. The gene list was then split into deciles. Enrichment analyses were separately conducted for the top and bottom gene deciles.
Extended Data Fig. 8 Cell enrichment of SCZ GWAS based on LDSC method.
Vertical bar plots show Lake (a) and ABA (b) cells enrichment of polygenic risk in SCZ from GWAS data. Cell-specific genes were defined using Lake data from the dorsal frontal cortex (DFC) and visual cortex (VIS), and ABA data from the middle temporal gyrus (MTG). The dashed line indicates unadjusted P < 0.05.
Supplementary information
Supplementary Information
Supplementary Figs. 1–18 and Tables 1–42.
Supplementary Data
Detailed statistical results that are not appropriate for inclusion in the Supplementary Information file.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, M., Yan, H., Tian, X. et al. Neuroimaging and multiomics reveal cross-scale circuit abnormalities in schizophrenia. Nat. Mental Health 1, 633–654 (2023). https://doi.org/10.1038/s44220-023-00110-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s44220-023-00110-3