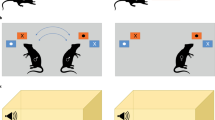
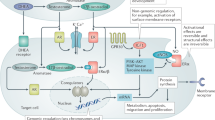
Abstract
In 2016, changes were mandated for basic research, including using sex as a biological variable. This policy change was due to the lack of research performed in female animals. This resulted in a mismatch between the sex of the subjects being used for drug development and the sex of the participants in subsequent clinical trials hampering the translational success of novel therapeutics, especially treatments for mood disorders. While it is now clear that sex differences exist, the field needs to move to the next frontier in sex-difference research. We need to start exploring why and how these sex differences exist. What are their functions? How do we harness this information to develop novel sex-specific treatments for mental illness? This Review will address what we have learned from using sex as a biological variable and how we can utilize these data to better understand and treat sex-based disparities in mental health.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$59.00 per year
only $4.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Gender Studies in Product Development: Historical Overview (FDA, 2018); https://www.fda.gov/science-research/womens-health-research/gender-studies-product-development-historical-overview
Mastroianni, A. C., Faden, R. & Federman, D. Women and health research: a report from the Institute of Medicine. Kennedy Inst. Ethics J. 4, 55–62 (1994).
Rechlin, R. K., Splinter, T. F. L., Hodges, T. E., Albert, A. Y. & Galea, L. A. M. An analysis of neuroscience and psychiatry papers published from 2009 and 2019 outlines opportunities for increasing discovery of sex differences. Nat. Commun. 13, 2137 (2022).
Beery, A. K. & Zucker, I. Sex bias in neuroscience and biomedical research. Neurosci. Biobehav. Rev. 35, 565–572 (2011).
Mamlouk, G. M., Dorris, D. M., Barrett, L. R. & Meitzen, J. Sex bias and omission in neuroscience research is influenced by research model and journal, but not reported NIH funding. Front. Neuroendocrinol. 57, 100835 (2020).
Becker, J. B., Prendergast, B. J. & Liang, J. W. Female rats are not more variable than male rats: a meta-analysis of neuroscience studies. Biol. Sex Differ. 7, 34 (2016).
Sensi, S., Pace Palitti, V. & Guagnano, M. T. Chronobiology in endocrinology. Ann. Ist. Super. Sanita 29, 613–631 (1993).
Keck, M. E., Welt, T., Müller, M. B., Landgraf, R. & Holsboer, F. The high-affinity non-peptide CRH1 receptor antagonist R121919 attenuates stress-induced alterations in plasma oxytocin, prolactin, and testosterone secretion in rats. Pharmacopsychiatry 36, 27–31 (2003).
Binneman, B. et al. A 6-week randomized, placebo-controlled trial of CP-316,311 (a selective CRH1 antagonist) in the treatment of major depression. Am. J. Psychiatry 165, 617–620 (2008).
Coric, V. et al. Multicenter, randomized, double-blind, active comparator and placebo-controlled trial of a corticotropin-releasing factor receptor-1 antagonist in generalized anxiety disorder. Depress. Anxiety 27, 417–425 (2010).
Ising, M. et al. High-affinity CRF1 receptor antagonist NBI-34041: preclinical and clinical data suggest safety and efficacy in attenuating elevated stress response. Neuropsychopharmacology 32, 1941–1949 (2007).
Zobel, A. W. et al. Effects of the high-affinity corticotropin-releasing hormone receptor 1 antagonist R121919 in major depression: the first 20 patients treated. J. Psychiatr. Res. 34, 171–181 (2000).
Heinrich, J., Gahart, M. T., Rowe, E. J. & Bradely, L. Drug Safety: Most Drugs Withdrawn in Recent Years Had Greater Health Risks for Women (United States General Accounting Office, 2001).
Arnegard, M. E., Whitten, L. A., Hunter, C. & Clayton, J. A. Sex as a biological variable: a 5-year progress report and call to action. J. Womens Health 29, 858–864 (2020).
Ernst, M. M., Kogan, B. A. & Lee, P. A. Gender identity: a psychosocial primer for providing care to patients with a disorder/difference of sex development and their families [individualized care for patients with intersex (disorders/differences of sex development): part 2]. J. Pediatr. Urol. 16, 606–611 (2020).
Rosenwohl-Mack, A. et al. A national study on the physical and mental health of intersex adults in the US. PLoS ONE 15, e0240088 (2020).
Salk, R. H., Hyde, J. S. & Abramson, L. Y. Gender differences in depression in representative national samples: meta-analyses of diagnoses and symptoms. Psychol. Bull. 143, 783–822 (2017).
Marcus, S. M. et al. Sex differences in depression symptoms in treatment-seeking adults: confirmatory analyses from the Sequenced Treatment Alternatives to Relieve Depression study. Compr. Psychiatry 49, 238–246 (2008).
Young, E. A. et al. Sex differences in response to citalopram: a STAR*D report. J. Psychiatr. Res. 43, 503–511 (2009).
Martin, L. A., Neighbors, H. W. & Griffith, D. M. The experience of symptoms of depression in men vs women: analysis of the National Comorbidity Survey Replication. JAMA Psychiatry 70, 1100–1106 (2013).
American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (5th ed.) https://doi.org/10.1176/appi.books.9780890425596 (American Psychiatric Association Publishing, 2013).
Labonte, B. et al. Sex-specific transcriptional signatures in human depression. Nat. Med. 23, 1102–1111 (2017).
Seney, M. L. et al. Opposite molecular signatures of depression in men and women. Biol. Psychiatry 84, 18–27 (2018).
Green, T., Flash, S. & Reiss, A. L. Sex differences in psychiatric disorders: what we can learn from sex chromosome aneuploidies. Neuropsychopharmacology 44, 9–21 (2019).
Engberg, H. et al. Increased psychiatric morbidity in women with complete androgen insensitivity syndrome or complete gonadal dysgenesis. J. Psychosom. Res. 101, 122–127 (2017).
Fliegner, M. et al. Sexual life and sexual wellness in individuals with complete androgen insensitivity syndrome (CAIS) and Mayer–Rokitansky–Kuster–Hauser Syndrome (MRKHS). J. Sex Med. 11, 729–742 (2014).
Jenkins-Jones, S. et al. Poor compliance and increased mortality, depression and healthcare costs in patients with congenital adrenal hyperplasia. Eur. J. Endocrinol. 178, 309–320 (2018).
Seney, M. L., Ekong, K. I., Ding, Y., Tseng, G. C. & Sibille, E. Sex chromosome complement regulates expression of mood-related genes. Biol. Sex Differ. 4, 20 (2013).
Seney, M. L. et al. The role of genetic sex in affect regulation and expression of GABA-related genes across species. Front. Psychiatry 4, 104 (2013).
Barko, K., Paden, W., Cahill, K. M., Seney, M. L. & Logan, R. W. Sex-specific effects of stress on mood-related gene expression. Mol. Neuropsychiatry 5, 162–175 (2019).
Post, C. & Leuner, B. The maternal reward system in postpartum depression. Arch. Womens Ment. Health 22, 417–429 (2019).
McCarthy, M. M., Arnold, A. P., Ball, G. F., Blaustein, J. D. & De Vries, G. J. Sex differences in the brain: the not so inconvenient truth. J. Neurosci. 32, 2241–2247 (2012).
Kinnear, H. M. et al. A mouse model to investigate the impact of testosterone therapy on reproduction in transgender men. Hum. Reprod. 34, 2009–2017 (2019).
Dela Cruz, C. et al. A mouse model mimicking gender-affirming treatment with pubertal suppression followed by testosterone in transmasculine youth. Hum. Reprod. 38, 256–265 (2023).
Lacasse, J. M., Gomez-Perales, E. & Brake, W. G. Modeling hormonal contraception in female rats: a framework for studies in behavioral neurobiology. Front. Neuroendocrinol. 67, 101020 (2022).
Skovlund, C. W., Morch, L. S., Kessing, L. V. & Lidegaard, O. Association of hormonal contraception with depression. JAMA Psychiatry 73, 1154–1162 (2016).
Rowniak, S., Bolt, L. & Sharifi, C. Effect of cross-sex hormones on the quality of life, depression and anxiety of transgender individuals: a quantitative systematic review. JBI Database System. Rev. Implement. Rep. 17, 1826–1854 (2019).
Eliot, L., Ahmed, A., Khan, H. & Patel, J. Dump the “dimorphism”: comprehensive synthesis of human brain studies reveals few male–female differences beyond size. Neurosci. Biobehav. Rev. 125, 667–697 (2021).
Williams, C. M., Peyre, H., Toro, R. & Ramus, F. Neuroanatomical norms in the UK Biobank: the impact of allometric scaling, sex, and age. Hum. Brain Mapp. 42, 4623–4642 (2021).
DeCasien, A. R., Guma, E., Liu, S. & Raznahan, A. Sex differences in the human brain: a roadmap for more careful analysis and interpretation of a biological reality. Biol. Sex Differ. 13, 43 (2022).
Liu, S., Seidlitz, J., Blumenthal, J. D., Clasen, L. S. & Raznahan, A. Integrative structural, functional, and transcriptomic analyses of sex-biased brain organization in humans. Proc. Natl Acad. Sci. USA 117, 18788–18798 (2020).
Joel, D. Beyond sex differences and a male–female continuum: mosaic brains in a multidimensional space. Handb. Clin. Neurol. 175, 13–24 (2020).
Kaczkurkin, A. N., Raznahan, A. & Satterthwaite, T. D. Sex differences in the developing brain: insights from multimodal neuroimaging. Neuropsychopharmacology 44, 71–85 (2019).
Ingalhalikar, M. et al. Sex differences in the structural connectome of the human brain. Proc. Natl Acad. Sci. USA 111, 823–828 (2014).
Abdallah, C. G. et al. Ketamine treatment and global brain connectivity in major depression. Neuropsychopharmacology 42, 1210–1219 (2017).
Arelin, K. et al. Progesterone mediates brain functional connectivity changes during the menstrual cycle—a pilot resting state MRI study. Front. Neurosci. 9, 44 (2015).
Cooke, B. M. & Woolley, C. S. Effects of prepubertal gonadectomy on a male-typical behavior and excitatory synaptic transmission in the amygdala. Dev. Neurobiol. 69, 141–152 (2009).
Cooke, B. M. & Woolley, C. S. Sexually dimorphic synaptic organization of the medial amygdala. J. Neurosci. 25, 10759–10767 (2005).
Estrada, C. M. et al. Estrogen signaling in the medial amygdala decreases emotional stress responses and obesity in ovariectomized rats. Horm. Behav. 98, 33–44 (2018).
Nordman, J. C. et al. Potentiation of divergent medial amygdala pathways drives experience-dependent aggression escalation. J. Neurosci. 40, 4858–4880 (2020).
Bangasser, D. A. & Shors, T. J. The bed nucleus of the stria terminalis modulates learning after stress in masculinized but not cycling females. J. Neurosci. 28, 6383–6387 (2008).
Dalla, C., Whetstone, A. S., Hodes, G. E. & Shors, T. J. Stressful experience has opposite effects on dendritic spines in the hippocampus of cycling versus masculinized females. Neurosci. Lett. 449, 52–56 (2009).
Salvatore, M. et al. Sex differences in circuits activated by corticotropin releasing factor in rats. Horm. Behav. 97, 145–153 (2018).
Bangasser, D. A. et al. Sex differences in corticotropin-releasing factor receptor signaling and trafficking: potential role in female vulnerability to stress-related psychopathology. Mol. Psychiatry 15, 896–904 (2010).
Bangasser, D. A. & Valentino, R. J. Sex differences in molecular and cellular substrates of stress. Cell. Mol. Neurobiol. 32, 709–723 (2012).
Bittar, T. P. et al. Chronic stress induces sex-specific functional and morphological alterations in corticoaccumbal and corticotegmental pathways. Biol. Psychiatry 90, 194–205 (2021).
Hodes, G. E. et al. Sex differences in nucleus accumbens transcriptome profiles associated with susceptibility versus resilience to subchronic variable stress. J. Neurosci. 35, 16362–16376 (2015).
Williams, E. S. et al. Androgen-dependent excitability of mouse ventral hippocampal afferents to nucleus accumbens underlies sex-specific susceptibility to stress. Biol. Psychiatry 87, 492–501 (2020).
Dion-Albert, L. et al. Vascular and blood–brain barrier-related changes underlie stress responses and resilience in female mice and depression in human tissue. Nat. Commun. 13, 164 (2022).
Zhang, S. et al. Sex differences in the neuroadaptations of reward-related circuits in response to subchronic variable stress. Neuroscience 376, 108–116 (2018).
Jain, A., Huang, G. Z. & Woolley, C. S. Latent sex differences in molecular signaling that underlies excitatory synaptic potentiation in the hippocampus. J. Neurosci. 39, 1552–1565 (2019).
Tabatadze, N., Huang, G., May, R. M., Jain, A. & Woolley, C. S. Sex differences in molecular signaling at inhibitory synapses in the hippocampus. J. Neurosci. 35, 11252–11265 (2015).
Zer-Aviv, T. M. & Akirav, I. Sex differences in hippocampal response to endocannabinoids after exposure to severe stress. Hippocampus 26, 947–957 (2016).
Bollinger, J. L., Bergeon Burns, C. M. & Wellman, C. L. Differential effects of stress on microglial cell activation in male and female medial prefrontal cortex. Brain Behav. Immun. 52, 88–97 (2016).
Wohleb, E. S., Terwilliger, R., Duman, C. H. & Duman, R. S. Stress-induced neuronal colony stimulating factor 1 provokes microglia-mediated neuronal remodeling and depressive-like behavior. Biol. Psychiatry 83, 38–49 (2018).
Gandal, M. J. et al. Transcriptome-wide isoform-level dysregulation in ASD, schizophrenia, and bipolar disorder. Science https://doi.org/10.1126/science.aat8127 (2018).
Gandal, M. J. et al. Shared molecular neuropathology across major psychiatric disorders parallels polygenic overlap. Science 359, 693–697 (2018).
Setiawan, E. et al. Role of translocator protein density, a marker of neuroinflammation, in the brain during major depressive episodes. JAMA Psychiatry 72, 268–275 (2015).
Kelly Barko, M. S. et al. Brain region- and sex-specific transcriptional profiles of microglia. Front. Psychiatry https://doi.org/10.3389/fpsyt.2022.945548 (2022).
Barreto, G., Veiga, S., Azcoitia, I., Garcia-Segura, L. M. & Garcia-Ovejero, D. Testosterone decreases reactive astroglia and reactive microglia after brain injury in male rats: role of its metabolites, oestradiol and dihydrotestosterone. Eur. J. Neurosci. 25, 3039–3046 (2007).
Villa, A. et al. Sex-specific features of microglia from adult mice. Cell Rep. 23, 3501–3511 (2018).
Woodburn, S. C., Bollinger, J. L. & Wohleb, E. S. Synaptic and behavioral effects of chronic stress are linked to dynamic and sex-specific changes in microglia function and astrocyte dystrophy. Neurobiol. Stress 14, 100312 (2021).
Tsyglakova, M. et al. Sex and region-specific effects of variable stress on microglia morphology. Brain Behav. Immun. Health 18, 100378 (2021).
Menard, C. et al. Social stress induces neurovascular pathology promoting depression. Nat. Neurosci. 20, 1752–1760 (2017).
Dion-Albert, L. et al. Sex differences in the blood–brain barrier: implications for mental health. Front. Neuroendocrinol. 65, 100989 (2022).
Lorsch, Z. S. et al. Estrogen receptor alpha drives pro-resilient transcription in mouse models of depression. Nat. Commun. 9, 1116 (2018).
Devan, B. D., Tobin, E. L., Dunn, E. N. & Magalis, C. Sex differences on the competitive place task in the water maze: the influence of peripheral pool time on spatial navigation performance in rats. Behav. Processes 132, 34–41 (2016).
Mueller, B. R. & Bale, T. L. Early prenatal stress impact on coping strategies and learning performance is sex dependent. Physiol. Behav. 91, 55–65 (2007).
Gruene, T. M., Flick, K., Stefano, A., Shea, S. D. & Shansky, R. M. Sexually divergent expression of active and passive conditioned fear responses in rats. Elife https://doi.org/10.7554/eLife.11352 (2015).
Maier, S. F. & Watkins, L. R. Stressor controllability and learned helplessness: the roles of the dorsal raphe nucleus, serotonin, and corticotropin-releasing factor. Neurosci. Biobehav. Rev. 29, 829–841 (2005).
Dalla, C., Edgecomb, C., Whetstone, A. S. & Shors, T. J. Females do not express learned helplessness like males do. Neuropsychopharmacology 33, 1559–1569 (2008).
Morrison, K. E. et al. Preadolescent adversity programs a disrupted maternal stress reactivity in humans and mice. Biol. Psychiatry 81, 693–701 (2017).
Zucker, I. & Prendergast, B. J. Sex differences in pharmacokinetics predict adverse drug reactions in women. Biol. Sex Differ. 11, 32 (2020).
Kornstein, S. G. et al. Gender differences in treatment response to sertraline versus imipramine in chronic depression. Am. J. Psychiatry 157, 1445–1452 (2000).
Sramek, J. J., Murphy, M. F. & Cutler, N. R. Sex differences in the psychopharmacological treatment of depression. Dialogues Clin. Neurosci. 18, 447–457 (2016).
Damoiseaux, V. A., Proost, J. H., Jiawan, V. C. & Melgert, B. N. Sex differences in the pharmacokinetics of antidepressants: influence of female sex hormones and oral contraceptives. Clin. Pharmacokinet. 53, 509–519 (2014).
Hodes, G. E., Hill-Smith, T. E., Suckow, R. F., Cooper, T. B. & Lucki, I. Sex-specific effects of chronic fluoxetine treatment on neuroplasticity and pharmacokinetics in mice. J. Pharmacol. Exp. Ther. 332, 266–273 (2010).
Alvarez, J. C., Bothua, D., Collignon, I., Advenier, C. & Spreux-Varoquaux, O. Determination of fluoxetine and its metabolite norfluoxetine in serum and brain areas using high-performance liquid chromatography with ultraviolet detection. J. Chromatogr. B 707, 175–180 (1998).
Ferguson, J. M. & Hill, H. Pharmacokinetics of fluoxetine in elderly men and women. Gerontology 52, 45–50 (2006).
Carrier, N. & Kabbaj, M. Sex differences in the antidepressant-like effects of ketamine. Neuropharmacology 70, 27–34 (2013).
Freeman, M. P. et al. Sex differences in response to ketamine as a rapidly acting intervention for treatment resistant depression. J. Psychiatr. Res. 110, 166–171 (2019).
Ponton, E., Turecki, G. & Nagy, C. Sex differences in the behavioral, molecular, and structural effects of ketamine treatment in depression. Int. J. Neuropsychopharmacol. 25, 75–84 (2022).
Anderson, G. D. Sex and racial differences in pharmacological response: where is the evidence? Pharmacogenetics, pharmacokinetics, and pharmacodynamics. J. Womens Health 14, 19–29 (2005).
Pinna, G., Costa, E. & Guidotti, A. Fluoxetine and norfluoxetine stereospecifically and selectively increase brain neurosteroid content at doses that are inactive on 5-HT reuptake. Psychopharmacology 186, 362–372 (2006).
Dalla, C., Pitychoutis, P. M., Kokras, N. & Papadopoulou-Daifoti, Z. Sex differences in animal models of depression and antidepressant response. Basic Clin. Pharmacol. Toxicol. 106, 226–233 (2010).
Berkley, K. J. Vive la difference! Trends Neurosci. 15, 331–332 (1992).
Lin, H. et al. The prevalence and factors associated with anxiety-like and depression-like behaviors in women with polycystic ovary syndrome. Front. Psychiatry 12, 709674 (2021).
Wang, Z. et al. Mapping global prevalence of depression among postpartum women. Transl. Psychiatry 11, 543 (2021).
Freeman, E. W. Associations of depression with the transition to menopause. Menopause 17, 823–827 (2010).
Mirin, A. A. Gender disparity in the funding of diseases by the US National Institutes of Health. J. Womens Health 30, 956–963 (2021).
Acknowledgments
This work was supported by National Institute of Mental Health grant no. R56MH124930 (G.E.H.). Figures 2 and 3 were created using https://BioRender.com.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Mental Health thanks the anonymous reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hodes, G.E., Kropp, D.R. Sex as a biological variable in stress and mood disorder research. Nat. Mental Health 1, 453–461 (2023). https://doi.org/10.1038/s44220-023-00083-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s44220-023-00083-3
This article is cited by
-
Sex differences in prenatal development of neural complexity in the human brain
Nature Mental Health (2024)