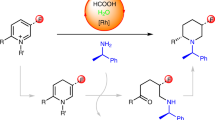
Abstract
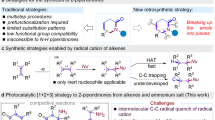
Piperidines are prevalent throughout Food and Drug Administration-approved drugs and current drug candidates, and thus robust methods for preparing these heterocycles are desirable for efficiently probing structure–activity relationships during drug discovery campaigns. N-(Hetero)aryl piperidines are particularly important in pharmaceutical applications, but remain difficult to synthesize because traditional approaches based on transition-metal-catalysed cross-coupling or SNAr reactions routinely fail. Here we report a general platform for the rapid synthesis of a range of densely functionalized N-(hetero)aryl piperidine derivatives using a common iminium ion precursor. An expeditious synthesis of bench-stable cyclic iminium salts starting from widely abundant heteroaryl-amine and ketoacrylate feedstocks is disclosed. We then show that these intermediates are readily functionalized, rapidly yielding a variety of complex piperidines.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
All data supporting the findings of this study are available within the Article and its Supplementary Information. The X-ray crystallographic coordinates for compound 1a reported in this study have been deposited at the Cambridge Crystallographic Data Centre (CCDC), under deposition number 2170178. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
References
Vitaku, E., Smith, D. T. & Njardarson, J. T. Analysis of the structural diversity, substitution patterns, and frequency of nitrogen heterocycles among US FDA approved pharmaceuticals. J. Med. Chem. 57, 10257–10274 (2014).
Taylor, R. D., MacCoss, M. & Lawson, A. D. G. Rings in drugs. J. Med. Chem. 57, 5845–5859 (2014).
Abdelshaheed, M. M., Fawzy, I. M., El-Subbagh, H. I. & Youssef, K. M. Piperidine nucleus in the field of drug discovery. Future J. Pharm. Sci. 7, 188 (2021).
Källström, S. & Leino, R. Synthesis of pharmaceutically active compounds containing a disubstituted piperidine framework. Bioorg. Med. Chem. 16, 601–635 (2008).
Liu, G.-Q. & Opatz, T. Recent advances in the synthesis of piperidines: functionalization of preexisting ring systems. Adv. Heterocycl. Chem. 125, 107–234 (2018).
Laschat, S. & Dickner, T. Stereoselective synthesis of piperidines. Synthesis 2000, 1781–1813 (2000).
Weintraub, P. M., Sabol, J. S., Kane, J. M. & Borcherding, D. R. Recent advances in the synthesis of piperidones and piperidines. Tetrahedron 59, 2953–2989 (2003).
Zhang, Z., Zhang, X. & Nagib, D. A. Chiral piperidines from acyclic amines via enantioselective radical-mediated δ C–H cyanation. Chem 5, 3127–3134 (2019).
Stateman, L. M., Dare, R. M., Paneque, A. N. & Nagib, D. A. Aza-heterocycles via copper-catalyzed remote C–H desaturation of amines. Chem 8, 210–224 (2021).
Qu, B. et al. Synthesis of enantioenriched 2-alkyl piperidine derivatives through asymmetric reduction of pyridinium salts. Org. Lett. 18, 4920–4923 (2016).
Qu, B. et al. Enantioselective synthesis of α-(hetero)aryl piperidines through asymmetric hydrogenation of pyridinium salts and its mechanistic insights. Org. Lett. 20, 1333–1337 (2018).
Chang, M. et al. Asymmetric hydrogenation of pyridinium salts with an iridium phosphole catalyst. Angew. Chem. Int. Ed. 53, 12761–12764 (2014).
Paruch, K. et al. Discovery of dinaciclib (SCH 727965): a potent and selective inhibitor of cyclin-dependent kinases. ACS Med. Chem. Lett. 1, 204–208 (2010).
Sakairi, M. et al. Synthesis and SAR studies of bicyclic amine series GPR119 agonists. Bioorg. Med. Chem. Lett. 22, 5123–5128 (2012).
Semple, G. et al. Discovery of the first potent and orally efficacious agonist of the orphan G-protein coupled receptor 119. J. Med. Chem. 51, 5172–5175 (2008).
Watson, K. G. et al. An orally bioavailable oxime ether capsid binder with potent activity against human rhinovirus. J. Med. Chem. 46, 3181–3184 (2003).
Goel, P. et al. Recent advancement of piperidine moiety in treatment of cancer—a review. Eur. J. Med. Chem. 157, 480–502 (2018).
Vlasov, V. M. Nucleophilic substitution of the nitro group, fluorine and chlorine in aromatic compounds. Russ. Chem. Rev. 72, 681–703 (2003).
Ruiz-Castillo, P. & Buchwald, S. L. Applications of palladium-catalyzed C–N cross-coupling reactions. Chem. Rev. 116, 12564–12649 (2016).
Park, N. H., Vinogradova, E. V., Surry, D. S. & Buchwald, S. L. Design of new ligands for the palladium-catalyzed arylation of α-branched secondary amines. Angew. Chem., Int. Ed. 54, 8259–8262 (2015).
Sather, A. C. & Martinot, T. A. Data-rich experimentation enables palladium-catalyzed couplings of piperidines and five-membered (hetero)aromatic electrophiles. Org. Process Res. Dev. 23, 1725–1739 (2019).
Bolleddula, J. et al. Biotransformation and bioactivation reactions of alicyclic amines in drug molecules. Drug Metab. Rev. 46, 379–419 (2014).
Kato, K., Jingu, S., Ogawa, N. & Higuchi, S. Differentiation between isomeric oxidative metabolites of a piperidine-containing drug by liquid chromatography–tandem mass spectrometry with stable-isotope tracer technique. Biomed. Chromatogr. 16, 25–30 (2002).
Lovering, F., Bikker, J. & Humblet, C. Escape from flatland: increasing saturation as an approach to improving clinical success. J. Med. Chem. 52, 6752–6756 (2009).
Hunt, J. A. et al. P38 inhibitors: piperidine- and 4-aminopiperidine-substituted naphthyridinones, quinolinones, and dihydroquinazolinones. Bioorg. Med. Chem. Lett. 13, 467–470 (2003).
Larsen, M. A. et al. A modular and diastereoselective 5 + 1 cyclization approach to N-(hetero)aryl piperidines. J. Am. Chem. Soc. 142, 726–732 (2020).
Bláha, K. & Červinka, O. Cyclic enamines and imines. Adv. Heterocycl. Chem. 6, 147–227 (1966).
Beng, T. K., Takeuchi, H., Weber, M. & Sarpong, R. Stereocontrolled synthesis of vicinally functionalized piperidines by nucleophilic β-addition of alkyllithiums to α-aryl substituted piperidine enecarbamates. Chem. Commun. 51, 7653–7656 (2015).
Chen, W., Ma, L., Paul, A. & Seidel, D. Direct α-C–H bond functionalization of unprotected cyclic amines. Nat. Chem. 10, 165–169 (2018).
Paul, A. & Seidel, D. α-Functionalization of cyclic secondary amines: Lewis acid promoted addition of organometallics to transient imines. J. Am. Chem. Soc. 141, 8778–8782 (2019).
Chen, W., Paul, A., Abboud, K. A. & Seidel, D. Rapid functionalization of multiple C–H bonds in unprotected alicyclic amines. Nat. Chem. 12, 545–550 (2020).
Holmberg-Douglas, N., Choi, Y., Aquila, B., Huynh, H. & Nicewicz, D. A. β-Functionalization of saturated aza-heterocycles enabled by organic photoredox catalysis. ACS Catal. 11, 3153–3158 (2021).
West, J. A. The chemistry of enamines. J. Chem. Edu. 40, 194 (1963).
Rappoport, Z. The Chemistry of Enamines (Wiley, 1994).
Roque, J. B., Kuroda, Y., Göttemann, L. T. & Sarpong, R. Deconstructive fluorination of cyclic amines by carbon–carbon cleavage. Science 361, 171–174 (2018).
Roque, J. B., Kuroda, Y., Göttemann, L. T. & Sarpong, R. Deconstructive diversification of cyclic amines. Nature 564, 244–248 (2018).
Jurczyk, J. et al. Photomediated ring contraction of saturated heterocycles. Science 373, 1004–1012 (2021).
Kerru, N., Gummidi, L., Maddila, S., Gangu, K. K. & Jonnalagadda, S. B. A review on recent advances in nitrogen-containing molecules and their biological applications. Molecules 25, 1909 (2020).
Taylor, A. P. et al. Modern advances in heterocyclic chemistry in drug discovery. Org. Biomol. Chem. 14, 6611–6637 (2016).
Su, M. & Buchwald, S. L. A bulky biaryl phosphine ligand allows for palladium-catalyzed amidation of five-membered heterocycles as electrophiles. Angew. Chem. Int. Ed. 51, 4710–4713 (2012).
Su, M., Hoshiya, N. & Buchwald, S. L. Palladium-catalyzed amination of unprotected five-membered heterocyclic bromides. Org. Lett. 16, 832–835 (2014).
Hartwig, J. F., Richards, S., Baranano, D. & Paul, F. Influences on the relative rates for C–N bond-forming reductive elimination and β-hydrogen elimination of amides. A case study on the origins of competing reduction in the palladium-catalyzed amination of aryl halides. J. Am. Chem. Soc. 118, 3626–3633 (1996).
Slagt, V. F., de Vries, A. H. M., de Vries, J. G. & Kellogg, R. M. Practical aspects of carbon–carbon cross-coupling reactions using heteroarenes. Org. Process Res. Dev. 14, 30–47 (2010).
Lad, U. P., Kulkarni, M. A., Desai, U. V. & Wadgaonkar, P. P. Lithium tetrafluoroborate catalyzed highly efficient inter- and intramolecular aza-Michael addition with aromatic amines. C. R. Chim. 14, 1059–1064 (2011).
Fedotova, A., Kondrashov, E., Legros, J., Maddaluno, J. & Rulev, A. Y. Solvent effects in the aza-Michael addition of anilines. C. R. Chim. 21, 639–643 (2018).
Schönherr, H. & Cernak, T. Profound methyl effects in drug discovery and a call for new C–H methylation reactions. Angew. Chem. Int. Ed. 52, 12256–12267 (2013).
Aynetdinova, D. et al. Installing the ‘magic methyl’—C–H methylation in synthesis. Chem. Soc. Rev. 50, 5517–5563 (2021).
Müller, K., Faeh, C. & Diederich, F. Fluorine in pharmaceuticals: looking beyond intuition. Science 317, 1881–1886 (2007).
Hagmann, W. K. The many roles for fluorine in medicinal chemistry. J. Med. Chem. 51, 4359–4369 (2008).
Zhou, Y. et al. Next generation of fluorine-containing pharmaceuticals, compounds currently in phase II–III clinical trials of major pharmaceutical companies: new structural trends and therapeutic areas. Chem. Rev. 116, 422–518 (2016).
Gritsenko, R. T. et al. Trifluoromethylation of enamines under acidic conditions. Tetrahedron Lett. 50, 2994–2997 (2009).
Lennox, A. J. J. et al. Electrochemical aminoxyl-mediated α-cyanation of secondary piperidines for pharmaceutical building block diversification. J. Am. Chem. Soc. 140, 11227–11231 (2018).
LaPlante, S. R. et al. Assessing atropisomer axial chirality in drug discovery and development. J. Med. Chem. 54, 7005–7022 (2011).
Wang, Z. et al. Recent progress toward developing axial chirality bioactive compounds. Eur. J. Med. Chem. 243, 114700 (2022).
Wang, Y.-B., Xiang, S.-H. & Tan, B. in Axially Chiral Compounds (ed Tan, B.) 297–315 (Wiley, 2021); https://doi.org/10.1002/9783527825172.ch11
Feng, T., Wang, S., Liu, Y., Liu, S. & Qiu, Y. Electrochemical desaturative β-acylation of cyclic N-aryl amines. Angew. Chem. Int. Ed. 61, e202115178 (2020).
Acknowledgements
We thank M. Gribble, E. Phillips, X. Ma, M. Mitcheltree, E. Edelstein, B. Sherry and L.-C. Campeau (Merck & Co., Inc., Rahway, NJ, USA) for helpful advice during the preparation of this manuscript. We thank D. Adpressa (Merck & Co., Inc., Rahway, NJ, USA) for preliminary NMR spectroscopy investigations. We also thank X. Wang (Merck & Co., Inc., Rahway, NJ, USA) for helpful discussions regarding NMR spectroscopy characterization.
Author information
Authors and Affiliations
Contributions
All authors have given approval to the final version of the manuscript. A.C.S. and J.W.G. conceived, performed and designed the experiments, analysed the data and wrote the manuscript. M.A.L. conceived, performed and designed the experiments. S.A.B. conceived, designed and performed the NMR spectroscopy experiments and analysed the data. J.A.N. performed the single-crystal X-ray diffraction experiments and analysed the data. Y.J. performed the high-resolution mass spectrometry experiments and analysed the data.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Synthesis thanks Bo Qu and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary handling editor: Peter Seavill, in collaboration with the Nature Synthesis team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Experimental details, Supplementary sections 1–9, Figs. 1–205, Tables 1–6 and Scheme 1.
Supplementary Data 1
Crystallographic data for 1a, CCDC 2170178.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Greenwood, J.W., Larsen, M.A., Burgess, S.A. et al. Isolable iminium ions as a platform for N-(hetero)aryl piperidine synthesis. Nat. Synth 2, 1059–1067 (2023). https://doi.org/10.1038/s44160-023-00313-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s44160-023-00313-7