Abstract
Lytic phages can be potent and selective inhibitors of microbial growth and can have profound impacts on microbiome composition and function. However, there is uncertainty about the biogeochemical conditions under which phage predation modulates microbial ecosystem function, particularly in terrestrial systems. Ionic strength is critical for infection of bacteria by many phages, but quantitative data is limited on the ion thresholds for phage infection that can be compared with environmental ion concentrations. Similarly, while carbon composition varies in the environment, we do not know how this variability influences the impact of phage predation on microbiome function. Here, we measured the half-maximal effective concentrations (EC50) of 80 different inorganic ions for the infection of E. coli with two canonical dsDNA and ssRNA phages, T4 and MS2, respectively. Many alkaline earth metals and alkali metals enabled lytic infection but the ionic strength thresholds varied for different ions between phages. Additionally, using a freshwater nitrate-reducing microbiome, we found that the ability of lytic phages to influence nitrate reduction end-products depended upon the carbon source as well as ionic strength. For all phage:host pairs, the ion EC50s for phage infection exceeded the ion concentrations found in many terrestrial freshwater systems. Thus, our findings support a model where phages most influence terrestrial microbial functional ecology in hot spots and hot moments such as metazoan guts, drought influenced soils, or biofilms where ion concentration is locally or transiently elevated and nutrients are available to support the growth of specific phage hosts.
Similar content being viewed by others
Introduction
Bacteriophages are ubiquitous in natural microbial communities and can play important roles in modulating microbial element cycling [1,2,3,4,5,6,7]. However, we lack robust mechanistic models to enable predictions of phage-host dynamics and ecological interactions, particularly in complex environments such as soils and sediments. While some studies have attempted to demonstrate an impact of phages on soil element cycling, results are variable and apparently context-dependent [1,2,3,4,5]. As such, the magnitude of the impact of phage predation on element cycling across terrestrial ecosystems and the mechanistic biogeochemistry of this process remain elusive.
In marine environments, phage lysis is responsible for ~40–50% of all bacterial mortality, approximately equivalent to the contribution of protozoal predation [6]. Carbon released from dead bacterial cells following phage predation drives a “viral shunt” in the marine carbon cycle [7]. In terrestrial systems, however, the contribution of phage to element cycling dynamics is much less well characterized and appears to be more variable, particularly in extremely heterogeneous environments such as soils [1,2,3,4,5].
In the absence of a mechanistic model of phage dynamics in terrestrial ecosystems it is difficult to interpret correlations between bacterial:viral ratios and environmental parameters to identify causal relationships [8]. Several studies have identified strong relationships between host bacterial blooms and predatory phage in natural systems [9, 10]. It thus follows that environmental parameters which influence host abundance are important for predatory phage success. For example, phage abundance can be influenced by the presence of essential microbial nutrients and energy sources required for host growth [11, 12] with the virus to microbial ratio increasing with microbial cell density [12]. Thus, in soils, because particles of detrital carbon or root exudates have higher microbial densities these should be hotspots of phage predation. Furthermore, the quality of carbon will determine the composition of the heterotrophic microbial population [13, 14]. It follows that as carbon composition shifts, the abundance of phage susceptible microbial populations will also shift to alter the possible impact of phages on microbial element cycling processes.
Inorganic ion gradients are also likely to play a major role in phage:host interactions in terrestrial ecosystems. It has been known for nearly a century that certain alkali metals and alkaline earth metals including calcium, sodium and magnesium are important for phage infectivity [15, 16]. This is in part because these cations interact with phage tail fibers to disaggregate them and enable phage binding to host cell surface receptors [17, 18]. Cations are also essential to neutralize negatively charged membranes and phage to enable phage binding [19, 20]. Most phages and bacteria are negatively charged under circumneutral environmental conditions [21]. For model phage:host pairs, greater than 1 mM Ca2+ or Mg2+ or greater than 10–40 mM Na+ was required for efficient phage binding [19]. Cations are also important for phage retention on soil and sediment particles via similar charge neutralization mechanisms [20, 22, 23]. Membrane fluidity is also influenced by ionic strength [24] and this may alter phage receptor binding, particularly for phages that bind to outer membrane lipids. Also, the persistence length of DNA packaged in phages is very sensitive to changes in ionic strength with more disordered DNA at lower ionic strength likely hampering infectivity [25,26,27]. Finally, the expression of some phage receptors are induced at higher ionic strength. The T4 phage receptor, OmpC is upregulated at ionic strengths between 10–500 mM [28] and the MS2 receptor, the type 1 conjugative pilus, is similarly upregulated with increasing ionic strength [29]. Thus, there are many indications that there are environmentally relevant ion thresholds below which phage predation will have a decreased impact on microbiomes. We anticipated that identifying these thresholds in well controlled laboratory systems would provide valuable constraints for models of environmental microbial ecology.
Measuring higher order interactions between geochemical parameters and microbial ecology requires high-throughput approaches to simulate and recapitulate environmental conditions under controlled laboratory settings [14, 30, 31]. Bacteria and phages can persist across broad ranges of ion and carbon complexity and concentration, and some studies have observed correlations between viral:host dynamics and environmental parameters [8]. However we lack detailed mechanistic understanding of how complex natural environmental gradients of carbon and inorganic ions influence phage predation. Furthermore, the use of non-standard media in previous laboratory low-throughput and low-resolution studies [15,16,17,18,19, 32] make systematic comparisons challenging.
In this study, we hypothesized that the nature and concentration of ions and carbon sources in a microbial ecosystem are major controls on phage-host dynamics and designed laboratory experiments to measure these influences on model phage:host interactions. We used the model interaction between T4 and MS2 phages and E. coli hosts to quantify inorganic ion thresholds on phage infectivity. We also used a cultured nitrate-reducing microbiome from freshwater aquatic sediment to study the higher order interactions between carbon composition, inorganic ion concentration and lytic phage in controlling microbial element cycling. We found that microbial community composition and respiratory activity is sensitive to changes in the composition and concentration of both carbon sources and inorganic ions. Specifically, we observed that phages can limit the growth and metabolic activity of populations carrying out a dominant element cycling function (dissimilatory nitrate reduction to ammonium, DNRA), but that phage predation is dependent upon both having a carbon source that can be utilized by the DNRA population and inorganic ion concentrations above that observed in most terrestrial freshwater environments. Therefore, we propose a mechanistic model in which phage predation in terrestrial systems occurs predominantly in hotspots where there are sufficient nutrients to favor host growth and inorganic ions are locally or transiently concentrated.
Results
Influence of inorganic ions and phage on the growth of model E. coli strains
To quantify the influence of inorganic ions on phage:host interactions, we first measured the growth of two model E. coli strains, BW25113 (the host for the dsDNA phage T4) and C3000 (the host for the ssRNA phage MS2) in the presence of an array of 80 different serially-diluted inorganic ions using a high-throughput assay platform [30, 31]. We then measured the growth of these host strains across the inorganic ion array in the presence of their corresponding lytic phages at a multiplicity of infection (MOI) of 1 phage per bacterial cell (Fig. 1A). Importantly, we used an ion-depleted chemically defined medium for our control cultures containing ~30 mM Na+ and sub-millimolar concentrations of other trace minerals and ions. In this ion-depleted media, E. coli growth was not inhibited by the addition of lytic phage. Thus, we were able to observe that many inorganic ions displayed similar inhibitory potencies (IC50) in the presence and absence of phages (Table S1). However, several alkali earth metals and alkali metals displayed a lower half-maximal inhibitory concentration in the presence of phage (Fig. 1B, C). These lower inhibitory potencies measured in the presence of phage represent the effective concentration required for phage infection (EC50). We also noticed a subtle, but reproducible increase in the IC50 of Al3+ in the presence of T4 phage (Fig. 1B, C). These results may suggest that phages can protect bacteria from metal toxicity in environments with high concentrations of transition metals.
A To measure inorganic ion influence on phage predation bacterial hosts are grown in an ion-depleted chemically defined medium in the presence and absence of phage and in the presence and absence of an array of inorganic ions serially diluted in microplates. Half maximal inhibitory concentrations are quantified in the presence and absence of phage to identify ion toxicity thresholds (IC50) and ion thresholds required for phage infection (EC50). B Growth of E. coli BW25113 cultures at 12 h as measured by optical density (OD 600) in the presence (closed symbols) or absence (open symbols) of T4 phage and varying concentrations of Ca2+ and Al3+ relative to control cultures lacking phage or additional ions. C Comparison of selected ion IC50 or phage-ion EC50 for E. coli BW25113 and phage T4 (black), and for E. coli C3000 and phage MS2 (red). Closed symbols indicate a significant difference between IC50/EC50 in the absence/presence of phage. D EC50 expressed as ionic strength for ions that enable lytic phage infection (open symbols = alkali metals, closed symbols = alkali earth metals).
Our dose-response assays indicate that the major divalent alkali earth metals (Ca2+ and Mg2+) and monovalent alkali metals (K+ and Na+) found in natural waters enable lytic infection by T4 and MS2 (Fig. 1C, D). Of the less common ions in natural waters, Sr2+ and Li+ enabled efficient phage infection, while Be2+, Rb+, and Cs+ were more toxic with similar inhibitory potencies in the presence and absence of phage. Cs+ and Rb+ likely interfere with K+ metabolism [33, 34] while Be2+ has the smallest ionic radius and most negative electron affinity of the alkali metal/alkali earth metals we tested. In general, MS2 phage required a lower ionic strength than was required for T4 infection (Fig. 1C, D). Of the ions that enable lytic phage infection, the monovalent ions required higher concentrations even when correcting for the difference in ionic strength conferred from the divalent ions (Fig. 1D). For example, Ca2+ EC50 were significantly lower than Na+ EC50 for both MS2 and T4. Others have reported both specific ion requirements and non-specific ion requirements for phages [35,36,37,38,39,40,41]. Compared to these previous studies, however, our results and methods show clear ion specificity across a wider range of inorganic species and concentration ranges. As such, our results demonstrate that phages differ in their ionic strength requirements and that for both MS2 and T4 phages the nature of the inorganic ion contributing to ionic strength influences the concentration of ion necessary for lytic infection. Only a small subset of ions (Ca2+, Mg2+, K+, Na+, Sr2+, Li+) enable lytic phage infection at concentrations lower than the direct ion toxicity to cells.
Influence of carbon composition and ionic strength on lytic phage modulation of microbial element cycling
We hypothesized that ionic strength also controls phage:host interactions and microbial element cycling in more complex microbiomes. To test this hypothesis we used a model nitrate-reducing microbial enrichment culture from aquatic freshwater sediment (FN enrichment culture). This cultured microbiome has diverse heterotrophic bacteria with distinct and varying respiratory capabilities including both denitrifiers and bacteria capable of dissimilatory nitrate reduction to ammonium (DNRA). Previously, we have used this microbiome to demonstrate that specific carbon sources can influence the end-products of microbial nitrate reduction by selectively enriching for microbial strains with particular respiratory enzymatic capabilities [14]. From our previous work [14], we have pure culture isolates from this enrichment including an Escherichia and two Citrobacter strains that depending on the primary electron donor/carbon source can dominate in catalyzing DNRA. To assess the phage influence on DNRA microbiome structure and function, we isolated and characterized dsDNA phages that can prey on either the Escherichia or the two Citrobacter isolates (Figure S1).
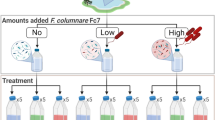
Next, we formulated a cocktail of three phage isolates each capable of lytic infection of one of the dominant DNRA organisms in the enrichment, two Citrobacter and one Escherichia, and measured the impact of this phage cocktail on ammonium production and strain composition of the enrichment culture (Fig. 2, Figure S2). We tested the addition of the phage cocktail on the enrichment culture recovered on different carbon sources/electron donors (Fig. 2A) and found that the impact of phage was greater for some carbon sources than others (Fig. 2B, C). For carbon sources in which the Escherichia or Citrobacter strains dominate, such as D-trehalose, D-glucose or D,L-lactate, the ammonium production was significantly decreased in the presence of the lytic phage cocktail (ANOVA, p < 0.05) (Fig. 2B, Figure S2). This corresponded to a subtle but significant decrease in the relative abundance of at least one of the dominant DNRA organisms. In contrast, cultures grown in D-cellobiose and L-sorbose enriched for a Klebsiella which is only capable of nitrate reduction to nitrite. Neither of the DNRA capable Citrobacter and Escherichia utilize D-cellobiose or L-sorbose and as such ammonium accumulation was already low in the absence of phages and the addition of the phage cocktails had little effect on ammonium production. Finally, ethanol and formate enriched for a Sulfurospirillum that is capable of DNRA. In these cultures, ammonium accumulation was largely driven by a strain not targeted by the lytic phage cocktail and hence was unaffected by the phage addition. Our findings demonstrate that the ability of lytic phages to influence microbiome structure and function depends upon the ability of of hosts to grow which is in turn controlled by changes in nutrients such as electron donors and carbon sources.
A A genomically-characterized nitrate-reducing microbiome is recovered in the presence of diverse carbon sources in the presence and absence of a phage cocktail formulated to target DNRA function in the microbiome. Ammonium and community composition are measured to determine the influence of each carbon source on phage modulation of the microbial element cycling function. B Ammonium production, DNRA genetic potential and relative abundances of dominant strains in the FN microbiome on selected carbon sources. Box and whiskers represent interquartile range. Shaded panels indicate significant differences (ANOVA, p < 0.05) between plus (+) and minus (−) phage conditions. In stacked bar plot: Dark Blue = Escherichia, Green = Citrobacter, Red = Klebsiella, Orange = Sulfurospirillum, Light Blue = Pseudomonas, Black = Gram-positive fermenters, Gray = other strains. C Ammonium production by the FN microbiome grown on different carbon sources in the presence and absence of phage cocktail. Colors of points reflect the dominant strain enriched on each carbon source and match color scheme in Panel A. D Dose-response assays to assess inorganic ion requirements for the phage cocktail to inhibit growth (OD 600) or ammonium production in the FN microbiome relative to ion depleted control cultures.
To test whether ionic strength influences the ability of our lytic phage cocktail to modulate the DNRA function of the freshwater microbial enrichment culture, we grew the enrichment on D-trehalose in a low ionic strength medium (~40 mM Na+) with varying concentrations of Na+ or Ca2+ (Fig. 2D). D-trehalose is produced by diverse soil microorganisms, including Enterobacteria, as an osmoprotectant under drought conditions [42]. Thus, this experiment may simulate the physiological states experienced by Enterobacteria in soils as water content and ionic strength varies. Ammonium concentrations in D-trehalose cultures were also extremely responsive to addition of the lytic phage cocktail. We measured both growth and ammonium accumulation by these cultures as a function of Na+ or Ca2+ concentration. We found that both ions inhibit ammonium accumulation in the presence of phage at lower concentrations than in the absence of phage, demonstrating their importance for lytic phage infection in this model freshwater microbiome. Furthermore, the EC50 of these ions against the FN culture were similar as those measured for MS2 or T4 (Fig. 1).
Comparison of ion concentrations necessary for phage infection with environmental ion concentrations
Having measured ion EC50 thresholds for lytic phage infection of bacterial hosts, we wondered if these concentrations are attained in terrestrial freshwaters. Na+, Ca2+ and Mg2+ concentrations in freshwaters vary by over three orders of magnitude from low ionic strength spring waters [43] to high ionic strength agricultural drainage waters [44] (Fig. 3), but ion concentrations are much higher in bacterial cytoplasm [45], the human gut or seawater [46]. For Na+, none of the freshwaters reached the necessary ion concentrations required for lytic phage infection we measured (Fig. 3A), but Na+ concentrations in the bacterial cytoplasm, human gut or seawater exceed the thresholds for phage infection that we measured. For both Ca2+ and Mg2+, a few of the extremely high ionic strength agricultural waters from tile drained soils reached the ion concentrations required for MS2 infection, but not the concentrations required for T4 or the freshwater nitrate-reducing enrichment culture phage cocktail (Fig. 3B, C). In seawater, Ca2+ and Mg2+ concentrations exceeded the MS2 phage EC50 and in the bacterial cytoplasm Mg2+ concentrations exceeded the MS2 phage EC50.
Discussion
Overall, our results along with those in the literature lead us to propose that the low ionic strength in many freshwater environments may limit successful lytic phage infection for the Enterobacterial phage host:pairs we studied. In contrast, in metazoan guts, in biofilms adjacent to recently lysed cells or in estuarine/marine environments, ionic strength is not a limitation on these phage:host interactions. While low ionic strength adaped phages may exist, our proposal is supported by ion EC50 measured for other soil and freshwater environmental phages of Bacillus and Rhodopseudomonas genera [39,40,41]. For example, Bacillus phage were shown to require cations above that found in many water systems (~1–10 mM Ca2+ or Mg2+, ~50–100 mM Na+) for maximal infectivity [41, 47]. Also, phages of the model freshwater bacterium, Caulobacter, have been shown to require Mg2+ at ~ 1 mM [48, 49]. A recent review of diverse phage studies concluded that on average 2.38 mM Ca2+, 5.08 mM Mg2+ or ~100 mM Na+ were required for efficient infection [32]. All of these measurements are consistent with the classical theoretical model that neutralization of membrane charge is required for phage binding and that this takes place >1 mM for divalent cations (e.g., Ca2+ or Mg2+) and >10 mM for monovalent cations (e.g., Na+) [19, 20]. Importantly, these concentrations are higher than are found in many freshwaters (Fig. 3). In fact the global mean Ca2+ concentration in freshwaters is ~0.1 mM and decreasing due to anthropogenic acidification [50]. Notably, while most phage lysis media rely on high concentrations of the likely non-physiological ions Ca2+ or Mg2+, our data suggests that Na+ may actually be a more important ion for enabling phage infection in many freshwaters because Ca2+ or Mg2+ concentrations are often too low to support lytic phage infection. Future work should focus on measuring ion thresholds for more phage using more standardized techniques as we have presented in this study to determine the extent to which ions are a controlling factor on their ecological range. Additionally, assessing the influence of more complex mixtures of inorganic ions and pH gradients on phage infection using high-throughput approaches as we have developed in this study will help characterize the complex higher-order interactions between geochemical context and phage:host dynamics.
A conceptual model of spatial and temporal constraints on phage dynamics in terrestrial environments
Our suggestion that most freshwater environments are sub-optimal for successful phage infection (Fig. 3) is ostensibly at odds with the prevalence of phage in some terrestrial soils [51] and lakes [8, 52]. However, in these environments, there are locations and times where ion concentrations locally or transiently will exceed the thresholds required for successful phage:host interactions. We propose that these “hot spots” and “hot moments” are critical sites of phage infection and should be considered in our conceptual models of phage ecology (Fig. 4, Figure S3).
A Major cytoplasmic ion concentrations as a function of diffusion distance from lysed E. coli cells compared with EC50 concentrations required for T4 phage infection (dashed lines) B Major ion concentrations in a typical Sierra Nevada spring water during evaporation as a function of concentration factor compared with phage EC50. Concentration factor is the ratio of the initial volume of water to the volume remaining after evaporation. Colors are consistent between EC50 and ion concentration lines. Colors and symbols are the same as in panel A. C Conceptual model for phage infection. Ionic strength in freshwater environments is too low to promote phage infection. Higher ionic strength is found in metazoan guts or brackish/marine waters and transiently observed in drought influenced soils or in a biofilm adjacent to a lysed cell. When other strains (blue) are present, such as when the dominant electron donor/carbon source or other niche dimensions shift, fewer productive phage:host interactions will proceed.
In bacterial biofilms, diffusion of ions from the cytoplasm of bacterial cells lysed by phage will locally maintain sufficient ionic strength for infection of new hosts. We can model the diffusion of these ions from cells (Fig. 4A). We know the ion concentrations in bacterial cytoplasms are ~212 mM Na+, 38 mM K+, 0.5 mM Ca2+, 1 mM Mg2+ and a cell can be approximated as a sphere with a radius of one micron. Thus, we can calculate how these concentrations will decrease if they diffuse evenly into larger volumes. Our analysis shows that beyond ~1 micron distance from lysed cells, the concentration of Na+ will drop below the EC50 for successful lytic infection by T4 phage (Fig. 4A). This suggests that phage blooms in biofilms are propagated by very local diffusion dynamics between adjacent cells. Interestingly, T4 phage begins to aggregate at concentrations near the lytic infection EC50 for Na+ [17]. As phage aggregates are more stable to environmental stressors, this may suggest a mechanism phage have evolved to survive outside of bacterial hosts at low ionic strength when infection is disfavored.
During drought, ionic solutes in soil pore water are concentrated until the solubility constants (Ksp) for insoluble inorganic compounds are exceeded (Fig. 4B). In many freshwater systems, the formation of magnesium silicates and calcium carbonates limit solubility of Ca2+ and Mg2+. Na+ and K+ salts are much more soluble and as such, the concentration of these ions will increase linearly with the concentration factor (Concentration factor is the ratio of the initial volume of water to the volume remaining after evaporation. A concentration factor of 3 means that 1/3 of the original water remains). Ion concentrations in the evaporation of a typical spring water (Sierra Nevada mountain springwater, CA) have been modeled [53] and when we overlay T4 phage EC50 on the ion concentration profiles we observe that between 100–1000 fold concentration of this water is necessary for phage to infect.
Thus, from our results and literature precedent, we propose a model in which phage infection is most favored when ionic strength is locally or transiently elevated (Fig. 4C). Some recent field observations are consistent with our model. Metagenomic surveys of soils suggest a strong influence of soil moisture on viral ecological dynamics [54,55,56]. For example, a strong distance-decay relationship was observed in similarity between viral populations across a grassland field site [54]. This could be explained by the strong limitations on viral dispersal we anticipate when freshwater ion concentrations are too low to enable infection. Other studies have observed a strong influence of soil moisture content on the viral community content [55], but this may reflect the well-established influence of drought on microbial community composition [57, 58]. Other field data support our model implicating a direct role of drought in controlling phage infection. Recently, in a timecourse from a soil undergoing rewetting after a period of drought, phage DNA was observed in the first hours after a rain event [59]. Based on our observations, this rapid phage bloom can best be explained by phage infection occurring during the early phases of the drought when host metabolism is slowing, with phage replication happening upon rewetting facilitated by the revival of host bacterial metabolism. Careful fieldwork to measure soil porewater ion concentrations and phage:host dynamics over a complete wet-dry-wet cycle would be helpful to further support this hypothesis.
Another feature of our conceptual model of phage predation is that the enrichment of non-host bacteria will decrease the probability of productive phage:host interactions. Our experiments with varying carbon sources provide support for this model. We observed that when Sulfurospirillum or Klebsiella were enriched in our cultures the predatory efficiency of the Escherichia and Citrobacter phage was decreased as well as the phage impact on DNRA activity. It follows that prebiotic nutrient amendments are potentially important to enhance the efficacy of phage cocktails, and that as carbon complexity increases (e.g., in soils) phage predation is likely to be less important in mediating microbiome composition and function. We anticipate that further work to assess how nutrient complexity influences phage:host interactions in natural systems will be important to support this hypothesis.
Conclusions
Understanding the environmental controls on phage ecology is essential for a predictive understanding of the impact of the viral fraction on biogeochemical cycling in terrestrial ecosystems. Furthermore, as there is optimism about the use of phage and other genetic editing technologies to control and manipulate microbial ecosystems, it is important to consider the conditions under which phage-based microbiome engineering will be successful. With improvements in our ability to simulate the higher order interactions between environmental parameters we can add important constraints to our models of microbial dynamics in complex terrestrial systems. We anticipate that the use of low complexity model microbiomes that are archived, genomically characterized and manipulable in laboratories in high-throughput will yield important insights into how selective pressures influence microbiome structure and element cycling function.
Materials and Methods
Cultivation of bacteria in the presence of phage and inorganic ions and dose-response analysis
To quantify the influence of inorganic ions on the growth of E. coli in the presence of T4 phage we used a low ionic strength culture media containing 30 mM PIPES buffer pH 7, 5 mM ammonium chloride, 1 mM sodium phosphate, 1 mM L-cysteine, 30 mM D-glucose and DL vitamins and minerals. All chemicals are from Sigma-Aldrich (St Louis, Mo, USA). Final Na+ concentration in this media was ~30 mM. In this culture media, phage were unable to infect and lyse E. coli in control cultures. All buffer and nutrient stock solutions were prepared with ultrapure water in plastic labware to minimize ion contamination from glassware. Cultures of E. coli recovered in LB were washed thrice in 2x concentrated medium prior to inoculation into growth assays. T4 and MS2 phage purified from E. coli LB cultures was dialyzed using Slidealyzer cassettes (Pierce, Thermo-Fisher, Waltham, MA, USA) into 30 mM PIPES buffer pH 7 to remove residual ions. Phage was mixed with E. coli cultures at a multiplicity of infection (MOI) of ~1 (108 phage per 108 bacteria) in 2x concentrated growth medium. 40 μL of phage and E. coli in 2x media were then transferred into 384 well microplates (Costar, Thermo Fisher Scientific, Waltham, MA, USA) containing 40 μL of serially-diluted aqueous solutions of inorganic ion [30, 31]. Cultures were incubated at 30 °C and shaken at 700 rpm in a multitron shaker/incubator (Infors, Annapolis Jn, MD, USA). After 12 h optical density was measured for dose-response analysis where normalized growth data was fit to a non-linear regression curve using GraphPad Prism (Graphpad software, Boston, MA, USA) to determine the half-maximal inhibitory concentrations for growth inhibition of the E. coli due to phage infection (EC50) and inorganic ion toxicity (IC50).
Phage isolation and characterization
Phages used in this study are listed in Figure S1. We enriched lab stock of T4 and MS2 phages on BW25113 and C3000 E. coli strains respectively using standard protocols [60]. All other phages were isolated from local wastewater samples (East Bay Municipal Utility District, Berkeley, CA) using E. coli and Citrobacter isolates as hosts following the standard phage isolation protocol [61]. Phage titer was estimated by spotting 10-fold serial dilution of each phage in SM buffer (Teknova) on a lawn of target host using top agar overlay method with 0.7% LB agar. For phage dilutions in plaque assays we used an SM buffer supplemented with 10 mM calcium chloride and magnesium sulfate (Sigma-Aldrich). We routinely stored phages as filter-sterilized (0.22 μm) lysates at 4 °C. For genome sequencing, phage genome was extracted using Wizard genomic DNA purification kit (Promega, Madison, WI) and sequenced at Massachusetts General Hospital next generation sequencing core facility. Genomes of both phage and bacteria used in this study are part of NCBI BioProject Accession PRJNA576510.
For transmission electron microscopy imaging, 3 µl of isolated bacteriophages were applied on a 300-mesh ultra-light carbon-coated copper grid for 5 min. The grid was briefly washed with sterile water and blotted with filter paper before staining with 3 µl of 2% aqueous uranyl-acetate. After 10 sec the grid was blotted to dryness. Preparations were examined with a 120KV Jeol1400-FLASH transmission electron microscope at Lawrence Berkeley National Laboratory. The sample was imaged at a magnification range of 12 and 50kX with the Oneview 16-Megapixel camera (Gatan®).
Cultivation of freshwater nitrate-reducing enrichment culture in the presence and absence of phage and inorganic ions
Freshwater nitrate-reducing enrichment cultures were recovered from glycerol stocks in anoxic chemically defined basal medium supplemented with 2 grams/liter yeast extract as the sole organic carbon source and electron donor and 20 mM sodium nitrate as the sole terminal electron acceptor as previously described [14]. Basal medium contained per liter: 1.5 g sodium chloride, 2.5 g ammonium chloride, 10 g sodium phosphate, 1 g potassium chloride and 30 mM HEPES buffer with vitamins and minerals added from 100x stock solutions. Vitamin stock solution contained per liter: 10 mg pyridoxine HCl, 5 mg 4-aminobenzoic acid, 5 mg lipoic acid, 5 mg nicotinic acid, 5 mg riboflavin, 5 mg thiamine HCl, 5 mg calcium D, L-pantothenate, 2 mg biotin, 2 mg folic acid, 0.1 mg cyanocobalamin. Mineral stock solution contained per liter: 3 g magnesium sulfate heptahydrate, 1.5 g nitrilotriacetic acid, 1 g sodium chloride, 0.5291 g manganese (II) chloride tetrahydrate, 0.05458 g cobalt chloride, 0.1 g zinc sulfate heptahydrate, 0.1 g calcium chloride dihydrate, 0.07153 g iron(II) chloride tetrahydrate, 0.02765 g nickel(II) sulfate hexahydrate, 0.02 g aluminum potassium sulfate dodecahydrate, 0.00683 g copper(II) chloride dihydrate, 0.01 g boric acid, 0.01 g sodium molybdate dihydrate, 0.000197 g sodium selenite pentahydrate. All chemicals are from Sigma-Aldrich.
To measure the influence of carbon sources on the end-products of the archived nitrate-reducing microbial communities, cells from recovered enrichment cultures were pelleted at 4000 RCF and washed three times with 2x concentrated basal medium lacking a carbon source. Washed cells were resuspended in 2x concentrated basal medium lacking a carbon source to an optical density (OD 600) of 0.04 and the cell suspension was transferred into either 384 well microplates (Costar) or 96 deep-well blocks (Costar) in which 94 carbon sources and water controls were arrayed (Table S1). Carbon source stock solutions were added to microplates using a Biomek FxP liquid handling robot (Beckman Coulter, Indianapolis, IN, USA) and kept in an anaerobic chamber (Coy, Coy Lab Products, Grass Lake, MI, USA) for 48 h to become anoxic prior to inoculation using an Avidien Micropro 200 pipettor (Mettler-Toledo, Columbus, OH USA). Inoculated microplates were sealed with silicon microplate seals (VWR, Radnor, PA) and incubated at 30 °C in an incubator in an anaerobic chamber (COY). Growth was monitored by optical density (OD 600) using a Tecan M1000 Pro microplate reader (Tecan Group Ltd., Männendorf, Switzerland) and cultures were harvested at 48 h for DNA sequencing and colorimetric assays to measure ammonium.
To measure the influence of inorganic ions on the efficacy of the phage cocktail, phage were dialyzed into low ionic strength buffer as described above and mixed with the washed enrichment culture and cultures containing 20 mM D-trehalose and 20 mM sodium nitrate in the presence of serially-diluted solutions of calcium chloride or sodium chloride. As above, culture were incubated for 48 h prior to measurements of optical density and ammonium.
Colorimetric assays to measure ammonium production in nitrate-reducing enrichment cultures
Ammonium production was measured as previously described [14]. Microplate seals were removed from 384-well microplates containing enrichment cultures and a Biomek FxP (Beckman Coulter) was used to transfer 4 µL of culture to assay microplates prefilled with 20 µL of distilled deionized. In sequential order, 4 µL of citrate reagent, 8 µL of salicylate/nitroprusside reagent, and 4 µL bleach reagent were added to assay plates which were then kept at 30 °C for 30 min. Citrate reagent contains 10 g trisodium citrate and 4 g sodium hydroxide in 200 mL water. Salicylate/nitroprusside reagent contains 15.626 g sodium salicylate and 0.250 g sodium nitroprusside in 200 mL water at pH 6–7. Bleach reagent contains 1 g sodium phosphate monobasic, 2 mL 2 M sodium hydroxide, 10 mL bleach (0.7 M NaOCl, Chlorox Company, Pleasanton, CA, USA) in 100 mL water at pH 12–13. For all colorimetric assays, we confirmed that interference of media additives (carbon sources and inorganic ions) was negligible. Using constants obtained from the BioNumbers database [62], we estimated the quantity of nitrogen assimilated into biomass by assuming 0.3 g/L of dry weight of bacterial culture at OD 600 = 1 [63, 64], and by assuming 12% nitrogen by weight in microbial biomass based on measured C:N:P ratios [65, 66].
16S rDNA amplicon sequencing of microbial enrichments to identify microbial community shifts associated with changes in element cycling end-products
For DNA extraction microbial cells from 500 µL cultures were pelleted by centrifugation at 4000 RCF after 48 h of growth at 30 °C. DNA (gDNA) extractions were performed as follows. Cell pellets were resuspended in 180 µL enzymatic lysis buffer (ELB) which contains 20 mM Tris-HCl pH 8.0, 1 mM sodium EDTA (TE) with 1.2% Triton X-100. 20 µL lyzosyme (25 mg/mL) was added and the samples incubated overnight at 37 °C. The following morning 20 µL of proteinase K (20 mg/mL) and 200 µL 4 M guanidinium HCL were added and the samples incubated overnight at 55 °C. The following morning 4 µL RNAse A (100 mg/mL) was added and samples incubated for 2 h at room temperature. 200 µL of ethanol was added to precipitate DNA and samples were then applied to 96 well silica membrane plates (BPI-Tech, San Diego, CA, USA). DNA bound to membranes was washed twice with 400 µL 70% ethanol:TE and eluted in 100 µL TE. An Avidien Micropro 200 (Avidien) was used to transfer large volumes of buffers and multichannel pipettes (Rainin) used to apply samples to 96 well columns and add low volume reagents. A vacuum manifold (Qiagen, Redwood City, CA, USA) was used to perform column purification steps. Extractions using this method were found to be similar in terms of DNA yield and recovery to using the Gene QIAamp 96 DNA QIAcube HT Kit (Qiagen).
gDNA template was added to a PCR reaction to amplify the V4/V5 16S gene region using the 515F/ 926R primers based on the Earth Microbiome Project primers [67, 68] but with in-line dual Illumina indexes [69, 70]. The amplicons were sequenced on an Illumina MiSeq (Illumina, San Diego, CA, USA) at the QB3 Genomics facility (QB3 Genomics, UC Berkeley, Berkeley, CA, RRID:SCR_022170) with 2×300 bp Ilumina v3 reagents. Reads were processed with custom Perl scripts implementing Pear for read merging [71], USearch [72] for filtering reads with more than one expected error, demultiplexing using inline indexes and Unoise [73] for filtering rare reads and chimeras. 16S sequences in the relative abundance table were searched against the RDP database [74] to assign taxonomy. The functional capacity for nitrate reduction enzymatic steps was determined in a previous publication [14]. The closest 16S rDNA ESVs from 16S taxonomy were matched to the GTDB-Tk [75] taxonomic bins [14].
Data Availability
DNA sequencing data are available under BioProject Accession PRJNA576510.
References
Braga LPP, Spor A, Kot W, Breuil M-C, Hansen LH, Setubal JC, et al. Impact of phages on soil bacterial communities and nitrogen availability under different assembly scenarios. Microbiome. 2020;8:1–14.
Breitbart M, Bonnain C, Malki K, Sawaya NA. Phage puppet masters of the marine microbial realm. Nat Microbiol. 2018;3:754–66.
Díaz-Muñoz SL, Koskella B. Bacteria-phage interactions in natural environments. Adv Appl Microbiol. 2014;89:135–83.
Albright MBN, Gallegos-Graves LV, Feeser KL, Montoya K, Emerson JB, Shakya M, et al. Experimental evidence for the impact of soil viruses on carbon cycling during surface plant litter decomposition. ISME Commun. 2022;2:1–8.
Clasen JL, Brigden SM, Payet JP, Suttle CA. Evidence that viral abundance across oceans and lakes is driven by different biological factors. Freshw Biol. 2008;53:1090–1100.
Fuhrman JA, Noble RT. Viruses and protists cause similar bacterial mortality in coastal seawater. Limnol Oceanogr. 1995;40:1236–42.
Puxty RJ, Millard AD, Evans DJ, Scanlan DJ. Viruses inhibit CO2 fixation in the most abundant phototrophs on earth. Curr Biol. 2016;26:1585–9.
Parikka KJ, Le Romancer M, Wauters N, Jacquet S. Deciphering the virus-to-prokaryote ratio (VPR): insights into virus-host relationships in a variety of ecosystems. Biol Rev Camb Philos Soc. 2017;92:1081–1100.
Daly RA, Roux S, Borton MA, Morgan DM, Johnston MD, Booker AE, et al. Viruses control dominant bacteria colonizing the terrestrial deep biosphere after hydraulic fracturing. Nat Microbiol. 2019;4:352–61.
Faruque SM, Naser IB, Islam MJ, Faruque ASG, Ghosh AN, Nair GB, et al. Seasonal epidemics of cholera inversely correlate with the prevalence of environmental cholera phages. Proc Natl Acad Sci USA. 2005;102:1702–7.
Modin O, Fuad N, Abadikhah M, I’Ons D, Ossiansson E, Gustavsson DJI, et al. A relationship between phages and organic carbon in wastewater treatment plant effluents. Water Res X. 2022;16:100146.
Wigington CH, Sonderegger D, Brussaard CPD, Buchan A, Finke JF, Fuhrman JA, et al. Re-examination of the relationship between marine virus and microbial cell abundances. Nat Microbiol. 2016;1:15024.
Zhalnina K, Louie KB, Hao Z, Mansoori N, da Rocha UN, Shi S, et al. Dynamic root exudate chemistry and microbial substrate preferences drive patterns in rhizosphere microbial community assembly. Nat Microbiol. 2018;3:470–80.
Carlson HK, Lui LM, Price MN, Kazakov AE, Carr AV, Kuehl JV, et al. Selective carbon sources influence the end products of microbial nitrate respiration. ISME J. 2020;14:2034–45.
Stassano H, de Beaufort AC. Action du citrate de soude sur la principe lytique transmissible. CR Soc Biol. 1925;93:1380–2.
Lark KG, Adams MH. The stability of phages as a function of the ionic environment. Cold Spring Harb Symp Quant Biol. 1953;18:171–83.
Szermer-Olearnik B, Drab M, Mąkosa M, Zembala M, Barbasz J, Dąbrowska K, et al. Aggregation/dispersion transitions of T4 phage triggered by environmental ion availability. J Nanobiotechnology. 2017;15:32.
Conley MP, Wood WB. Bacteriophage T4 whiskers: a rudimentary environment-sensing device. Proc Natl Acad Sci USA. 1975;72:3701–5.
Beumer J, Dirkx J, Beumer-Jochman MP. Role of cations in phage adsorption to sensitive bacteria. Nature. 1957;180:83–85.
Zemb O, Manefield M, Thomas F, Jacquet S. Phage adsorption to bacteria in the light of the electrostatics: a case study using E. coli, T2 and flow cytometry. J Virol Methods. 2013;189:283–9.
Michen B, Graule T. Isoelectric points of viruses. J Appl Microbiol. 2010;109:388–97.
Pham M, Mintz EA, Nguyen TH. Deposition kinetics of bacteriophage MS2 to natural organic matter: role of divalent cations. J Colloid Interface Sci. 2009;338:1–9.
Valentine RC, Allison AC. Virus particle adsorption. I. Theory of adsorption and experiments on the attachment of particles to non-biological surfaces. Biochim Biophys Acta. 1959;34:10–23.
Quinn PJ. Models of haloadaptation in bacterial membranes. FEMS Microbiol Rev. 1986;2:87–94.
Bauer DW, Evilevitch A. Influence of internal DNA pressure on stability and infectivity of phage λ. J Mol Biol. 2015;427:3189–3200.
Li D, Liu T, Zuo X, Li T, Qiu X, Evilevitch A. Ionic switch controls the DNA state in phage λ. Nucleic Acids Res. 2015;43:6348–58.
Liu T, Sae-Ueng U, Li D, Lander GC, Zuo X, Jönsson B, et al. Solid-to-fluid-like DNA transition in viruses facilitates infection. Proc Natl Acad Sci USA. 2014;111:14675–80.
Villarejo M, Davis JL, Granett S. Osmoregulation of alkaline phosphatase synthesis in Escherichia coli K-12. J Bacteriol. 1983;156:975–8.
Schwan WR, Lee JL, Lenard FA, Matthews BT, Beck MT. Osmolarity and pH growth conditions regulate fim gene transcription and type 1 pilus expression in uropathogenic Escherichia coli. Infect Immun. 2002;70:1391–402.
Carlson HK, Price MN, Callaghan M, Aaring A, Chakraborty R, Liu H, et al. The selective pressures on the microbial community in a metal-contaminated aquifer. ISME J. 2019;13:937–49.
Carlson H, Deutschbauer A, Coates J. Microbial metal resistance and metabolism across dynamic landscapes: high-throughput environmental microbiology. F1000Res. 2017.
Christi K, Elliman J, Owens L A synthesis of the divalent cation requirements for efficient adsorption of bacteriophage onto bacterial cells. In: Bacteriophages An Overview And Synthesis of A Re-Emerging Field. 2017. pp 43–70.
Bronowska M, Stęborowski R, Bystrzejewska-Piotrowska G. Estimation of the acute cesium toxicity by the microbial assay for risk assessment (MARA) test. Nukleonika 2013;58:481–85.
Avery SV. Caesium accumulation by microorganisms: uptake mechanisms, cation competition, compartmentalization and toxicity. J Ind Microbiol. 1995;14:76–84.
Garen A, Puck TT. The first two steps of the invasion of host cells by bacterial viruses. II. J Exp Med. 1951;94:177–89.
Tzagoloff H, Pratt D. The initial steps in infection with coliphage M13. Virology. 1964;24:372–80.
Danziger RE, Paranchych W. Stages in phage R17 infection. Virology. 1970;40:554–64.
Sinsheimer RL Bacteriophage ϕx174 and Related Viruses. In: Davidson JN, Cohn WE (eds). Progress in Nucleic Acid Research and Molecular Biology. 1968. Academic Press, pp 115–69.
Bertani G, Choe BK, Lindahl G. Calcium sensitive and other mutants of bacteriophage P2. J Gen Virol. 1969;5:97–104.
Abeliovich A, Kaplan S. Bacteriophages of Rhodopseudomonas spheroides: isolation and characterization of a Rhodopseudomonas spheroides bacteriophage. J Virol. 1974;13:1392–9.
Landry EF, Zsigray RM. Effects of calcium on the lytic cycle of Bacillus subtilis phage 41c. J Gen Virol. 1980;51:125–35.
Purvis JE, Yomano LP, Ingram LO. Enhanced trehalose production improves growth of Escherichia coli under osmotic stress. Appl Environ Microbiol. 2005;71:3761–9.
Azoulay A, Garzon P, Eisenberg MJ. Comparison of the mineral content of tap water and bottled waters. J Gen Intern Med. 2001;16:168–75.
Rhoades JD. Use of saline drainage water for irrigation. Agronomy Monographs. 2015. American Society of Agronomy, Crop Science Society of America, Soil Science Society of America, Madison, WI, USA, pp 615–57.
Szatmári D, Sárkány P, Kocsis B, Nagy T, Miseta A, Barkó S, et al. Intracellular ion concentrations and cation-dependent remodelling of bacterial MreB assemblies. Sci Rep. 2020;10:12002.
Hovanec TA, Coshland JL. A chemical analysis of select trace elements in synthetic sea salts and natural seawater. Sea Scope. Aquarium Syst. 2004;21:1–6.
Bandara N, Jo J, Ryu S, Kim K-P. Bacteriophages BCP1-1 and BCP8-2 require divalent cations for efficient control of Bacillus cereus in fermented foods. Food Microbiol. 2012;31:9–16.
Johnson RC, Wood NB, Ely B. Isolation and characterization of bacteriophages for Caulobacter crescentus. J Gen Virol. 1977;37:323–35.
Inakaren B, Shapiro L. Properties of caulobacter ribonucleic acid bacteriophage φCb5. J Virol. 1970;6:847–54.
Weyhenmeyer GA, Hartmann J, Hessen DO, Kopáček J, Hejzlar J, Jacquet S, et al. Widespread diminishing anthropogenic effects on calcium in freshwaters. Sci Rep. 2019;9:10450.
Williamson KE. Soil Phage Ecology: Abundance, Distribution, and Interactions with Bacterial Hosts. In: Witzany G, (ed). Biocommunication in Soil Microorganisms. Berlin, Heidelberg: Springer Berlin Heidelberg; 2011. p. 113–36.
Bergh O, Børsheim KY, Bratbak G, Heldal M. High abundance of viruses found in aquatic environments. Nature. 1989;340:467–8.
Garrels RM, Mackenzie FT. Origin of the chemical compositions of some springs and lakes. Equilibrium Concepts in Natural Water Systems. 1967. American Chemical Society, pp 222–42.
Santos-Medellín C, Estera-Molina K, Yuan M, Pett-Ridge J, Firestone MK, Emerson JB Spatial turnover of soil viral populations and genotypes overlain by cohesive responses to moisture in grasslands. bioRxiv . 2022. 2022.03.24.485562
Wu R, Davison MR, Gao Y, Nicora CD, Mcdermott JE, Burnum-Johnson KE, et al. Moisture modulates soil reservoirs of active DNA and RNA viruses. Commun Biol. 2021;4:992.
Van Goethem MW, Swenson TL, Trubl G, Roux S, Northen TR. Characteristics of wetting-induced bacteriophage blooms in biological soil crust. MBio 2019;10:10–1128
Naylor D, DeGraaf S, Purdom E, Coleman-Derr D. Drought and host selection influence bacterial community dynamics in the grass root microbiome. ISME J. 2017;11:2691–704.
Santos-Medellín C, Liechty Z, Edwards J, Nguyen B, Huang B, Weimer BC, et al. Prolonged drought imparts lasting compositional changes to the rice root microbiome. Nat Plants. 2021;7:1065–77.
Nicolas AM, Sieradzki ET, Pett-Ridge J, Banfield JF, Taga ME, Firestone MK, et al. Isotope-enrichment reveals active viruses follow microbial host dynamics during rewetting of a California grassland soil. bioRxiv. 2022. 2022.09.30.510406
Bonilla N, Rojas MI, Netto Flores Cruz G, Hung S-H, Rohwer F, Barr JJ. Phage on tap-a quick and efficient protocol for the preparation of bacteriophage laboratory stocks. PeerJ. 2016;4:e2261.
Van Twest R, Kropinski AM. Bacteriophage enrichment from water and soil. In: Clokie MRJ, Kropinski AM (eds). Bacteriophages: Methods and Protocols, Volume 1: Isolation, Characterization, and Interactions. 2009. Humana Press, Totowa, NJ, pp 15–21.
Milo R, Jorgensen P, Moran U, Weber G, Springer M. BioNumbers—the database of key numbers in molecular and cell biology. Nucleic Acids Res. 2009;38:D750–D753.
Soini J, Ukkonen K, Neubauer P. High cell density media for Escherichia coli are generally designed for aerobic cultivations – consequences for large-scale bioprocesses and shake flask cultures. Microbial Cell Factories. 2008;7:1-11.
Glazyrina J, Materne E-M, Dreher T, Storm D, Junne S, Adams T, et al. High cell density cultivation and recombinant protein production with Escherichia coli in a rocking-motion-type bioreactor. Microb Cell Fact. 2010;9:42.
Redfield AC. The biological control of chemical factors in the environment. Am Sci. 1958;46:230A–221.
Paul EA 1 - Soil microbiology, ecology, and biochemistry in perspective. In: Paul EA (ed). Soil Microbiology, Ecology and Biochemistry (Third Edition). 2007. Academic Press, San Diego, pp 3–24.
Parada AE, Needham DM, Fuhrman JA. Every base matters: assessing small subunit rRNA primers for marine microbiomes with mock communities, time series and global field samples. Environ Microbiol. 2016;18:1403–14.
Quince C, Lanzen A, Davenport RJ, Turnbaugh PJ. Removing noise from pyrosequenced amplicons. BMC Bioinformatics. 2011;12:38.
Sharpless W, Sander K, Song F, Kuehl J, Arkin AP. Towards environmental control of microbiomes. bioRxiv . 2022. 2022.11.04.515211
Price MN, Wetmore KM, Waters RJ, Callaghan M, Ray J, Liu H, et al. Mutant phenotypes for thousands of bacterial genes of unknown function. Nature. 2018;557:503–9.
Zhang J, Kobert K, Flouri T, Stamatakis A. PEAR: a fast and accurate Illumina Paired-End reAd mergeR. Bioinformatics. 2014;30:614–20.
Edgar RC. Search and clustering orders of magnitude faster than BLAST. Bioinformatics. 2010;26:2460–1.
Edgar RC. UNOISE2: improved error-correction for Illumina 16S and ITS amplicon sequencing. bioRxiv . 2016. 081257
Cole JR, Wang Q, Fish JA, Chai B, McGarrell DM, Sun Y, et al. Ribosomal Database Project: data and tools for high throughput rRNA analysis. Nucleic Acids Res. 2014;42:D633–42.
Parks DH, Chuvochina M, Waite DW, Rinke C, Skarshewski A, Chaumeil P-A, et al. A standardized bacterial taxonomy based on genome phylogeny substantially revises the tree of life. Nat Biotechnol. 2018;36:996–1004.
Funding
This work was funded by ENIGMA, a Scientific Focus Area Program supported by the U.S. Department of Energy, Office of Science, Office of Biological and Environmental Research, Genomics: GTL Foundational Science through contract DE-AC02-05CH11231 between Lawrence Berkeley National Laboratory and the U.S. Department of Energy. Initial part of this research was supported by the DOE Office of Science through the National Virtual Biotechnology Laboratory, a consortium of DOE national laboratories focused on response to COVID-19, with funding provided by the Coronavirus CARES Act.
Author information
Authors and Affiliations
Contributions
HKC and VKM conceived of the experiments and wrote the manuscript. HKC, VKM, DP, MLM, NLE and RTP conducted experiments. All authors edited and revised the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Carlson, H.K., Piya, D., Moore, M.L. et al. Geochemical constraints on bacteriophage infectivity in terrestrial environments. ISME COMMUN. 3, 78 (2023). https://doi.org/10.1038/s43705-023-00297-7
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s43705-023-00297-7
This article is cited by
-
Soil properties that affect the adsorption of ΦITL-1 and ΦRSP bacteriophages
Journal of Soils and Sediments (2024)