Abstract
Skeletal muscle plays a central role in the regulation of systemic metabolism during lifespan. With aging, this function is perturbed, initiating multiple chronic diseases. Our knowledge of mechanisms responsible for this decline is limited. Glycerophosphocholine phosphodiesterase 1 (Gpcpd1) is a highly abundant muscle enzyme that hydrolyzes glycerophosphocholine (GPC). The physiological functions of Gpcpd1 remain largely unknown. Here we show, in mice, that the Gpcpd1–GPC metabolic pathway is perturbed in aged muscles. Further, muscle-specific, but not liver- or fat-specific, inactivation of Gpcpd1 resulted in severely impaired glucose metabolism. Western-type diets markedly worsened this condition. Mechanistically, Gpcpd1 muscle deficiency resulted in accumulation of GPC, causing an ‘aged-like’ transcriptomic signature and impaired insulin signaling in young Gpcpd1-deficient muscles. Finally, we report that the muscle GPC levels are markedly altered in both aged humans and patients with type 2 diabetes, displaying a high positive correlation between GPC levels and chronological age. Our findings reveal that the muscle GPCPD1–GPC metabolic pathway has an important role in the regulation of glucose homeostasis and that it is impaired during aging, which may contribute to glucose intolerance in aging.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The authors declare that the data supporting the findings of this study are available within the paper and its source data files, and are available from the corresponding authors upon request. Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contacts Josef M. Penninger (josef.penninger@ubc.ca) or Domagoj Cikes (domagoj.cikes@imba.oeaw.ac.at). The original datasets used in the RNA sequencing analysis can be accessed at National Center for Biotechnology Information archived under Gene Expression Omnibus accession codes GSE158850 and GSE40551 for human studies. For the mouse study, the quant-seq analysis can be obtained under the accession number GSE245285. For pan tissue analysis in Extended Data Fig. 2, GeneAtlas MOE430, probeset 1429144_at on GeneChip HT Mouse Genome MG-430A (Mus musculus) from the BioGPS portal (http://biogps.org/#goto=welcome) was used. Mass spectrometry lipidomics source data for Extended Data Fig. 6 is provided under a supplementary data file with the paper. Proteomics data can be accessed at PRIDE, with accession code PXD047373.
References
Cruz-Jentoft, A. J. & Sayer, A. A. Sarcopenia. Lancet 393, 2636–2646 (2019).
Yang, M., Jiang, J., Zeng, Y. & Tang, H. Sarcopenia for predicting mortality among elderly nursing home residents: SARC-F versus SARC-CalF. Medicine 98, e14546 (2019).
Yanagi, N. et al. Assessment of sarcopenia in the intensive care unit and 1-year mortality in survivors of critical illness. Nutrients 13, 2726 (2021).
Baskin, K. K., Winders, B. R. & Olson, E. N. Muscle as a ‘mediator’ of systemic metabolism. Cell Metab. 21, 237–248 (2015).
Jue, T. et al. Direct observation of glycogen synthesis in human muscle with 13C NMR. Proc. Natl Acad. Sci. USA 86, 4489–4491 (1989).
DeFronzo, R. A. et al. The effect of insulin on the disposal of intravenous glucose. Results from indirect calorimetry and hepatic and femoral venous catheterization. Diabetes 30, 1000–1007 (1981).
Demontis, F., Piccirillo, R., Goldberg, A. L. & Perrimon, N. Mechanisms of skeletal muscle aging: insights from Drosophila and mammalian models. Dis. Model Mech. 6, 1339–1352 (2013).
Kern, M., Dolan, P. L., Mazzeo, R. S., Wells, J. A. & Dohm, G. L. Effect of aging and exercise on GLUT-4 glucose transporters in muscle. Am. J. Physiol. 263, E362–E367 (1992).
Chia, C. W., Egan, J. M. & Ferrucci, L. Age-related changes in glucose metabolism, hyperglycemia, and cardiovascular risk. Circ. Res. 123, 886–904 (2018).
Cowie, C. C. et al. Full accounting of diabetes and pre-diabetes in the US population in 1988–1994 and 2005–2006. Diabetes Care 32, 287–294 (2009).
Corda, D. et al. The emerging physiological roles of the glycerophosphodiesterase family. FEBS J. 28, 998–1016 (2014).
Okazaki, Y. et al. A novel glycerophosphodiester phosphodiesterase, GDE5, controls skeletal muscle development via a non-enzymatic mechanism. J. Biol. Chem. 285, 27652–27663 (2010).
Stewart, J. D. et al. Choline-releasing glycerophosphodiesterase EDI3 drives tumor cell migration and metastasis. Proc. Natl Acad. Sci. USA 109, 8155–8160 (2012).
Houtkooper, R. H. et al. The metabolic footprint of aging in mice. Sci. Rep. 1, 134 (2011).
Pilling, L. C. et al. Human longevity is influenced by many genetic variants: evidence from 75,000 UK Biobank participants. Aging 8, 547–560 (2016).
Shavlakadze, T. et al. Age-related gene expression signature in rats demonstrate early, late, and linear transcriptional changes from multiple tissues. Cell Rep. https://doi.org/10.1016/j.celrep.2019.08.043 (2019).
Bruning, J. C. & Michael, M. D. A muscle-specific insulin receptor knockout exhibits features of metabolic syndrome. Mol. Cell 2, 559–569 (1998).
Fagone, P. & Jackowski, S. Phosphatidylcholine and the CDP-choline cycle. Biochim. Biophys. Acta 1831, 523–532 (2013).
Gallazzini, M., Ferraris, J. D. & Burg, M. B. GDPD5 is a glycerophosphocholine phosphodiesterase that osmotically regulates the osmoprotective organic osmolyte GPC. Proc. Natl Acad. Sci. USA 105, 11026–11031 (2008).
Demontis, F., Piccirillo, R., Goldberg, A. L. & Perrimon, N. The influence of skeletal muscle on systemic aging and lifespan. Aging Cell 12, 943–949 (2013).
Bolognesi, B. & Lehner, B. Protein overexpression: reaching the limit. eLife 7, e39804 (2018).
Moriya, H. Quantitative nature of overexpression experiments. Mol. Biol. Cell 26, 3932–3939 (2015).
Kulak, N. A., Pichler, G., Paron, I., Nagaraj, N. & Mann, M. Minimal, encapsulated proteomic-sample processing applied to copy-number estimation in eukaryotic cells. Nat. Methods 11, 319–324 (2014).
Matsubara, K. et al. The delaying effect of alpha-glycerophosphocholine on senescence, transthyretin deposition, and osteoarthritis in senescence-accelerated mouse prone 8 mice. Biosci. Biotechnol., Biochem. 82, 647–653 (2018).
Park, S. S. & Seo, Y. K. Excess accumulation of lipid impairs insulin sensitivity in skeletal muscle. Int. J. Mol. Sci. 21, 1949 (2020).
Wu, H. & Ballantyne, C. M. Skeletal muscle inflammation and insulin resistance in obesity. J. Clin. Invest. 127, 43–54 (2017).
Rovira Gonzalez, Y. I. et al. Mss51 deletion enhances muscle metabolism and glucose homeostasis in mice. JCI Insight 4, e122247 (2019).
Fujita, R. et al. Zmynd17 controls muscle mitochondrial quality and whole-body metabolism. FASEB J. 32, 5012–5025 (2018).
Gual, P. et al. Hyperosmotic stress inhibits insulin receptor substrate-1 function by distinct mechanisms in 3T3-L1 adipocytes. J. Biol. Chem. 278, 26550–26557 (2003).
Chen, D., Fucini, R. V., Olson, A. L., Hemmings, B. A. & Pessin, J. E. Osmotic shock inhibits insulin signaling by maintaining Akt/protein kinase B in an inactive dephosphorylated state. Mol. Cell. Biol. 19, 4684–4694 (1999).
Stookey, J., Pieper, C. & Cohen, H. Hypertonic hyperglycemia progresses to diabetes faster than normotonic. Eur. J. Epidemiol. 19, 935–944 (2004).
Lee, P. G. & Halter, J. B. The pathophysiology of hyperglycemia in older adults: clinical considerations. Diabetes Care 40, 444–452 (2017).
Lattin, J. E. et al. Expression analysis of G protein-coupled receptors in mouse macrophages. Immunome Res. 4, 5 (2008).
Wu, C., Jin, X., Tsueng, G., Afrasiabi, C. & Su, A. I. BioGPS: building your own mash-up of gene annotations and expression profiles. Nucleic Acids Res. 44, D313–D316 (2016).
Dorfer V., et al.MS Amanda, a universal identification algorithm optimized for high accuracy tandem mass spectra. J. Proteome Res. 13, 3679–3684 (2014).
Käll, L., Canterbury, J. D., Weston, J., Noble, W. S. & MacCoss, M. J. Semi-supervised learning for peptide identification from shotgun proteomics datasets. Nat. Methods 4, 923–925 (2007).
Taus, T. et al. Universal and confident phosphorylation site localization using phosphoRS. J. Proteome Res. 10, 5354–5362 (2011).
Doblmann, J. et al. ApQuant: accurate label-free quantification by quality filtering. J. Proteome Res. 18, 535–541 (2019).
Schwanhüusser, B. et al. Global quantification of mammalian gene expression control. Nature 473, 337–342 (2011).
Cox, J. et al. Accurate proteome-wide label-free quantification by delayed normalization and maximal peptide ratio extraction, termed MaxLFQ. Mol. Cell. Proteomics 13, 2513–2526 (2014).
Laurens, C. et al. G0/G1 Switch Gene 2 controls adipose triglyceride lipase activity and lipid metabolism in skeletal muscle. Mol. Metab. 5, 527–537 (2016).
Laurens, C. et al. Perilipin 5 fine-tunes lipid oxidation to metabolic demand and protects against lipotoxicity in skeletal muscle. Sci. Rep. 6, 38310 (2016).
Kraegen, E. W., James, D. E., Jenkins, A. B. & Chisholm, D. J. Dose–response curves for in vivo insulin sensitivity in individual tissues in rats. Am. J. Physiol. 11, E353–E362 (1985).
Bergström, J. & Hultman, E. A study of the glycogen metabolism during exercise in man. Scand. J. Clin. Lab. Invest. https://doi.org/10.3109/00365516709090629 (1967).
Besse-Patin, A. et al. Effect of endurance training on skeletal muscle myokine expression in obese men: identification of apelin as a novel myokine. Int. J. Obes. 38, 707–713 (2014).
Vion, J. et al. Metabolic and cardiovascular adaptations to an 8-wk lifestyle weight loss intervention in younger and older obese men. Am. J. Physiol. 321, E325–E337 (2021).
Cikes, D. et al. PCYT2-regulated lipid biosynthesis is critical to muscle health and ageing. Nat. Metab. https://doi.org/10.1038/s42255-023-00766-2 (2023).
Acknowledgements
We thank all members of our laboratories for helpful discussions. We are grateful to the Transgenic Unit, Comparative Medicine and Metabolomics Unit from Vienna Biocenter Core Facilities for their service. We also thank M.G. from the Histopathology unit in Vienna Biocenter Core Facilities for histological processing. We also thank the lipidomic service of Center for Molecular Medicine. We thank P. Krumpolec from the Biomedical Research Center, Institute of Experimental Endocrinology, Slovak Academy of Sciences, Bratislava, Slovakia, for the processing of MRS data from young and senior participants. We thank S. Trattnig, High Field MR Centre, Department of Biomedical Imaging and Image Guided Therapy for logistic support. We thank J. D. Johnson from the University of British Columbia, Vancouver, Canada for critical reading of the manuscript. J.M.P. is supported by the Institute of Molecular Biotechnology of Austrian Academy of Sciences, a Wittgenstein award, the T. von Zastrow foundation and a Canada 150 Research Chair in functional genetics. D.C. is supported by the Austrian Academy of Sciences and the T. von Zastrow foundation. M. Krssak, R.K. and L.P. are supported by Austrian Science Foundation (KLI-904-B). The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
D.C. and J.M.P. conceived, coordinated and designed the study. D.C. and M.O. performed experiments and analyzed the data with contributions from M.L. and S.J.F.C. M.N. performed the bioinformatic analysis. A.H. assisted in tissue sampling. E. Ru. and T.G. collected human muscle biopsies. L.P., R.K. and M. Krssak performed and analyzed in vivo MRS measurements. B.L., M.L., C.B., C.K., G.T., V.B. and C.M. performed glucose uptake experiments. N.V. generated and analyzed mRNA levels in the clinical cohort. G.D. and E. Rö. performed mass spectrometry experiments. M.G. performed histological processing and stainings. M.L., M. Krebs, M. Krssak and A.K.-W. designed, coordinated and oversaw the human T2DM experiments. D.C. and J.M.P. wrote the manuscript. All authors edited the manuscript and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors have declared that no conflict of interest exists.
Peer review
Peer review information
Nature Aging thanks Thomas Jensen and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Assessment of age-related Gpcpd1 levels in rat tissues.
Correlation of mRNA expression of Gpcpd1 in the indicated rat tissues with age. Data are shown as means ± SEM. Student’s two tailed, unpaired t-test was used for statistical analysis unless otherwise stated. There were no statistically significant differences.
Extended Data Fig. 2 Gpcpd1 mRNA expression across different cell and tissue type.
Gpcpd1 mRNA transcript per million in different tissues and cell types. Data represent mean Gpcpd1expression levels (z-scores) ± SD of from microarray analysis. A value bigger than 5 indicates expression in the corresponding tissue.
Extended Data Fig. 3 Validation of Gpcpd1 protein disruption in the skeletal muscle from MckCre-Gpcpd1flox/flox mice by mass spectrometry.
a) Scheme of the KO strategy upon Cre activation b) Representative extracted ion chromatograms (XICs) of Gpcpd1 peptides downstream of exon 8 of the Gpcpd1 locus from skeletal and heart muscle proteome of Control and MckCre-Gpcpd1flox/flox mice. Integrated signal used for quantification is indicated in grey. Peptide identifications are represented by red dashed lines. XICs from N = 3 mice per group are shown. c) RT-PCR based validation of Gpcpd1 deletion from various tissues in the Mck-CreGpcpd1flox/flox mice. N = 5 animals per each group. Data are shown as means ± SEM. Student’s two tailed, unpaired t-test was used for statistical analysis. ***p < 0.001, ****p < 0.0001.
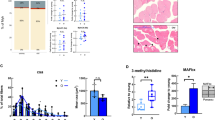
Extended Data Fig. 4 Grip strength, muscle weights, fiber type, and muscle ultrastructure assessment of Mck-CreGpcpd1flox/flox mice.
a) Skeletal muscle weights (quadriceps, QA; gastrocnemicus, GC; and Tibialis anterior, TA) of 20 months old Control and Mck-CreGpcpd1flox/flox mice N = 6 per group, respectively. b) Representative images and quantification of MyHC!, MyhCIIA and MyHCIIB fibers in skeletal muscle (quadriceps) from 20 months old Control and Mck-CreGpcpd1flox/flox mice. Images were taken under 5x magnification, and ≥100 myofibers were counted at 3 different matching histological areas. N = 4 animals per group. Scale bar 500µm. c) Representative cross sections of skeletal muscle (quadriceps) and myofiber diameter size from 20 months old Control (N = 570 myofibers) and Mck-CreGpcpd1flox/flox mice (N = 640 myofibers). Myofibers were imaged using 10X magnification with ≥ 100 myofibers analyzed per mouse, isolated from 4 Control and 4 Mck-CreGpcpd1flox/flox mice. Scale bar 100µm. d) Ultrastructure of skeletal muscle (quadriceps) and e) intermyofibrillar mitochondria from 20 months old control and Mck-CreGpcpd1flox/flox mice. No apparent structural defects were observed in the muscles of Mck-CreGpcpd1flox/flox mice. n = 3 animals per group. Scale bar = 1µm and scale bar = 500nm respectively. f, g) Muscle strength evaluation of 20 months old Control (N = 6) and MckCre-Gpcpd1 flox/flox mice (N = 9). Unless otherwise stated, each dot represents one individual animal. Data are shown as means ± SEM. Student’s two tailed, unpaired t-test with Welch correction was used for statistical analysis unless otherwise stated, ns, not significant.
Extended Data Fig. 5 Fasting glucose levels and tissue glucose uptake in Mck-CreGpcpd1flox/flox mice.
a) Fasting blood glucose levels in 12 weeks old Control (N = 16) and Mck-CreGpcpd1flox/flox mice (N = 17). b) Blood glucose levels and c) Area under curve (AUC) after an oral glucose tolerance test (OGTT) in 20 month old months old Control and MckCre-Gpcpd1flox/flox mice. N = 6 per each group. Student’s two tailed un-paired t test with Welch correction was used for AUC statistical analysis. d) and e) Radioactive 2DG glucose uptake in heart muscle of 12 weeks old Control (N = 6) and Mck-CreGpcpd1flox/flox mice (N = 11) after bolus glucose feeding. f) Radioactive 2DG glucose uptake in white fat (eWAT), beige fat (iWAT), brown fat (BAT), and liver of 12 weeks old control (N = 6) and Mck-CreGpcpd1flox/flox mice (N = 11) after bolus glucose feeding. Each dot represents one individual animal. Data are shown as means ± SEM. Student’s two tailed, unpaired t-test with Welch correction was used for statistical analysis unless otherwise stated; ns, not significant; *p < 0.05, **p < 0.01, ***p < 0.001.
Extended Data Fig. 6 Muscle lipidome evaluation upon Gpcpd1 loss and C2C12 derived myotubes GPC treatment.
a) Mass spectrometry based un-targeted lipidomic analysis in quadriceps muscles isolated from 3 months old Control and Mck-Cre-Gpcpd1flox/flox littermate mice. Abbreviations denote triacylglycerols (TAG), sphingomyelins (SM), phosphatidylinositols (PI), phosphatydilethanolamines (PE), phosphatydilcholines (PC), lysophosphatydilethanolamines (LPE), lysophosphatydilcholines (LPC), diacylglycerols (DAG), ceramides (Cer), and cholesterolesther (CE). Numbers denote the carbon numbers, heatmap denotes higher and lower abundant lipid species in the muscles. No significant differences were found in the indicated lipid species abundance between muscles isolated from Control and Mck-Cre-Gpcpd1flox/flox mice. N = 5 per group. b) Choline levels in skeletal muscle (quadriceps) of 3 months old Control (N = 10) and MckCre-Gpcpd1flox/flox mice (N = 12). c) Correlation between tissue osmolarity and rising GPC levels in young and old mice. Each dot represents individual mice. d) GPC levels in C2C12 differentiated myotubes without (N = 5) or with GPC pretreatment (N = 5). Each dot represents one cell culture; error bars depict standard deviation. e) Cellular osmolarity of C2C12 derived myofibers treated with vehicle (N = 9) or GPC (N = 9) for 7 days ; error bars depict ; error bars depict standard deviation. f) Glucose uptake of C2C12 differentiated myotubes treated with vehicle or GPC for 7 days. Each dot represents one cell culture replicate (N = 6 per each condition); error bars depict Standard Deviation. Two-way ANOVA followed by Tukey’s multiple comparison test was used to analyze this data set. Data are shown as means ± SEM unless stated otherwise. Student’s two tailed, unpaired t-test was used for statistical analysis unless otherwise stated, ns, not significant, ****p < 0.0001.
Extended Data Fig. 7 Insulin signaling pathway s is affected in muscles from young Mck-CreGpcpd1flox/flox mice.
Analysis of insulin signaling network on transcriptional level in quadriceps from young 3 months old Control and MckCre-Gpcpd1flox/flox mice. The significantly downregulated genes (P < 0,05) are labelled in green.
Extended Data Fig. 8 Muscles from young Mck-CreGpcpd1flox/flox mice display an ‘aged-like’ transcriptomic signature.
Comparison of upregulated (red) and downregulated (blue) gene expression changes in aged skeletal muscles in rats (27 month old versus 6 month old) 16 with gene expression changes of skeletal muscles from 3 month old Control and Mck-CreGpcpd1flox/flox mice. Overlapping dysregulated genes are highlighted.
Extended Data Fig. 9 Correlation of GPC and GPC-PDE levels in humans with fat mass, muscle mass and body mass index.
Correlation of skeletal muscle Glycerophosphocholine (GPC) and Glycerophosphocholine phosphodiester (GPC-PDE) levels with fat mass, muscle mass and body mass index in humans, irrespective of age. No significant correlation was found for any of the parameters. Two-tailed Person correlation test was used for the analysis.
Supplementary information
Supplementary Data
Untargeted lipidome analysis of skeletal muscle at P10.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 4
Unprocessed western blots.
Source Data Fig. 5
Statistical source data.
Source Data Extended Data Fig. 1
Statistical source data.
Source Data Extended Data Fig. 3
Statistical source data.
Source Data Extended Data Fig. 4
Statistical source data.
Source Data Extended Data Fig. 5
Statistical source data.
Source Data Extended Data Fig. 6
Statistical source data.
Source Data Extended Data Fig. 9
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Cikes, D., Leutner, M., Cronin, S.J.F. et al. Gpcpd1–GPC metabolic pathway is dysfunctional in aging and its deficiency severely perturbs glucose metabolism. Nat Aging 4, 80–94 (2024). https://doi.org/10.1038/s43587-023-00551-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s43587-023-00551-6
This article is cited by
-
Dysfunctional lipid metabolism in ageing muscle: effects on glucose tolerance
Nature Reviews Endocrinology (2024)