Abstract
While autophagy genes are required for lifespan of long-lived animals, their tissue-specific roles in aging remain unclear. Here, we inhibited autophagy genes in Caenorhabditis elegans neurons, and found that knockdown of early-acting autophagy genes, except atg-16.2, increased lifespan, and decreased neuronal PolyQ aggregates, independently of autophagosomal degradation. Neurons can secrete protein aggregates via vesicles called exophers. Inhibiting neuronal early-acting autophagy genes, except atg-16.2, increased exopher formation and exopher events extended lifespan, suggesting exophers promote organismal fitness. Lifespan extension, reduction in PolyQ aggregates and increase in exophers were absent in atg-16.2 null mutants, and restored by full-length ATG-16.2 expression in neurons, but not by ATG-16.2 lacking its WD40 domain, which mediates noncanonical functions in mammalian systems. We discovered a neuronal role for C. elegans ATG-16.2 and its WD40 domain in lifespan, proteostasis and exopher biogenesis. Our findings suggest noncanonical functions for select autophagy genes in both exopher formation and in aging.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
The authors declare that all data supporting the findings of this study are available within this article, extended data and Supplementary Information. Source data are available with this paper. Additional information on data is available from the corresponding authors upon reasonable request. The unique identifiers for ATG-16.2 sequences are stated in the figure legends.
References
Hansen, M., Rubinsztein, D. C. & Walker, D. W. Autophagy as a promoter of longevity: insights from model organisms. Nat. Rev. Mol. Cell Biol. 19, 579–593 (2018).
Miller, H. A., Dean, E. S., Pletcher, S. D. & Leiser, S. F. Cell non-autonomous regulation of health and longevity. Elife https://doi.org/10.7554/eLife.62659 (2020).
Simonsen, A. et al. Promoting basal levels of autophagy in the nervous system enhances longevity and oxidant resistance in adult Drosophila. Autophagy 4, 176–184 (2008).
Ulgherait, M., Rana, A., Rera, M., Graniel, J. & Walker, D. W. AMPK modulates tissue and organismal aging in a non-cell-autonomous manner. Cell Rep. 8, 1767–1780 (2014).
Hara, T. et al. Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice. Nature 441, 885–889 (2006).
Komatsu, M. et al. Loss of autophagy in the central nervous system causes neurodegeneration in mice. Nature 441, 880–884 (2006).
Jia, K., Hart, A. C. & Levine, B. Autophagy genes protect against disease caused by polyglutamine expansion proteins in Caenorhabditis elegans. Autophagy 3, 21–25 (2007).
Kumsta, C. et al. The autophagy receptor p62/SQST-1 promotes proteostasis and longevity in C. elegans by inducing autophagy. Nat. Commun. 10, 5648 (2019).
Kumsta, C., Chang, J. T., Schmalz, J. & Hansen, M. Hormetic heat stress and HSF-1 induce autophagy to improve survival and proteostasis in C. elegans. Nat. Commun. 8, 14337 (2017).
Kamath, R. S. & Ahringer, J. Genome-wide RNAi screening in Caenorhabditis elegans. Methods 30, 313–321 (2003).
Timmons, L., Court, D. L. & Fire, A. Ingestion of bacterially expressed dsRNAs can produce specific and potent genetic interference in Caenorhabditis elegans. Gene 263, 103–112 (2001).
Winston, W. M., Molodowitch, C. & Hunter, C. P. Systemic RNAi in C. elegans requires the putative transmembrane protein SID-1. Science 295, 2456–2459 (2002).
Feinberg, E. H. & Hunter, C. P. Transport of dsRNA into cells by the transmembrane protein SID-1. Science 301, 1545–1547 (2003).
Mizushima, N., Yoshimori, T. & Ohsumi, Y. The role of Atg proteins in autophagosome formation. Annu. Rev. Cell Dev. Biol. 27, 107–132 (2011).
Noda, T., Fujita, N. & Yoshimori, T. The late stages of autophagy: how does the end begin? Cell Death Differ. 16, 984–990 (2009).
Noda, N. N. & Inagaki, F. Mechanisms of autophagy. Annu. Rev. Biophys. 44, 101–122 (2015).
Klionsky, D. J. et al. Guidelines for the use and interpretation of assays for monitoring autophagy (3rd edition). Autophagy 12, 1–222 (2016).
Zhang, H. et al. Guidelines for monitoring autophagy in Caenorhabditis elegans. Autophagy 11, 9–27 (2015).
Cadwell, K. & Debnath, J. Beyond self-eating: the control of nonautophagic functions and signaling pathways by autophagy-related proteins. J. Cell Biol. 217, 813–822 (2018).
Fletcher, K. et al. The WD40 domain of ATG16L1 is required for its non-canonical role in lipidation of LC3 at single membranes. EMBO J. https://doi.org/10.15252/embj.201797840 (2018).
Martinez, J. et al. Molecular characterization of LC3-associated phagocytosis reveals distinct roles for Rubicon, NOX2 and autophagy proteins. Nat. Cell Biol. 17, 893–906 (2015).
Heckmann, B. L. et al. Noncanonical function of an autophagy protein prevents spontaneous Alzheimer’s disease. Sci. Adv. 6, eabb9036 (2020).
Nieto-Torres, J. L., Leidal, A. M., Debnath, J. & Hansen, M. Beyond autophagy: the expanding roles of ATG8 proteins. Trends Biochem. Sci 46, 673–686 (2021).
Melentijevic, I. et al. C. elegans neurons jettison protein aggregates and mitochondria under neurotoxic stress. Nature 542, 367–371 (2017).
Turek, M. et al. Muscle-derived exophers promote reproductive fitness. EMBO Rep. 22, e52071 (2021).
Nicolas-Avila, J. A. et al. A network of macrophages supports mitochondrial homeostasis in the heart. Cell 183, 94–109 (2020).
Siddique, I. et al. Exophers are components of mammalian cell neurobiology in health and disease. Preprint at bioRxiv https://doi.org/10.1101/2021.12.06.471479 (2021).
Calixto, A., Chelur, D., Topalidou, I., Chen, X. & Chalfie, M. Enhanced neuronal RNAi in C. elegans using SID-1. Nat. Methods 7, 554–559 (2010).
Roux, A. E. et al. Individual cell types in C. elegans age differently and activate distinct cell-protective responses. Cell Rep. 42, 112902 (2023).
WormBase WS283. http://www.wormbase.org/db/get?name=WBGene00006843;class=Gene (2023).
Wilhelm, T. et al. Neuronal inhibition of the autophagy nucleation complex extends lifespan in post-reproductive C. elegans. Genes Dev. 31, 1561–1572 (2017).
Apfeld, J. & Kenyon, C. Regulation of lifespan by sensory perception in Caenorhabditis elegans. Nature 402, 804–809 (1999).
Tank, E. M., Rodgers, K. E. & Kenyon, C. Spontaneous age-related neurite branching in Caenorhabditis elegans. J. Neurosci. 31, 9279–9288 (2011).
Toth, M. L. et al. Neurite sprouting and synapse deterioration in the aging Caenorhabditis elegans nervous system. J. Neurosci. 32, 8778–8790 (2012).
Melendez, A. et al. Autophagy genes are essential for dauer development and life-span extension in C. elegans. Science 301, 1387–1391 (2003).
Gelino, S. et al. Intestinal autophagy improves healthspan and longevity in C. elegans during dietary restriction. PLoS Genet. 12, e1006135 (2016).
Manil-Segalen, M. et al. The C. elegans LC3 acts downstream of GABARAP to degrade autophagosomes by interacting with the HOPS subunit VPS39. Dev. Cell 28, 43–55 (2014).
Chang, J. T., Kumsta, C., Hellman, A. B., Adams, L. M. & Hansen, M. Spatiotemporal regulation of autophagy during Caenorhabditis elegans aging. Elife https://doi.org/10.7554/eLife.18459 (2017).
Kuma, A., Matsui, M. & Mizushima, N. LC3, an autophagosome marker, can be incorporated into protein aggregates independent of autophagy: caution in the interpretation of LC3 localization. Autophagy 3, 323–328 (2007).
Chang, J. T., Hansen, M. & Kumsta, C. Assessing tissue-specific autophagy flux in adult Caenorhabditis elegans. Methods Mol. Biol. 2144, 187–200 (2020).
Yamamoto, A. et al. Bafilomycin A1 prevents maturation of autophagic vacuoles by inhibiting fusion between autophagosomes and lysosomes in rat hepatoma cell line, H-4-II-E cells. Cell Struct. Funct. 23, 33–42 (1998).
Wilkinson, D. S. et al. Phosphorylation of LC3 by the Hippo kinases STK3/STK4 is essential for autophagy. Mol. Cell 57, 55–68 (2015).
Arnold, M. L., Cooper, J., Grant, B. D. & Driscoll, M. Quantitative approaches for scoring in vivo neuronal aggregate and organelle extrusion in large exopher vesicles in C. elegans. J. Vis. Exp. https://doi.org/10.3791/61368 (2020).
Wu, F., Li, Y., Wang, F., Noda, N. N. & Zhang, H. Differential function of the two Atg4 homologues in the aggrephagy pathway in Caenorhabditis elegans. J. Biol. Chem. 287, 29457–29467 (2012).
Zhang, H. et al. The two C. elegans ATG-16 homologs have partially redundant functions in the basal autophagy pathway. Autophagy 9, 1965–1974 (2013).
Matsushita, M. et al. Structure of Atg5.Atg16, a complex essential for autophagy. J. Biol. Chem. 282, 6763–6772 (2007).
Arnold, M. L. et al. Intermediate filaments associate with aggresome-like structures in proteostressed C. elegans neurons and influence large vesicle extrusions as exophers. Nat. Commun. 14, 4450 (2023).
Durgan, J. et al. Non-canonical autophagy drives alternative ATG8 conjugation to phosphatidylserine. Mol. Cell 81, 2031–2040 (2021).
Hooper, K. M. et al. V-ATPase is a universal regulator of LC3-associated phagocytosis and non-canonical autophagy. J. Cell Biol. https://doi.org/10.1083/jcb.202105112 (2022).
Ishibashi, K., Uemura, T., Waguri, S. & Fukuda, M. Atg16L1, an essential factor for canonical autophagy, participates in hormone secretion from PC12 cells independently of autophagic activity. Mol. Biol. Cell 23, 3193–3202 (2012).
Guo, H. et al. Atg5 disassociates the V1V0-ATPase to promote exosome production and tumor metastasis independent of canonical macroautophagy. Dev. Cell 43, 716–730 (2017).
Varga, K. et al. Loss of Atg16 delays the alcohol-induced sedation response via regulation of Corazonin neuropeptide production in Drosophila. Sci. Rep. 6, 34641 (2016).
Choi, A. M., Ryter, S. W. & Levine, B. Autophagy in human health and disease. N. Engl. J. Med. 368, 651–662 (2013).
Wong, E. & Cuervo, A. M. Autophagy gone awry in neurodegenerative diseases. Nat. Neurosci. 13, 805–811 (2010).
Cortes, C. J. & La Spada, A. R. Autophagy in polyglutamine disease: imposing order on disorder or contributing to the chaos? Mol. Cell Neurosci. 66, 53–61 (2015).
Kim, D. K. et al. Cell-to-cell transmission of polyglutamine aggregates in C. elegans. Exp. Neurobiol. 26, 321–328 (2017).
Pearce, M. M. P., Spartz, E. J., Hong, W., Luo, L. & Kopito, R. R. Prion-like transmission of neuronal huntingtin aggregates to phagocytic glia in the Drosophila brain. Nat. Commun. 6, 6768 (2015).
Jeon, I. et al. Human-to-mouse prion-like propagation of mutant huntingtin protein. Acta Neuropathol. 132, 577–592 (2016).
Takeuchi, T. & Nagai, Y. Emerging roles of extracellular vesicles in polyglutamine diseases: mutant protein transmission, therapeutic potential, and diagnostics. Neurochem. Int. 157, 105357 (2022).
Zhang, X. et al. Potential transfer of polyglutamine and CAG-repeat RNA in extracellular vesicles in Huntington’s disease: background and evaluation in cell culture. Cell Mol. Neurobiol. 36, 459–470 (2016).
Fussi, N. et al. Exosomal secretion of alpha-synuclein as protective mechanism after upstream blockage of macroautophagy. Cell Death Dis. 9, 757 (2018).
Abdulrahman, B. A., Abdelaziz, D. H. & Schatzl, H. M. Autophagy regulates exosomal release of prions in neuronal cells. J. Biol. Chem. 293, 8956–8968 (2018).
Miranda, A. M. et al. Neuronal lysosomal dysfunction releases exosomes harboring APP C-terminal fragments and unique lipid signatures. Nat. Commun. 9, 291 (2018).
Nakamura, S. et al. Suppression of autophagic activity by Rubicon is a signature of aging. Nat. Commun. 10, 847 (2019).
Brenner, S. The genetics of Caenorhabditis elegans. Genetics 77, 71–94 (1974).
Shen, K. & Bargmann, C. I. The immunoglobulin superfamily protein SYG-1 determines the location of specific synapses in C. elegans. Cell 112, 619–630 (2003).
Rual, J. F. et al. Toward improving Caenorhabditis elegans phenome mapping with an ORFeome-based RNAi library. Genome Res. 14, 2162–2168 (2004).
Hansen, M., Hsu, A. L., Dillin, A. & Kenyon, C. New genes tied to endocrine, metabolic, and dietary regulation of lifespan from a Caenorhabditis elegans genomic RNAi screen. PLoS Genet. 1, 119–128 (2005).
Wang, Y. et al. Large vesicle extrusions from C. elegans neurons are consumed and stimulated by glial-like phagocytosis activity of the neighboring cell. Elife https://doi.org/10.7554/eLife.82227 (2023).
Kauffman, A. et al. C. elegans positive butanone learning, short-term, and long-term associative memory assays. J. Vis. Exp. https://doi.org/10.3791/2490 (2011).
Kauffman, A. L., Ashraf, J. M., Corces-Zimmerman, M. R., Landis, J. N. & Murphy, C. T. Insulin signaling and dietary restriction differentially influence the decline of learning and memory with age. PLoS Biol. 8, e1000372 (2010).
Han, S. K. et al. OASIS 2: online application for survival analysis 2 with features for the analysis of maximal lifespan and healthspan in aging research. Oncotarget 7, 56147–56152 (2016).
Acknowledgements
We thank members of the M.H. and C.K. laboratories for comments on the manuscript. We thank X. She, E. P. Tan, S. Lim and K. Schilling from the M.H. laboratory for creating some of the strains used in this study. We thank H. Zhang for sharing construct atg-16.2p::atg-16.2::gfp, R. Legouis for the GFP::LGG-1(Gly116Ala) strain and B. Grant for sharing the hygromycin resistance-containing plasmid. We thank O. Florey for help with designing the Phe394 mutation in the C. elegans ATG-16.2 WD40 domain. We thank N. Riehs and C. Kenyon for sharing unpublished information on neuronal-only RNAi of autophagy genes. Some of the nematode strains used in this work were provided by the Caenorhabditis Genetics Center (University of Minnesota), which is supported by the NIH Office of Research Infrastructure Program (P40OD010440). We acknowledge the C. elegans Gene Knockout Project at the Oklahoma Medical Research Foundation, which was part of the International C. elegans Gene Knockout Consortium, for generating atg-16.2(ok3224) mutation and WormBase WS283. This work was funded by NIA/NIH fellowship F31AG066405 to M.L.A., a Paul F. Glenn Center Fellowship and Larry L. Hillblom Fellowship 2023-A-019-FEL to H.E., a Taiwan Global Fellowship to L.-H.S., NIH/NIA grants R37AG056510 (to M.D.), R01AG038664 and R01AG039756 and Breakthrough in Gerontology Award BIG 20016 from American Association for Aging Research (to M.H.). The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Author information
Authors and Affiliations
Contributions
Y.Y., M.L.A., C.K. and M.H. designed the experiments. Y.Y., M.L.A., T.M.M., C.K. and M.H. constructed strains. Y.Y., C.M.L., L.-H.S., H.E., M.B., S.D., E.H.C., K.P., T.M.M., A.S., C.K. and M.H. performed lifespan experiments. Y.Y. and C.K. counted polyQ aggregates. Y.Y. and E.H.C. conducted healthspan assays. Y.Y., C.M.L., S.D. and C.K. measured RNAi efficiency. Y.Y. conducted neuronal branching, dye-filling experiments and cloning. C.M.L. and C.K. performed chemotaxis experiments. M.L.A., C.M.L., L.-H.S., H.E. and C.K. monitored exopher formation. L.H.S. monitored exopher formation in polyQ-expressing animals. C.K. performed all autophagy measurements and RT–qPCR. Y.Y., C.K. and M.H. wrote the manuscript with input from all co-authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests. The funders had no role in the study design, data collection and analysis, decision to publish or preparation of the manuscript.
Peer review
Peer review information
Nature Aging thanks Evandro Fang and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
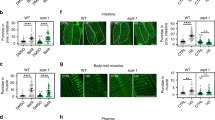
Extended Data Fig. 1 Neuronal expression of SID-1, an RNA channel protein, leads to RNAi-competent neurons.
(a) Mean GFP fluorescence intensity in head region of rgef-1p::gfp animals on day 1 to day 12 of adulthood, relative to day 1. Error bars are s.d. of n = 3 experiments, with n = 29, 34, 32, 24, 28, 19 animals over 3 independent experiments. ns P = 0.78, P = 0.05, P = 0.116, ****P < 0.0001, **P = 0.005, by one-way ANOVA with Dunnett’s multiple comparisons test. (b-f) Wild-type animals (N2, WT), sid-1; rgef-1p::sid-1 + rgef-1p::gfp, and sid-1 mutants after whole-life RNAi against the indicated gene with tissue-specific functions, compared to control (CTRL). (b) Representative animals are shown with WT animals displaying paralysis (Prz) on unc-112 RNAi, larval arrest (Lva) on rpl-2 RNAi, blister formation (Bli) and larval arrest (Lva) on bli-1 RNAi, and clear (Clr) and larval arrest on elt-2 RNAi. Mean percent phenotypic penetrance after knockdown of genes with functions in (c) body-wall muscle; unc-22 – twitching and uncoordinated movement (Unc) (n = 8 experiments, ****P < 0.0001) and unc-112 – paralysis (n = 7 experiments, ****P < 0.0001), (d) a ubiquitous manner; rpl-2 – larval arrest (n = 8 experiments, ****P < 0.0001), (e) hypodermis; tsp-15 – blisters (n = 8 experiments, ****P < 0.0001) and bli-1 – blisters and larval arrest; (f) intestine; elt-2 – clear and larval arrest. Error bars are s.d. (g) Mean GFP fluorescence intensity in head region of day 1 sid-1; rgef-1p::sid-1 + rgef-1p::gfp rgef-1p::gfp animals after whole-life gfp RNAi compared to control (CTRL). Error bars are s.d. with n = 30 over 3 independent experiments. ****P < 0.0001, **P = 0.005, by two-tailed Student’s t-test. Scale bar: 100 µm. (h) Mean GFP fluorescence intensity in head region of day 1 sid-1; rgef-1p::sid-1 + rgef-1p::gfp rgef-1p::gfp animals after whole-life lgg-1 RNAi (n = 32) compared to control (CTRL) (n = 35). Error bars are s.d. over 3 independent experiments. ****P < 0.0001 by two-tailed Student’s t-test. Scale bar: 100 µm. (i) Mean percent of shrinker phenotype in day 2 WT, rde-1; unc-47p::rde-1::SL2::sid-1 (capable of GABA neuron-specific RNAi), sid-1; rgef-1p::sid-1 + rgef-1p::gfp, or sid-1 animals after two generations of whole-life snb-1 or unc-13 RNAi compared to control (CTRL). Error bars are s.d. with ****P < 0.0001 and ns P > 0.99 by one-way ANOVA with Dunnett’s multiple comparisons test.
Extended Data Fig. 2 Healthspan and neuronal phenotypes of animals after neuronal inhibition of atg-7 and lgg-1/ATG8.
(a) Mean body bends per 20 s of sid-1; rgef-1p::sid-1 + rgef-1p::gfp animals after whole-life atg-7, or lgg-1/ATG8 RNAi compared to control (CTRL). Error bars are s.e.m. of one representative experiment, each with n = 16 animals. Experiment was performed three times with similar results. Linear regression comparison versus CTRL: atg-7 RNAi: Pslope = 0.5; Py-intercept = 0.02; lgg-1/ATG8 RNAi: Pslope = 0.3; Py-intercept = 0.01. (b) Mean number of contractions in the terminal pharyngeal bulb per 30 s of sid-1; rgef-1p::sid-1 + rgef-1p::gfp animals after whole-life atg-7, or lgg-1/ATG8 RNAi compared to control (CTRL). Error bars are s.d. of n = 30 animals over 3 independent experiments. Linear regression comparison versus CTRL: atg-7 RNAi: Pslope = 0.4; Py-intercept = 0.4; lgg-1 RNAi: Pslope = 0.3; Py-intercept = 0.5. (c) Mean number of progeny produced per day in sid-1; rgef-1p::sid-1 + rgef-1p::gfp animals after atg-7 (n = 19 animals), or lgg-1/ATG8 RNAi (n = 18 animals) compared to control (CTRL) (n = 22 animals) over 2 independent experiments. Error bars are s.d. CTRL versus atg-7 RNAi: ns P = 0.72, 0.89, 0.91, 0.82, >0.99; CTRL versus lgg-1/ATG8 RNAi: ns P = 0.95, 0.96,0.60, 0.70, >0.99, by two-way ANOVA with Dunnett’s multiple comparisons test. (d) Analysis of integrity of sensory neurons in day 5 sid-1; rgef-1p::sid-1 + rgef-1p::gfp animals after atg-7, or lgg-1/ATG8 RNAi compared to control (CTRL). Sensory mutants daf-10(e1387) and osm-6(p811) are negative controls. Shown are representative images of n = 10 animals. Experiment was performed three times with similar results. Scale bar, 20 μm. (e) Representative image of neuronal branch (arrowhead) from ALM neuron in day 15 sid-1; rgef-1p::sid-1+ rgef-1p::gfp animals. Scale bar: 20 µm. Mean percent of animals with branches after whole-life atg-7, or lgg-1/ATG8 dsRNA compared to control (CTRL) of n = 4 experiments. Error bars are s.d. ***P = 0.0002, ****P < 0.0001 by Cochran-Mantel-Haenszel test. (f) Mean chemotaxis index of day 5 sid-1; rgef-1p::sid-1 + rgef-1p::gfp animals after whole-life atg-7, or lgg-1/ATG8 RNAi using the chemoattractant butanone. Error bars are 95% C.I. of n = 4 experiments. *P = 0.048, **P = 0.0094, by one-way ANOVA with Dunnett’s multiple comparisons test.
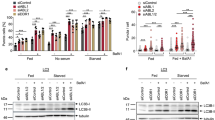
Extended Data Fig. 3 Autophagy status is unchanged in animals expressing sid-1 and in non-neuronal tissues after neuronal knockdown of early-acting autophagy genes.
(a) Mean neuronal GFP::LGG-1 and GFP::LGG-1(G116A) punctae in day 1 wild-type (sid-1 + /+) (n = 28, 29) and sid-1(qt9) (sid-1-/-) animals (n = 27, 27) with or without rgef-1p::sid-1 transgene (rgef-1p::sid-1 (+)) (n = 26, 29). Error bars are s.d. over 3 independent experiments. Comparison between strains: LGG-1: ns P = 0.75, P = 0.97, G116A: ns P = 0.98, P > 0.99. Comparison of lipidated and unlipidated structures: ****P < 0.0001, by two-way ANOVA with Tukey’s multiple comparisons test. (b) Mean sqst-1p::sqst-1::gfp fluorescence intensity in head region of day 1 wild-type (sid-1 + /+), and sid-1(qt9) (sid-1(-/-)) animals with or without rgef-1p::sid-1 transgene (rgef-1p::sid-1 (+)) on day 1 of adulthood. Error bars are s.d. of n = 31 animals over 3 independent experiments. ns P = 0.18 and P = 0.59 by one-way ANOVA with Dunnett’s multiple comparisons test. (c) GFP::LGG-1 punctae in intestinal cells of day 1 wild-type (sid-1 + /+) (n = 59) and sid-1(qt9) (sid-1-/-) animals (n = 62) with or without rgef-1p::sid-1 transgene (rgef-1p::sid-1 (+)) (n = 62). Violin plots with solid line indicating median and dashed lines indicating quartiles. ns P = 0.79, P = 0.99 by one-way ANOVA with Dunnett’s multiple comparisons test. (d) GFP::LGG-1 punctae in body-wall muscle areas of day 1 wild-type (sid-1 + /+) (n = 48) and sid-1(qt9) (sid-1-/-) animals (n = 45) with or without rgef-1p::sid-1 transgene (rgef-1p::sid-1 (+)) (n = 52). Violin plots with solid line indicating median and dashed lines indicating quartiles. ns P = 0.64, P = 0.70 by one-way ANOVA with Dunnett’s multiple comparisons test. (e) GFP::LGG-1 punctae in intestinal cells of day 1 sid-1; rgef-1p::sid-1 + rgef-1p::gfp; lgg-1p::gfp::lgg-1 animals after whole-life atg-7 (n = 65), or lgg-1/ATG8 (n = 48) RNAi compared to control (CTRL) (n = 81). Violin plots with solid line indicating median and dashed lines indicating quartiles. ns P = 0.98, P = 0.71 by one-way ANOVA with Dunnett’s multiple comparisons test. (f) GFP::LGG-1 punctae in body-wall muscle areas of day 1 sid-1; rgef-1p::sid-1 + rgef-1p::gfp; lgg-1p::gfp::lgg-1 animals after whole-life atg-7 (n = 54), or lgg-1/ATG8 (n = 53) RNAi compared to control (CTRL) (n = 49). Violin plots with solid line indicating median and dashed lines indicating quartiles. ns P = 0.34, P = 0.96 by one-way ANOVA with Dunnett’s multiple comparisons test.
Extended Data Fig. 4 Neuronal PolyQ aggregation, exopher formation and lifespan extension are correlated.
(a) Number of neuronal PolyQ aggregates in day 7 rgef-1::Q40::yfp wild-type (sid-1 + /+) (n = 41) and sid-1(qt9) (sid-1-/-) animals (n = 48 with or without rgef-1p::sid-1 transgene (rgef-1p::sid-1 (+)) (n = 42). Violin plots with solid line indicating median and dashed lines indicating quartiles. ns P = 0.79, P = 0.82 by one-way ANOVA with Dunnett’s multiple comparisons test. (b) Mean percent of ALMR neurons with exophers of day 2 mec-4p::mCherry wild-type (sid-1 + /+) (n = 228 animals) and sid-1(qt9) (sid-1-/-) (n = 262 animals) with or without rgef-1p::sid-1 transgene (rgef-1p::sid-1 (+)) (n = 295 animals). Error bars are s.d. of n = 7 experiments, ns P = 0.62, P = 0.54 by two-sided Cochran-Mantel-Haenszel test. (c) Mean percent of ALMR neurons with exophers of day 2 sid-1; rgef-1p::sid-1 + rgef-1p::gfp; mec-4p::mCherry animals (n = 247 animals) after adult-only atg-7 (n = 271 animals), or lgg-1/ATG8 (n = 285 animals) RNAi compared to control (CTRL). Error bars are s.d. of n = 6 experiments, *P = 0.028, P = 0.00006 by two-sided Cochran-Mantel-Haenszel test. (d) Mean percent of ALMR neurons with exophers of day 2 mec-4p::mCherry animals (n = 151 animals) after whole-life atg-7 (n = 157 animals), or lgg-1/ATG8 (n = 180 animals) RNAi compared to control (CTRL). Error bars are s.d. of n = 5 experiments, ns P = 0.39, P = 0.53 by two-sided Cochran-Mantel-Haenszel test. (e-g) Percent mean lifespan (LS) change (Fig. 1b), number of neuronal PolyQ aggregates (Fig. 3b), and mean percent of AMLR neurons with exophers (Fig. 4b) plotted against each other with simple linear regression (solid line with 95% C.I. as dashed lines). Numbers refer to specific RNAi treatment; 1unc-51/ATG1, 2atg-13, 3bec-1/BECN1, 4atg-9, 5atg-16.2, 6atg-7, 7atg-4.1, 8lgg-1/ATG8, 9cup-5, 10epg-5, 11vha-13, 12vha-15, 13vha-16. P values determined by two-sided Spearman correlation test.
Extended Data Fig. 5 atg-16.2 and atg-4.1 mutants display similar RNAi phenotypes.
(a-d) Phenotypes of day 2 WT, atg-16.2(ok3224), atg-4.1(bp501), and sid-1(qt9) animals after whole-life RNAi against a specific gene expressed in different tissues. Mean percent phenotypic penetrance after knockdown of genes with functions in (a) body-wall muscle; unc-22 – twitching and uncoordinated movement (Unc) and unc-112 – paralysis, (b) a ubiquitous manner; rpl-2 – larval arrest, (c) hypodermis; tsp-15 – blisters and bli-1 – blisters and larval arrest; (d) intestine; elt-2 – clear and larval arrest. Error bars are s.d. of n = 4 experiments. (a) unc-22 RNAi: ns P = 0.59, P = 0.87, ****P < 0.0001. unc-112 RNAi: ns P = 0.92, P = 0.96, ****P < 0.0001. (b) rpl-2 RNAi: ns P = 0.82, P = 0.96, ****P < 0.0001. (c) tsp-15 RNAi: ns P = 0.99, P = 0.64, ****P < 0.0001. bli-1 RNAi: ns P = 0.24, P = 0.99, ****P < 0.0001. (d) elt-2 RNAi: ns P = 0.76, P = 0.18, ****P < 0.0001 by one-way one-way ANOVA with Dunnett’s multiple comparisons test.
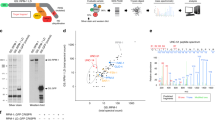
Extended Data Fig. 6 Multiple sequence alignment of ATG16 proteins, and atg-16.2 mRNA expression levels in C. elegans.
(a) Primary sequences of ATG16 proteins from S. cerevisiae (1 isoform, Sc_ATG16), C. elegans (2 isoforms, Ce_ATG-16.1, Ce_ATG-16.2), Mus musculus (2 isoforms, Mm_ATG16L1, MmATG16L2), and humans (2 isoforms, Hs_ATG16L1, Hs_ATG16L2) were aligned by the Clustal Omega and colored by conservation with darker shades indicated increased conservation (Jalview). Protein elements of ATG16 proteins are drawn under the alignment and phenylalanine 349 and isoleucine 509 are indicated with an arrow. Sequences showed are ATG16 (NP_013882.1) in S. cerevisiae, ATG16.2 (NP_495299.2) in C. elegans, ATG16 (NP_001138124.2) in D. melanogaster, ATG16L1 (NP_001192320.1) in M. musculus, and ATG16L1 (NP_001350671.1) in H. sapiens. (b) Transcript levels of atg-16.2 in wild-type (WT), atg-16.2(ok3224), and atg-16.2(ok3224) animals expressing full-length atg-16.2, atg-16.2(∆WD40), or atg-16.2(Phe394A, Ile509Met) from the neuronal rgef-1 promoter. Schematic of atg-16.2 cDNA indicates the ok3224 deletion, the WD40 domain, the position of the Phe394Ala and Ile509Met point mutations, and the amplicons produced by the primers used in this experiment. Data are the mean and s.e.m. of three biological replicates, each with three technical replicates, and are normalized to the mean expression levels of three housekeeping genes. Amplicon 1: **P = 0.0013, ns P = 0.23, P = 0.36, P = 0.49, Amplicon 2: *P = 0.03, ns P = 0.16, *P = 0.03, ns P = 0.89 by unpaired two-sided t-test with control.
Supplementary information
Supplementary Table 1
Summary of lifespan experiments of comparing WT, sid-1 and neuronal-specific RNAi strains.
Supplementary Table 2
Summary of statistical information for figures testing >10 conditions (Figs. 1–4).
Supplementary Table 3
Summary of lifespan experiments in WT animals after neuronal gene knockdown.
Supplementary Table 4
Summary of lifespan experiments in transgenic strains after neuronal knockdown of atg-7 and lgg-1/ATG8.
Supplementary Table 5
Summary of lifespan experiments of animals clustered ± exopher formation in touch neurons.
Supplementary Table 6
Summary of lifespan experiments in autophagy mutants atg-16.2 and atg-4.1.
Supplementary Table 7
Summary of lifespan experiments with neuronal atg-16.2 reconstitution.
Supplementary Table 8
C. elegans strains used in this study.
Supplementary Table 9
Plasmids used in this study.
Supplementary Table 10
Primers used in this study.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Statistical source data.
Source Data Fig. 6
Statistical source data.
Source Data Fig. 7
Statistical source data.
Source Data Extended Data Fig. 1
Statistical source data.
Source Data Extended Data Fig. 2
Statistical source data.
Source Data Extended Data Fig. 3
Statistical source data.
Source Data Extended Data Fig. 4
Statistical source data.
Source Data Extended Data Fig. 5
Statistical source data.
Source Data Extended Data Fig. 6
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yang, Y., Arnold, M.L., Lange, C.M. et al. Autophagy protein ATG-16.2 and its WD40 domain mediate the beneficial effects of inhibiting early-acting autophagy genes in C. elegans neurons. Nat Aging 4, 198–212 (2024). https://doi.org/10.1038/s43587-023-00548-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s43587-023-00548-1
This article is cited by
-
Pheromone-based communication influences the production of somatic extracellular vesicles in C. elegans
Nature Communications (2024)