Abstract
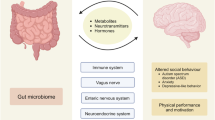
Alzheimer’s disease (AD) is characterized by amyloid-β accumulation in the brain and hyperphosphorylated tau aggregation, as well as neuroinflammation. The gut–brain axis has emerged as a therapeutic target in neurodegenerative diseases by modulating metabolic activity, neuroimmune functions and sensory neuronal signaling. Here we investigate interactions between orally ingested chiral Au nanoparticles and the gut microbiota in AD mice. Oral administration of chiral Au nanoparticles restored cognitive abilities and ameliorated amyloid-β and hyperphosphorylated tau pathologies in AD mice via alterations in the gut microbiome composition and an increase in the gut metabolite, indole-3-acetic acid, which was lower in serum and cerebrospinal fluid of patients with AD compared with age-matched controls. Oral administration of indole-3-acetic acid was able to penetrate the blood–brain barrier and alleviated cognitive decline and pathology including neuroinflammation in AD mice. These findings provide a promising therapeutic target for the amelioration of neuroinflammation and treatment of neurodegenerative diseases.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
Source data are provided with this paper. Any additional data generated and analyzed in this study are available from the corresponding authors upon reasonable request. Raw reads of 16S rRNA-sequencing were uploaded to the National Center for Biotechnology Information Sequence Read Archive database (accession number: PRJNA1014784; http://www.ncbi.nlm.nih.gov/bioproject/1014784) or Figshare (https://figshare.com/articles/online_resource/16S_rRNA_zip/24187827).
References
Venegas, C. et al. Microglia-derived ASC specks cross-seed amyloid-β in Alzheimer’s disease. Nature 552, 355–361 (2017).
Franceschi, C., Garagnani, P., Parini, P., Giuliani, C. & Santoro, A. Inflammaging: a new immune–metabolic viewpoint for age-related diseases. Nat. Rev. Endocrinol. 14, 576–590 (2018).
Sun, M.-F. & Shen, Y.-Q. Dysbiosis of gut microbiota and microbial metabolites in Parkinson’s disease. Ageing Res. Rev. 45, 53–61 (2018).
Sampson, T. R. et al. Gut microbiota regulate motor deficits and neuroinflammation in a model of Parkinson’s disease. Cell 167, 1469–1480.e12 (2016).
Ling, Z. et al. Structural and functional dysbiosis of fecal microbiota in Chinese patients with Alzheimeras disease. Front. Cell Dev. Biol. 8, 634069 (2021).
Needham, B. D., Kaddurah-Daouk, R. & Mazmanian, S. K. Gut microbial molecules in behavioural and neurodegenerative conditions. Nat. Rev. Neurosci. 21, 717–731 (2020).
Mayer, E. A., Nance, K. & Chen, S. The gut–brain axis. Annu. Rev. Med. 73, 439–453 (2022).
Main, B. S. & Minter, M. R. Microbial immuno-communication in neurodegenerative diseases. Front. Neurosci. 11, 151 (2017).
Boehme, M. et al. Microbiota from young mice counteracts selective age-associated behavioral deficits. Nat. Aging 1, 666–676 (2021).
Seo, D. O. et al. ApoE isoform- and microbiota-dependent progression of neurodegeneration in a mouse model of tauopathy. Science 379, eadd1236 (2023).
Singh, R. et al. Enhancement of the gut barrier integrity by a microbial metabolite through the Nrf2 pathway. Nat. Commun. 10, 89 (2019).
Rothhammer, V. et al. Type I interferons and microbial metabolites of tryptophan modulate astrocyte activity and central nervous system inflammation via the aryl hydrocarbon receptor. Nat. Med. 22, 586–597 (2016).
Platten, M., Nollen, E. A. A., Röhrig, U. F., Fallarino, F. & Opitz, C. A. Tryptophan metabolism as a common therapeutic target in cancer, neurodegeneration and beyond. Nat. Rev. Drug Discov. 18, 379–401 (2019).
Krautkramer, K. A., Fan, J. & Bäckhed, F. Gut microbial metabolites as multi-kingdom intermediates. Nat. Rev. Microbiol. 19, 77–94 (2020).
Needham, B. D. et al. A gut-derived metabolite alters brain activity and anxiety behaviour in mice. Nature 602, 647–653 (2022).
Liu, X. et al. High-fiber diet mitigates maternal obesity-induced cognitive and social dysfunction in the offspring via gut–brain axis. Cell Metab. 33, 923–938.e6 (2021).
Mossad, O. et al. Gut microbiota drives age-related oxidative stress and mitochondrial damage in microglia via the metabolite N6-carboxymethyllysine. Nat. Neurosci. 25, 295–305 (2022).
Serger, E. et al. The gut metabolite indole-3 propionate promotes nerve regeneration and repair. Nature 607, 585–592 (2022).
Tintelnot, J. et al. Microbiota-derived 3-IAA influences chemotherapy efficacy in pancreatic cancer. Nature 615, 168–174 (2023).
Brakedal, B. et al. The NADPARK study: a randomized phase I trial of nicotinamide riboside supplementation in Parkinson’s disease. Cell Metab. 34, 396–407 (2022).
Ma, N., He, T., Johnston, L. J. & Ma, X. Host–microbiome interactions: the aryl hydrocarbon receptor as a critical node in tryptophan metabolites to brain signaling. Gut Microbes 11, 1203–1219 (2020).
Fung, T. C., Olson, C. A. & Hsiao, E. Y. Interactions between the microbiota, immune and nervous systems in health and disease. Nat. Neurosci. 20, 145–155 (2017).
Mancuso, C. & Santangelo, R. Alzheimer’s disease and gut microbiota modifications: the long way between preclinical studies and clinical evidence. Pharmacol. Res. 129, 329–336 (2018).
Zhao, L. P. et al. Gut bacteria selectively promoted by dietary fibers alleviate type 2 diabetes. Science 359, 1151–1156 (2018).
Pan, R.-Y. et al. Intermittent fasting protects against Alzheimer’s disease in mice by altering metabolism through remodeling of the gut microbiota. Nat. Aging 2, 1024–1039 (2022).
Bell, H. N. et al. Reuterin in the healthy gut microbiome suppresses colorectal cancer growth through altering redox balance. Cancer Cell 40, 185–200.e186 (2022).
Blacher, E. et al. Potential roles of gut microbiome and metabolites in modulating ALS in mice. Nature 572, 474–480 (2019).
Wang, Q. et al. IL-27 signalling promotes adipocyte thermogenesis and energy expenditure. Nature 600, 314–318 (2021).
Chen, P. B. et al. Directed remodeling of the mouse gut microbiome inhibits the development of atherosclerosis. Nat. Biotechnol. 38, 1288–1297 (2020).
Liu, Y. et al. Gut microbiome alterations in high-fat-diet-fed mice are associated with antibiotic tolerance. Nat. Microbiol. 6, 874–884 (2021).
Sun, J. et al. Microbiota-derived metabolite indoles induced aryl hydrocarbon receptor activation and inhibited neuroinflammation in APP/PS1 mice. Brain Behav. Immun. 106, 76–88 (2022).
Chu, J. N. & Traverso, G. Foundations of gastrointestinal-based drug delivery and future developments. Nat. Rev. Gastroenterol. Hepatol. 19, 219–238 (2021).
Gonzalez-Rubio, G. et al. Micelle-directed chiral seeded growth on anisotropic gold nanocrystals. Science 368, 1472–1477 (2020).
Ben-Moshe, A. et al. The chain of chirality transfer in tellurium nanocrystals. Science 372, 729–733 (2021).
Hananel, U., Ben-Moshe, A., Tal, D. & Markovich, G. Enantiomeric control of intrinsically chiral nanocrystals. Adv. Mater. 32, e1905594 (2020).
Zhang, Q. et al. Unraveling the origin of chirality from plasmonic nanoparticle-protein complexes. Science 365, 1475–1478 (2019).
Lu, J. et al. Enhanced optical asymmetry in supramolecular chiroplasmonic assemblies with long-range order. Science 371, 1368–1374 (2021).
Zhou, C., Duan, X. & Liu, N. A plasmonic nanorod that walks on DNA origami. Nat. Commun. 6, 8102 (2015).
Cai, J. et al. Polarization-sensitive optoionic membranes from chiral plasmonic nanoparticles. Nat. Nanotechnol. 17, 408–416 (2022).
Nguyen, H. V. et al. Bottlebrush polymers with flexible enantiomeric side chains display differential biological properties. Nat. Chem. 14, 85–93 (2022).
Hou, K. et al. Chiral gold nanoparticles enantioselectively rescue memory deficits in a mouse model of Alzheimer’s disease. Nat. Commun. 11, 4790 (2020).
Xu, L. et al. Enantiomer-dependent immunological response to chiral nanoparticles. Nature 601, 366–373 (2022).
Qu, A. et al. Stimulation of neural stem cell differentiation by circularly polarized light transduced by chiral nanoassemblies. Nat. Biomed. Eng. 5, 103–113 (2021).
Xin, Q., Liu, Q., Geng, L., Fang, Q. & Gong, J. R. Chiral nanoparticle as a new efficient antimicrobial nanoagent. Adv. Healthc. Mater. 6, 1601011 (2017).
Sun, M. et al. Site-selective photoinduced cleavage and profiling of DNA by chiral semiconductor nanoparticles. Nat. Chem. 10, 821–830 (2018).
Gao, R. et al. Site-selective proteolytic cleavage of plant viruses by photoactive chiral nanoparticles. Nat. Catal. 5, 694–707 (2022).
Cao, Z., Wang, X., Pang, Y., Cheng, S. & Liu, J. Biointerfacial self-assembly generates lipid membrane coated bacteria for enhanced oral delivery and treatment. Nat. Commun. 10, 5783 (2019).
Zheng, D.-W. et al. Phage-guided modulation of the gut microbiota of mouse models of colorectal cancer augments their responses to chemotherapy. Nat. Biomed. Eng. 3, 717–728 (2019).
Dong, X. et al. Bioinorganic hybrid bacteriophage for modulation of intestinal microbiota to remodel tumor-immune microenvironment against colorectal cancer. Sci. Adv. 6, eaba1590 (2020).
Shi, C. et al. A nanoparticulate dual scavenger for targeted therapy of inflammatory bowel disease. Sci. Adv. 8, eabj2372 (2022).
Peng, G. et al. Graphene oxide elicits microbiome-dependent type 2 immune responses via the aryl hydrocarbon receptor. Nat. Nanotechnol. 18, 42–48 (2023).
Erny, D. et al. Microbiota-derived acetate enables the metabolic fitness of the brain innate immune system during health and disease. Cell Metab. 33, 2260–2276.e7 (2021).
De Miguel, Z. et al. Exercise plasma boosts memory and dampens brain inflammation via clusterin. Nature 600, 494–499 (2021).
Rothhammer, V. et al. Microglial control of astrocytes in response to microbial metabolites. Nature 557, 724–728 (2018).
Ising, C. et al. NLRP3 inflammasome activation drives tau pathology. Nature 575, 669–673 (2019).
Baruch, K. et al. Breaking immune tolerance by targeting Foxp3+ regulatory T cells mitigates Alzheimer’s disease pathology. Nat. Commun. 6, 7967 (2015).
Baek, H. et al. Neuroprotective effects of CD4+CD25+Foxp3+ regulatory T cells in a 3xTg–AD Alzheimer’s disease model. Oncotarget 7, 69347–69357 (2016).
Quintana, F. J. et al. Control of Treg and TH17 cell differentiation by the aryl hydrocarbon receptor. Nature 453, 65–71 (2008).
Zhu, X. et al. Dectin-1 signaling on colonic gammadelta T cells promotes psychosocial stress responses. Nat. Immunol. 24, 625–636 (2023).
Hezaveh, K. et al. Tryptophan-derived microbial metabolites activate the aryl hydrocarbon receptor in tumor-associated macrophages to suppress anti-tumor immunity. Immunity 55, 324–340.e8 (2022).
Su, X. et al. Gut microbiota-derived metabolite 3-idoleacetic acid together with LPS induces IL-35+ B cell generation. Microbiome 10, 13 (2022).
Lamas, B. et al. CARD9 impacts colitis by altering gut microbiota metabolism of tryptophan into aryl hydrocarbon receptor ligands. Nat. Med. 22, 598–605 (2016).
Stockinger, B., Shah, K. & Wincent, E. AHR in the intestinal microenvironment: safeguarding barrier function. Nat. Rev. Gastroenterol. Hepatol. 18, 559–570 (2021).
Scott, S. A., Fu, J. & Chang, P. V. Microbial tryptophan metabolites regulate gut barrier function via the aryl hydrocarbon receptor. Proc. Natl Acad. Sci. USA 117, 19376–19387 (2020).
Roager, H. M. & Licht, T. R. Microbial tryptophan catabolites in health and disease. Nat. Commun. 9, 3294 (2018).
Shinde, R. et al. Apoptotic cell-induced AhR activity is required for immunological tolerance and suppression of systemic lupus erythematosus in mice and humans. Nat. Immunol. 19, 571–582 (2018).
Secombe, K. R. et al. Guidelines for reporting on animal fecal transplantation (GRAFT) studies: recommendations from a systematic review of murine transplantation protocols. Gut Microbes 13, 1979878 (2021).
Scheiman, J. et al. Meta-omics analysis of elite athletes identifies a performance-enhancing microbe that functions via lactate metabolism. Nat. Med. 25, 1104–1109 (2019).
Martino, C. et al. Acetate reprograms gut microbiota during alcohol consumption. Nat. Commun. 13, 4630 (2022).
Chen, X. et al. Microglia-mediated T cell infiltration drives neurodegeneration in tauopathy. Nature 615, 668–677 (2023).
Acknowledgements
H.K., C.X. and L.X. acknowledge support from the National Natural Science Foundation of China, grant nos. 21925402, 92156003 and 32071400, respectively.
Author information
Authors and Affiliations
Contributions
L.X., H.K. and C.X. conceived the project and planned the experiments. X.G. and C.L. fabricated and characterized chiral NPs. X.G. and J.Z. carried out experiments in vivo and in vitro. M.S. assisted with the animal behavioral tests. J.X. provided assistance in collecting clinical samples and information. L.X., H.K. and C.X. conceptualized the work. All authors wrote the manuscript and compiled figures, with discussion of results and feedback on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interest.
Peer review
Peer review information
Nature Aging thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Pathologies of APP/PS1 AD mice through FMT (n = 6).
(A) Immunofluorescence of Aβ (red) and p-tau (green) in the hippocampus. (B) Representative immunostaining for Aβ (black arrows) and p-tau (black arrows) protein. (C) Nissl staining of neuro cells in the brains (hippocampus of mice with treatments for 60 days). l-Au→AD: fecal microbiota was transplanted from the donor mice, APP/PS1 AD mice orally administered l-Au NPs, into receptor APP/PS1 AD mice. HT of l-Au→AD: Heat-treatment (HT) of fecal microbiota from the donor mice, APP/PS1 AD mice orally administered l-Au NPs, were transplanted into receptor APP/PS1 AD mice. Scale bars, 50 μm. ‘AD’ stands for APP/PS1 AD model mice. Each ‘n’ represents an independent biological sample.
Extended Data Fig. 2 Restoration of cognition abilities and pathologies of 3xTg AD mice through oral administration of IAA (n = 6).
(A) Immunofluorescence of Aβ (red) and p-tau (green) in the hippocampus. (B) Representative immunostaining for Aβ (black arrows) and p-tau (black arrows) protein. (C) Nissl staining of neuro cells in the brains (hippocampus of mice with treatments for 45 days). Scale bars, 50 μm. CH-223191, the AHR inhibitor. Each ‘n’ represents an independent biological sample.
Extended Data Fig. 3 Restoration of cognition abilities and pathologies of APP/PS1 AD mice through oral administration of IAA and co-housed with WT or APP/PS1 AD mice (n = 6).
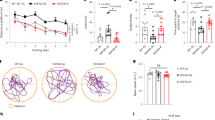
(A) Overview of the experimental design in the effects of microbiota on IAA function. (B) The latent period to find the escape platform in water maze of mice with different treatments for 15 days, 30 days, and 45 days, respectively. (C) Track sheets in water maze of mice with treatments for 45 days. (D) Novel object recognition (NOR) test of mice with different treatments for 45 days. (E) Track sheets in NOR test of mice with treatments for 45 days. (F) Aβ and (G) p-tau concentrations in CSF in 45 days. (H) Immunofluorescence of Aβ (red) and p-tau (green) in the hippocampus. (I) Representative immunostaining for Aβ (black arrows) and p-tau (black arrows) protein. (J) Nissl staining of neuro cells in the brains (hippocampus of mice with treatments for 45 days). i.g: Intragastric administration. IAA co-housed with AD: APP/PS1 AD mice oral administrated of IAA that were housed with APP/PS1 AD mice without treatment. Scale bars, 50 μm. ‘AD’ stands for APP/PS1 AD model mice. Each ‘n’ represents an independent biological sample. One dot represents one mouse. Data are represented as the mean ± SD. *p < 0.05, **p < 0.01, ***p < 0.001. Two-tailed unpaired Student’s t-test (D), one-way (B, F, and G) ANOVA, followed by Tukey’s multiple comparisons test.
Extended Data Fig. 4 Alteration of the ability of tryptophan metabolism to produce IAA in APP/PS1 AD mice after oral administration l-Au NPs (n = 6).
(A) Schematic of the 13C10-IAA produced by 13C11- Trp in APP/PS1 AD mice experimental design. (B-C) Relative abundance of 13C10-IAA in APP/PS1 AD mice serum after oral administration of 13C11- Trp, or 13C11- Trp and l-Au NPs 8 h. i.g: Intragastric administration. Each ‘n’ represents an independent biological sample. One dot represents one mouse. Data are represented as the mean ± SD. ***p < 0.001. Two-tailed unpaired Student’s t-test.
Extended Data Fig. 5 Neuroinflammations in mice oral administration with Au NPs and FMT mice (n = 6).
Contents of (A) IL6, (B) TNF-α, and (C) IL1β in the CSF of APP/PS1 AD mice after oral administration of l-, d-, and t-Au NPs. Contents of (D) IL6, (E) TNF-α, and (F) IL1β in the CSF of FMT mice. l-Au→AD: fecal microbiota were transplanted from the donor mice, APP/PS1 AD mice orally administered l-Au NPs, into receptor APP/PS1 AD mice. HT of l-Au→AD: Heat-treatment (HT) of fecal microbiota from the donor mice, APP/PS1 AD mice orally administered l-Au NPs, were transplanted into receptor APP/PS1 AD mice. ‘AD’ stands for APP/PS1 AD model mice. Each ‘n’ represents an independent biological sample. One dot represents one mouse. Data are represented as the mean ± SD. *p < 0.05, **p < 0.01, ***p < 0.001. One-way ANOVA followed by Tukey’s multiple comparisons test.
Extended Data Fig. 6 Activated state of microglia cells line BV2, astrocytes cells line MA-c in vitro after IAA treatment (n = 6).
Detection of activated (A) M1(CD68+CD86+), (B) M2 (CD206+CD163+) microglia cell line BV2 and (C) A1, (D) A2 astrocyte cell line MA-c by flow cytometry. (E) Detection of activated M1, M2 microglia cell line BV2 and A1, A2 astrocyte cell line MA-c by confocal. Scale bars, 20 μm. CH-223191, the AHR inhibitor. Box plots extend from the 25th to the 75th percentile with the median value shown as a black line in the middle, and whiskers denote the minima and maxima values. Each ‘n’ represents an independent biological sample. One dot represents one sample. Data are represented as the mean ± SD. *p < 0.05, **p < 0.01, ***p < 0.001. One-way (A-D) ANOVA followed by Tukey’s multiple comparisons test.
Extended Data Fig. 7 The cognition abilities and pathologies of 3xTg AD mice through oral administration of IAA after depleted Treg or microglia cells (n = 6).
(A) Overview of the experimental design to explore the key targets of IAA. (B) The latent period to find the escape platform in water maze of mice with different treatments for 15 days, 30 days, and 45 days, respectively. (C) Track sheets in water maze of mice with treatments for 45 days. (D) Novel object recognition (NOR) test of mice with different treatments for 45 days. (E) Track sheets in NOR test of mice with treatments for 45 days. (F) Aβ and (G) p-tau concentrations in CSF in 45 days. (H) Immunofluorescence of Aβ (red) and p-tau (green) in the hippocampus. (I) Representative immunostaining for Aβ (black arrows) and p-tau (black arrows) protein. (J) Nissl staining of neuro cells in the brains (hippocampus of mice with treatments for 45 days). Scale bars, 50 μm. PLX, PLX3397. i.g: Intragastric administration. Each ‘n’ represents an independent biological sample. One dot represents one mouse. Data are represented as the mean ± SD. *p < 0.05, **p < 0.01, ***p < 0.001. Two-tailed unpaired Student’s t-test (D), one-way (B, F, and G) ANOVA, followed by Tukey’s multiple comparisons test. To explore the role of cells, we transiently depleted Treg or microglia cells in 3xTg AD mice (Extended Data Fig. 7a). After depletion of Treg by anti-CD25, 3xTg AD mice showed the improved cognitive function in both Morris water maze experiment and novel object recognition test after treatment with IAA for approximately 45 days, whereas 3xTg AD depleted microglia cells did not (Extended Data Fig. 7b–e). The contents of Aβ and p-tau in the CSF of 3xTg AD mice depleted Treg cells after treatment with IAA for 45 days were the same as those of the WT mice (Extended Data Fig. 7f,g), which also verified by immunohistochemical and immunofluorescence analyses (Extended Data Fig. 7h,i). Nissl staining of the hippocampus in 3xTg AD mice depleted Treg cells showed that the nuclei of the neurons were intact and that the number of neurons had increased after oral administrated of IAA for 45 days, compared with those of 3xTg AD mice (Extended Data Fig. 7j). To be noticed, the concentrations of Aβ and p-tau in CSF, immunohistochemical and immunofluorescence analysis of 3xTg AD mice depleted microglia cells have no any improvements on oral administration with IAA for 45 days.
Extended Data Fig. 8 Impact of IAA on the systemic immune cell population of the 3xTg AD mice (n = 6).
Percentages of (A)Treg cells in CD4+ T cells, (B)TH17 in CD4+ T cells, and (C) IL17+γδT cells in γδ T cells in the colon of 3xTg AD mice. Percentages of (D)Treg cells in CD4+ T cells, (E)TH17 in CD4+ T cells, and (F) IL17+γδT cells in γδ T cells in the periphery of 3xTg AD mice. Percentages of (G)Treg cells in CD4+ T cells, (H)TH17 in CD4+ T cells, and (I) IL17+γδT cells in γδ T cells in the meninges of 3xTg AD mice. Percentages of (J)Treg cells in CD4+ T cells, (K)TH17 in CD4+ T cells, and (L) IL17+γδT cells in γδ T cells in the brain of 3xTg AD mice. CH-223191, the AHR inhibitor. Each ‘n’ represents an independent biological sample. One dot represents one mouse. Data are represented as the mean ± SD. *p < 0.05, **p < 0.01, ***p < 0.001. One-way ANOVA followed by Tukey’s multiple comparisons test. The results displayed the increase in Treg cells and decrease in TH17 and IL17γδT cells in the colon, peripheral and meninges of 3xTg AD mice after treatment with IAA for 45 days. On the contrary, 3xTg AD mice without IAA treatment triggered the decrease in Treg cells and the increase in TH17 and IL17γδT cells in colon, peripheral and meninges. While 3xTg AD mice treated with IAA and CH-223191(an AHR inhibitor), the percentage of Treg cells, TH17 cells, and IL17γδT cells had no changes, which was the same as 3xTg AD mice without IAA treatment. Noticeably, these cells in brain parenchyma were almost no affected.
Extended Data Fig. 9 The inflammasome activity of primary microglia, primary astrocytes cell, microglial cells line BV2, and astrocyte cell line MA-c after treated with IAA in vitro (n = 6).
(A) levels of NLRP3 in microglia cell line BV2 and astrocyte cell line MA-c were determined confocally. Scale bars, 20 μm. (B) Western blot analysis of microglia cell line BV2 and astrocyte cell line MA-c for NLRP3, pro-caspase 1, caspase 1, ASC, pro-IL18, IL18, pro-IL1β, IL1β, NF-κB and β-actin, respectively. The expression levels of NLRP3, caspase-1, IL1β and IL18 in (C) microglial cell line BV2 and (D) astrocyte cell line MA-c were detected by RT-qPCR. Contents of IL18 and IL1β in (E) primary microglia and (F) primary astrocytes cell were determined by ELISA. Contents of IL18 and IL1β in (G) microglia cell line BV2 and (H) astrocyte cell line MA-c were determined by ELISA. CH-223191, the AHR inhibitor. Each ‘n’ represents an independent biological sample. One dot represents one sample. Data are represented as the mean ± SD. *p < 0.05, **p < 0.01, ***p < 0.001. One-way (C-H) ANOVA followed by Tukey’s multiple comparisons test.
Extended Data Fig. 10 The way of Au NPs interacts with bacteria.
(A) Zeta potential of PEGylated Au NPs (n = 3). (B) Confocal and (C) SEM images of L.reuteri incubated with l-Au NPs (n = 6). ITC data and integrated heat data with respect to time for the titration of Trp to (D) l-Au NPs (E) d-Au NPs (F) t-Au NPs. The (G) absorbance (H) CD and (I) Fluorescence of remaining Trp in the supernatant after coincubation of Au NPs with Trp. Scale bars, 2 μm. Each ‘n’ represents an independent sample. Data are represented as the mean ± SD. *p < 0.05, **p < 0.01, ***p < 0.001. One-way (A) followed by Tukey’s multiple comparisons test.
Supplementary information
Supplementary Information
Supplementary materials and methods, Figs. 1–18 and references.
Supplementary Data 1
Statistical source data for Supplementary Fig. 3.
Supplementary Data 2
Statistical source data for Supplementary Fig. 4.
Supplementary Data 3
Statistical source data for Supplementary Fig. 7.
Supplementary Data 4
Statistical source data for Supplementary Fig. 8.
Supplementary Data 5
Statistical source data for Supplementary Fig. 10.
Supplementary Data 6
Statistical source data for Supplementary Fig. 11.
Supplementary Data 7
Statistical source data for Supplementary Fig. 12.
Supplementary Table 1
KEGG pathway enrichment analysis of feces metabolites in APP/PS1 AD mice with or without oral administration of ʟ-Au NPs.
Supplementary Table 2
RT–qPCR primer sequence.
Source data
Source Data Fig. 1
Statistical source data for Fig. 1.
Source Data Fig. 2
Statistical source data for Fig. 2.
Source Data Fig. 3
Statistical source data for Fig. 3.
Source Data Fig. 4
Statistical source data for Fig. 4.
Source Data Fig. 5
Statistical source data for Fig. 5.
Source Data Fig. 6
Statistical source data for Fig. 6.
Source Data
All unprocessed western blots.
Source Data Extended Data Fig. 3
Statistical source data for Extended Data Fig. 3.
Source Data Extended Data Fig. 4
Statistical source data for Extended Data Fig. 4.
Source Data Extended Data Fig. 5
Statistical source data for Extended Data Fig. 5.
Source Data Extended Data Fig. 6
Statistical source data for Extended Data Fig. 6.
Source Data Extended Data Fig. 7
Statistical source data for Extended Data Fig. 7.
Source Data Extended Data Fig. 8
Statistical source data for Extended Data Fig. 8.
Source Data Extended Data Fig. 9
Statistical source data for Extended Data Fig. 9.
Source Data Extended Data Fig. 10
Statistical source data for Extended Data Fig. 10.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Guo, X., Li, C., Zhang, J. et al. Chiral nanoparticle-remodeled gut microbiota alleviates neurodegeneration via the gut–brain axis. Nat Aging 3, 1415–1429 (2023). https://doi.org/10.1038/s43587-023-00516-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s43587-023-00516-9
This article is cited by
-
Gold nanoparticles exhibit anti-osteoarthritic effects via modulating interaction of the “microbiota-gut-joint” axis
Journal of Nanobiotechnology (2024)