Abstract
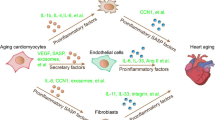
Aging is a major risk factor contributing to pathophysiological changes in the heart, yet its intrinsic mechanisms have been largely unexplored in primates. In this study, we investigated the hypertrophic and senescence phenotypes in the hearts of aged cynomolgus monkeys as well as the transcriptomic and proteomic landscapes of young and aged primate hearts. SIRT2 was identified as a key protein decreased in aged monkey hearts, and engineered SIRT2 deficiency in human pluripotent stem cell-derived cardiomyocytes recapitulated key senescence features of primate heart aging. Further investigations revealed that loss of SIRT2 in human cardiomyocytes led to the hyperacetylation of STAT3, which transcriptionally activated CDKN2B and, in turn, triggered cardiomyocyte degeneration. Intra-myocardial injection of lentiviruses expressing SIRT2 ameliorated age-related cardiac dysfunction in mice. Taken together, our study provides valuable resources for decoding primate cardiac aging and identifies the SIRT2–STAT3–CDKN2B regulatory axis as a potential therapeutic target against human cardiac aging and aging-related cardiovascular diseases.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
The transcriptomic data obtained in the study have been deposited in the Genome Sequence Archive97 in the National Genomics Data Center, Beijing Institute of Genomics (China National Center for Bioinformation), of the Chinese Academy of Sciences, under accession number HRA002883 (human cardiomyocytes) and CRA007858 (monkey hearts). The proteomic data have been deposited to the ProteomeXchange Consortium via the iProX partner repository98 under accession numbers PXD036689 and PXD036693.
Code availability
Codes used for the analysis of RNA sequencing in this study are available at https://github.com/Ykk151/Primate_Heart_aging.
References
Lakatta, E. G. & Levy, D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: part II: the aging heart in health: links to heart disease. Circulation 107, 346–354 (2003).
Stern, S., Behar, S. & Gottlieb, S. Aging and diseases of the heart. Circulation 108, E99–E101 (2003).
Lakatta, E. G. & Levy, D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: part I: aging arteries: a ‘set up’ for vascular disease. Circulation 107, 139–146 (2003).
Paneni, F., Canestro, C. D., Libby, P., Luscher, T. F. & Camici, G. G. The aging cardiovascular system understanding it at the cellular and clinical levels. J. Am. Coll. Cardiol. 69, 1952–1967 (2017).
Li, H. B. et al. Targeting age-related pathways in heart failure. Circ. Res. 126, 533–551 (2020).
Chiao, Y. A. & Rabinovitch, P. S. The aging heart. Cold Spring Harb. Perspect. Med. 5, a025148 (2015).
Nakamura, M. & Sadoshima, J. Mechanisms of physiological and pathological cardiac hypertrophy. Nat. Rev. Cardiol. 15, 387–407 (2018).
Boon, R. A. et al. MicroRNA-34a regulates cardiac ageing and function. Nature 495, 107–110 (2013).
Cai, Y. et al. The landscape of aging. Sci. China Life Sci. 65, 2354–2454 (2022).
Johnson, S. C., Rabinovitch, P. S. & Kaeberlein, M. mTOR is a key modulator of ageing and age-related disease. Nature 493, 338–345 (2013).
Chen, M. S., Lee, R. T. & Garbern, J. C. Senescence mechanisms and targets in the heart. Cardiovasc. Res. 118, 1173–1187 (2022).
Wang, L. et al. Circular RNAs in cardiovascular diseases: regulation and therapeutic applications. Research 6, 0038 (2023).
Zhu, X. et al. Fine-tuning of PGC1alpha expression regulates cardiac function and longevity. Circ. Res. 125, 707–719 (2019).
Chen, R. et al. Personal omics profiling reveals dynamic molecular and medical phenotypes. Cell 148, 1293–1307 (2012).
Zhang, B. et al. Proteogenomic characterization of human colon and rectal cancer. Nature 513, 382–387 (2014).
Li, J. et al. Determining a multimodal aging clock in a cohort of Chinese women. Med https://doi.org/10.1016/j.medj.2023.06.010 (2023).
Wessels, A. & Sedmera, D. Developmental anatomy of the heart: a tale of mice and man. Physiol. Genomics 15, 165–176 (2003).
Cox, L. A. et al. Nonhuman primates and translational research—cardiovascular disease. ILAR J. 58, 235–250 (2017).
Colman, R. J. et al. Caloric restriction delays disease onset and mortality in rhesus monkeys. Science 325, 201–204 (2009).
Biernacka, A. & Frangogiannis, N. G. Aging and cardiac fibrosis. Aging Dis. 2, 158–173 (2011).
Ma, S. et al. Caloric restriction reprograms the single-cell transcriptional landscape of Rattus norvegicus aging. Cell 180, 984–1001 (2020).
Aging Biomarker Consortium et al. Biomarkers of aging. Sci. China Life Sci. 66, 893–1066 (2023).
Engbers, M. J., van Hylckama Vlieg, A. & Rosendaal, F. R. Venous thrombosis in the elderly: incidence, risk factors and risk groups. J. Thromb. Haemost. 8, 2105–2112 (2010).
Li, L., Zhao, Q. & Kong, W. Extracellular matrix remodeling and cardiac fibrosis. Matrix Biol. 68-69, 490–506 (2018).
North, B. J. & Verdin, E. Sirtuins: Sir2-related NAD-dependent protein deacetylases. Genome Biol. 5, 224 (2004).
He, M. et al. An acetylation switch of the NLRP3 inflammasome regulates aging-associated chronic inflammation and insulin resistance. Cell Metab. 31, 580–591 (2020).
Aging Atlas, C. Aging Atlas: a multi-omics database for aging biology. Nucleic Acids Res. 49, D825–D830 (2021).
Cheng, X. L. et al. Overexpression of Kininogen-1 aggravates oxidative stress and mitochondrial dysfunction in DOX-induced cardiotoxicity. Biochem. Biophys. Res. Commun. 550, 142–150 (2021).
Ricklin, D., Reis, E. S., Mastellos, D. C., Gros, P. & Lambris, J. D. Complement component C3—the ‘Swiss Army Knife’ of innate immunity and host defense. Immunol. Rev. 274, 33–58 (2016).
Kamath, S. & Lip, G. Y. H. Fibrinogen: biochemistry, epidemiology and determinants. QJM 96, 711–729 (2003).
Bauman, S. J., Whinna, H. C. & Church, F. C. Serpins (serine protease inhibitors). Curr. Protoc. Protein Sci. Chapter 21, 21.7.1–21.7.14 (2002).
Janciauskiene, S. Conformational properties of serine proteinase inhibitors (serpins) confer multiple pathophysiological roles. Biochim. Biophys. Acta 1535, 221–235 (2001).
Lehallier, B. et al. Undulating changes in human plasma proteome profiles across the lifespan. Nat. Med. 25, 1843–1850 (2019).
Ganz, P. et al. Development and validation of a protein-based risk score for cardiovascular outcomes among patients with stable coronary heart disease. JAMA 315, 2532–2541 (2016).
Burridge, P. W. et al. Human induced pluripotent stem cell-derived cardiomyocytes recapitulate the predilection of breast cancer patients to doxorubicin-induced cardiotoxicity. Nat. Med. 22, 547–556 (2016).
Lee, J. et al. Activation of PDGF pathway links LMNA mutation to dilated cardiomyopathy. Nature 572, 335–340 (2019).
Karakikes, I., Ameen, M., Termglinchan, V. & Wu, J. C. Human induced pluripotent stem cell-derived cardiomyocytes: insights into molecular, cellular, and functional phenotypes. Circ. Res. 117, 80–88 (2015).
Burridge, P. W., Holmstrom, A. & Wu, J. C. Chemically defined culture and cardiomyocyte differentiation of human pluripotent stem cells. Curr. Protoc. Hum. Genet. 87, 21.3.1–21.3.15 (2015).
Tohyama, S. et al. Distinct metabolic flow enables large-scale purification of mouse and human pluripotent stem cell-derived cardiomyocytes. Cell Stem Cell 12, 127–137 (2013).
Eisner, D. A., Caldwell, J. L., Kistamas, K. & Trafford, A. W. Calcium and excitation-contraction coupling in the heart. Circ. Res. 121, 181–195 (2017).
Su, S. A. et al. Cardiac Piezo1 exacerbates lethal ventricular arrhythmogenesis by linking mechanical stress with Ca2+ handling after myocardial infarction. Research 6, 0165 (2023).
Han, H. et al. TRRUST v2: an expanded reference database of human and mouse transcriptional regulatory interactions. Nucleic Acids Res. 46, D380–D386 (2018).
Hillmer, E. J., Zhang, H., Li, H. S. & Watowich, S. S. STAT3 signaling in immunity. Cytokine Growth Factor Rev. 31, 1–15 (2016).
Rufini, A., Tucci, P., Celardo, I. & Melino, G. Senescence and aging: the critical roles of p53. Oncogene 32, 5129–5143 (2013).
Sreedhar, R. et al. Role of MAPK-mediated endoplasmic reticulum stress signaling in the heart during aging in senescence-accelerated prone mice. Biofactors 42, 368–375 (2016).
Shao, B., Zheng, L., Shi, J. & Sun, N. Acetylation of ANXA1 reduces caspase-3 activation by enhancing the phosphorylation of caspase-9 under OGD/R conditions. Cell. Signal. 88, 110157 (2021).
Wang, Y., Yang, J., Hong, T., Chen, X. & Cui, L. SIRT2: controversy and multiple roles in disease and physiology. Ageing Res. Rev. 55, 100961 (2019).
Yuan, Z. L., Guan, Y. J., Chatterjee, D. & Chin, Y. E. Stat3 dimerization regulated by reversible acetylation of a single lysine residue. Science 307, 269–273 (2005).
Zhao, H. et al. Destabilizing heterochromatin by APOE mediates senescence. Nat. Aging 2, 303–316 (2022).
Ling, C. et al. Modeling CADASIL vascular pathologies with patient-derived induced pluripotent stem cells. Protein Cell 10, 249–271 (2019).
Kim, W. Y. & Sharpless, N. E. The regulation of INK4/ARF in cancer and aging. Cell 127, 265–275 (2006).
Gil, J. & Peters, G. Regulation of the INK4b–ARF–INK4a tumour suppressor locus: all for one or one for all. Nat. Rev. Mol. Cell Biol. 7, 667–677 (2006).
Krimpenfort, P. et al. p15Ink4b is a critical tumour suppressor in the absence of p16Ink4a. Nature 448, 943–946 (2007).
Wang, W. et al. A genome-wide CRISPR-based screen identifies KAT7 as a driver of cellular senescence. Sci. Transl. Med. 13, eabd2655 (2021).
Houtkooper, R. H., Pirinen, E. & Auwerx, J. Sirtuins as regulators of metabolism and healthspan. Nat. Rev. Mol. Cell Biol. 13, 225–238 (2012).
Michishita, E., Park, J. Y., Burneskis, J. M., Barrett, J. C. & Horikawa, I. Evolutionarily conserved and nonconserved cellular localizations and functions of human SIRT proteins. Mol. Biol. Cell 16, 4623–4635 (2005).
Korotkov, A., Seluanov, A. & Gorbunova, V. Sirtuin 6: linking longevity with genome and epigenome stability. Trends Cell Biol. 31, 994–1006 (2021).
Sundaresan, N. R. et al. The sirtuin SIRT6 blocks IGF-Akt signaling and development of cardiac hypertrophy by targeting c-Jun. Nat. Med. 18, 1643–1650 (2012).
Matsushima, S. & Sadoshima, J. The role of sirtuins in cardiac disease. Am. J. Physiol. Heart Circ. Physiol. 309, H1375–H1389 (2015).
de Oliveira, R. M., Sarkander, J., Kazantsev, A. G. & Outeiro, T. F. SIRT2 as a therapeutic target for age-related disorders. Front. Pharmacol. 3, 82 (2012).
Serrano, L. et al. The tumor suppressor SirT2 regulates cell cycle progression and genome stability by modulating the mitotic deposition of H4K20 methylation. Genes Dev. 27, 639–653 (2013).
Sarikhani, M. et al. SIRT2 deacetylase regulates the activity of GSK3 isoforms independent of inhibitory phosphorylation. eLife 7, e32952 (2018).
North, B. J. et al. SIRT2 induces the checkpoint kinase BubR1 to increase lifespan. EMBO J. 33, 1438–1453 (2014).
Tang, X. et al. SIRT2 acts as a cardioprotective deacetylase in pathological cardiac hypertrophy. Circulation 136, 2051–2067 (2017).
Sarikhani, M. et al. SIRT2 deacetylase represses NFAT transcription factor to maintain cardiac homeostasis. J. Biol. Chem. 293, 5281–5294 (2018).
Wang, R., Cherukuri, P. & Luo, J. Activation of Stat3 sequence-specific DNA binding and transcription by p300/CREB-binding protein-mediated acetylation. J. Biol. Chem. 280, 11528–11534 (2005).
Ren, J. et al. The Aging Biomarker Consortium represents a new era for aging research in China. Nat. Med. https://doi.org/10.1038/s41591-023-02444-y (2023).
Wang, S. et al. Single-cell transcriptomic atlas of primate ovarian aging. Cell 180, 585–600 (2020).
Li, J. et al. A single-cell transcriptomic atlas of primate pancreatic islet aging. Natl Sci. Rev. 8, nwaa127 (2021).
Huang, D. et al. A single-nucleus transcriptomic atlas of primate testicular aging reveals exhaustion of the spermatogonial stem cell reservoir and loss of Sertoli cell homeostasis. Protein Cell https://doi.org/10.1093/procel/pwac057 (2022).
Zhang, Y. et al. Single-nucleus transcriptomics reveals a gatekeeper role for FOXP1 in primate cardiac aging. Protein Cell 14, 279–293 (2023).
Zhang, W. et al. A single-cell transcriptomic landscape of primate arterial aging. Nat. Commun. 11, 2202 (2020).
Ma, S. et al. Single-cell transcriptomic atlas of primate cardiopulmonary aging. Cell Res. 31, 415–432 (2021).
Ma, S. et al. Heterochronic parabiosis induces stem cell revitalization and systemic rejuvenation across aged tissues. Cell Stem Cell 29, 990–1005 (2022).
Zhang, W. et al. Aging stem cells. A Werner syndrome stem cell model unveils heterochromatin alterations as a driver of human aging. Science 348, 1160–1163 (2015).
Lei, J. et al. Human ESC-derived vascular cells promote vascular regeneration in a HIF-1α dependent manner. Protein Cell https://doi.org/10.1093/procel/pwad027 (2023).
Wang, C. et al. MAVS antagonizes human stem cell senescence as a mitochondrial stabilizer. Research 6, 0192 (2023).
Yan, P. Z. et al. FOXO3-Engineered Human ESC-Derived Vascular Cells Promote Vascular Protection and Regeneration. Cell Stem Cell 24, 447–461.e8 (2019).
Zhang, X., Cao, H., Bai, S., Huo, W. & Ma, Y. Differentiation and characterization of rhesus monkey atrial and ventricular cardiomyocytes from induced pluripotent stem cells. Stem Cell Res. 20, 21–29 (2017).
Lian, X. et al. Directed cardiomyocyte differentiation from human pluripotent stem cells by modulating Wnt/β-catenin signaling under fully defined conditions. Nat. Protoc. 8, 162–175 (2013).
Zhao, H. et al. APOE-mediated suppression of the lncRNA MEG3 protects human cardiovascular cells from chronic inflammation. Protein Cell https://doi.org/10.1093/procel/pwad017 (2023).
Yang, X. et al. Tri-iodo-l-thyronine promotes the maturation of human cardiomyocytes-derived from induced pluripotent stem cells. J. Mol. Cell. Cardiol. 72, 296–304 (2014).
Liang, C. et al. Stabilization of heterochromatin by CLOCK promotes stem cell rejuvenation and cartilage regeneration. Cell Res. 31, 187–205 (2021).
Liu, X. et al. Deacetylation of NAT10 by Sirt1 promotes the transition from rRNA biogenesis to autophagy upon energy stress. Nucleic Acids Res. 46, 9601–9616 (2018).
Zhang, W. Q. et al. A Werner syndrome stem cell model unveils heterochromatin alterations as a driver of human aging. Science 348, 1160–1168 (2015).
Mattila, M., Koskenvuo, J., Soderstrom, M., Eerola, K. & Savontaus, M. Intramyocardial injection of SERCA2a-expressing lentivirus improves myocardial function in doxorubicin-induced heart failure. J. Gene Med. 18, 124–133 (2016).
Wang, S. et al. A single-cell transcriptomic landscape of the lungs of patients with COVID-19. Nat. Cell Biol. 23, 1314–1328 (2021).
Kim, D., Langmead, B. & Salzberg, S. L. HISAT: a fast spliced aligner with low memory requirements. Nat. Methods 12, 357–360 (2015).
Anders, S., Pyl, P. T. & Huber, W. HTSeq—a Python framework to work with high-throughput sequencing data. Bioinformatics 31, 166–169 (2015).
Pertea, M. et al. StringTie enables improved reconstruction of a transcriptome from RNA-seq reads. Nat. Biotechnol. 33, 290–295 (2015).
Love, M. I., Huber, W. & Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550 (2014).
Mure, L. S. et al. Diurnal transcriptome atlas of a primate across major neural and peripheral tissues. Science 359, eaao0318 (2018).
Li, M. L. et al. 547 transcriptomes from 44 brain areas reveal features of the aging brain in non-human primates. Genome Biol. 20, 258 (2019).
Zhou, Y. et al. Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat. Commun. 10, 1523 (2019).
Subramanian, A., Kuehn, H., Gould, J., Tamayo, P. & Mesirov, J. P. GSEA-P: a desktop application for gene set enrichment analysis. Bioinformatics 23, 3251–3253 (2007).
Lei, J. et al. FOXO3-engineered human mesenchymal progenitor cells efficiently promote cardiac repair after myocardial infarction. Protein Cell 12, 145–151 (2021).
Chen, T. T. et al. The genome sequence archive family: toward explosive data growth and diverse data types. Genomics Proteomics Bioinformatics 19, 578–583 (2021).
Ma, J. et al. iProX: an integrated proteome resource. Nucleic Acids Res. 47, D1211–D1217 (2019).
Acknowledgements
We would like to thank Ruoqi Wang (Institute of Biophysics, Chinese Academy of Sciences) for the help with the generation of SIRT2-deficient hESCs. We thank the Center for Biological Imaging, Institute of Biophysics, Chinese Academy of Sciences, for the TEM analysis, and we are grateful to C. Peng for the TEM sample preparation and X. Li for help with the TEM operation. We thank S. Li, X. Zhu, J. Hao and Y. Teng (the Center of Imaging facility of the Institute of Zoology and Institute of Biophysics, Chinese Academy of Sciences) for help with imaging scanning as well as L. Wang for help with the echocardiography analysis. We thank J. Wang for help with the LC–MS/MS assay and J. Jia for help with the FACS experiment. We thank F. Liu, J. Jia and C. Xie for help with animal experiments; D. Lv and C. Zhang for help with the H&E assay; and L. Bai, Q. Chu, J. Lu, L. Tian, S. Qiao, Y. Yang, X. Chen, J. Chen, R. Bai and S. Ma for administrative assistance. Figures 1a, 3a, 5a,g and 7a,e,i, Extended Data Fig. 9a and Supplementary Fig. 1 were created with BioRender. This work was supported by the National Key Research and Development Program of China (2022YFA1103700 to W.Z. and J.Q.), the National Natural Science Foundation of China (91949209 to J.Q. and 32000510 to Y.F.), the National Key Research and Development Program of China (2022YFC3602500 to Y. Ye; 2020YFA0804000 to G.-H.L. and S.W.; 2020YFA0112200 to G.-H.L.; 2021YFF1201000 to W.Z.; the STI2030-Major Projects-2021ZD0202400 to S.W.; and 2022YFA1104700 to Y. Ye), the National Natural Science Foundation of China (81921006 to G.-H.L., J.Q. and M.S.; 82125011 to J.Q.; 92149301 to G.-H.L. and S.W.; 92168201 to G.-H.L.; 92049304 to J.Q.; 92049116 to W.Z.; 32121001 to W.Z.; 82192863 to W.Z.; 82122024 to S.W.; 82071588 to S.W.; 81601233 to Y. Ye; and 82201714 to D.H.), the Strategic Priority Research Program of the Chinese Academy of Sciences (XDA16000000 to G.-H.L., J.Q., M.S., W.Z. and S.W.), the Chinese Academy of Sciences Project for Young Scientists in Basic Research (YSBR-076 to G.-H.L., J.Q. and M.S. and YSBR-012 to W.Z.), the Program of the Beijing Natural Science Foundation (Z190019 to G.-H.L. and W.Z. and JQ20031 to M.S.), the Project for Technology Development of Beijing-affiliated Medical Research Institutes (11000023T000002036310 to S.W.), the Youth Innovation Promotion Association of the Chinese Academy of Sciences (E1CAZW0401 to W.Z.), the Young Elite Scientists Sponsorship Program by CAST (YESS20200012 to S.W.), the Informatization Plan of the Chinese Academy of Sciences (CAS-WX2021SF-0301 to G.-H.L.; CAS-WX2022SDC-XK14 to G.-H.L.; and CAS-WX2021SF-0101 to J.Q.), the Tencent Foundation (2021-1045 to G.-H.L.), the Excellent Young Talents Program of Capital Medical University (12300927 to S.W.), the Excellent Young Talents Training Program for the Construction of Beijing Municipal University Teacher Team (BPHR202203105 to S.W.), the Pilot Project for Public Welfare Development and Reform of Beijing-affiliated Medical Research Institutes (11000022T000000461062 to S.W.) and the Fellowship of China Postdoctoral Science Foundation (2022M712216 to D.H.). The funders had no role in study design, data collection and analysis, the decision to publish or the preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
G.-H.L., J.Q. and W.Z. conceived the work and supervised the overall experiments. Y. Ye performed the experiments related to the phenotypic and mechanistic analyses, including western blotting, ChIP–qPCR, co-IP, immunofluorescence and immunohistochemistry staining, cardiomyocyte differentiation, plasmid construction and animal experiments. K.Y. performed bioinformatic analyses. H.L., Y. Yu and M.S. performed the manuscript writing. D.H. performed the co-IP experiments. J.L. performed the animal experiments. Y.Z. performed the H&E, immunofluorescence, Masson’s trichrome staining in cynomolgus monkeys and Ki67 staining in hESCs. Z.L. helped with the bioinformatic analyses. Q.C. performed the immunofluorescence staining of teratomas. Y.F., S.Z. and Y.J. helped with the sample preparation. C.R.E., S.W. and J.C.I.B. helped with supervision of the project. G.-H.L., J.Q., W.Z., Y. Ye, K.Y., H.L., Y. Yu and M.S. performed manuscript writing, reviewing and editing. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
J.C.I.B. is an employee of Altos Labs. The other authors declare no competing interests.
Peer review
Peer review information
Nature Aging thanks Laura Cox, Mark Sussman and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
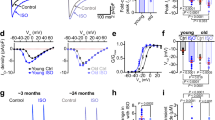
Extended Data Fig. 1 Proteomic analysis of aged and young monkey hearts.
a, Box plot showing the normalized intensities for each replicate in proteomics of young and aged monkey hearts. The boxes show the median values (centre line) and interquartile ranges. The whiskers extend from the quartile to the minimum and maximum values. b, Bar plot showing the distribution of the number of peptides in identified proteins of proteomics of young and aged monkey hearts. c, Heatmap showing the Euclidean distance for proteomic data between young and aged monkey hearts. The color key of the Euclidean distance from blue to white indicates strong to weak correlations. d, Principal component analysis of proteomic data between young and aged monkey hearts. e, Heatmap showing the DEPs overlapped with the epigenetic regulation-associated genes in aged monkey hearts. The color key from blue to purple indicates log2(fold change) from low to high. f, Left, box plot showing the protein level of SIRT2 in young and aged female monkey hearts. Right, box plot showing the protein level of SIRT2 in young and aged male monkey hearts. The boxes show the median values (centre line) and interquartile ranges. The whiskers extend from the quartile to the minimum and maximum values. Two-tailed Student’s t-test, n = 3 replicates per group. g, Western blotting of SIRT2 in young and aged female or male monkey hearts. Data are presented as the mean ± s.e.m., two-tailed Student’s t-test. n = 4 biological replicates. h, Network plot showing DEPs that are common to the aged monkey hearts and plasma of aging individuals or patients with cardiovascular diseases. Node colors from blue to purple indicate log2(fold change) from low to high. i, Point plot showing DEPs that have not been annotated with four aging-related cardiovascular diseases. Point size indicates absolute log2(fold change).
Extended Data Fig. 2 Characterizing hESCs and analysis of p21 expression in human cardiomyocytes.
a, Schematic diagram of SIRT2 knockout strategy through CRISPR-Cas9-mediated non-homologous end joining (NHEJ) in SIRT2+/+ hESCs. b, Western blotting of SIRT2 in SIRT2+/+ and SIRT2-/- hESCs. GAPDH was used as the control. c, Karyotype analysis of SIRT2-/- hESCs (female). d, Immunofluorescence analysis of TuJ1, SMA, and FOXA2 to evaluate the differentiation potential of SIRT2+/+ and SIRT2-/- hESCs toward lineages of all three germ layers, that is, ectoderm, mesoderm, and endoderm, respectively. Scale bars, 50 μm. e, Immunofluorescence analysis of Ki67 expression in SIRT2+/+ and SIRT2-/- hESCs. Scale bars, 25 μm. f, Flow cytometry gating strategy for Fig. 3b. g, Western blotting of p21 in SIRT2+/+ and SIRT2-/- human cardiomyocytes (whole blot). GAPDH was used as the control. h, Flow cytometry gating strategy for Fig. 3l. Two-tailed Student’s t-test was used for statistical analysis. Data are presented as the mean ± s.e.m. Data are representative of 3 independent experiments in b. n = 5 biological replicates in e. Data are representative of 5 independent experiments in g. hCardiomyocyte, human cardiomyocyte.
Extended Data Fig. 3 Transcriptomic analysis of human cardiomyocytes and monkey hearts.
a, Bar plot showing the percentage of duplicate reads, GC content and total sequences for RNA-seq of SIRT2-/- and SIRT2+/+ human cardiomyocytes. b, Bar plot showing the percentage of duplicate reads, GC content and total sequences for RNA-seq of young and aged monkey hearts. c, Bar plot showing representative GO terms enriched by DEGs in SIRT2-/- human cardiomyocytes. Key DEGs enriched in the indicated terms are listed. d, Bar plot showing representative GO terms enriched by DEGs in aged monkey hearts. Key DEGs enriched in the indicated terms are listed. hCardiomyocyte, human cardiomyocyte; mkHeart, monkey heart.
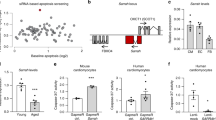
Extended Data Fig. 4 Gene set analysis and TF analysis in aged monkey hearts and SIRT2-deficient human cardiomyocytes.
a, Gene set enrichment analysis (GSEA) plot showing the upregulated calcium signaling pathway and downregulated lipid metabolism shared in aged monkey hearts and SIRT2-deficient human cardiomyocytes (hCM). b, Network plot showing STAT3 target DEGs in aged monkey hearts. Node colors from white to purple indicate log2(fold change) from low to high. c, Left, bar plot showing the TFs enriched by DEGs in aged female monkey hearts. Right, bar plot showing the TFs enriched by DEGs in aged male monkey hearts. d, Left, GSEA plot showing STAT3 target DEGs in aged female monkey hearts; Right, GSEA plot showing STAT3 target DEGs in aged male monkey hearts. hCardiomyocyte, human cardiomyocyte; mkHeart, monkey heart; NES, normalized enrichment score.
Extended Data Fig. 5 STAT3 interacts with the deacetylation-defective mutant SIRT2 (H187Y).
Co-IP analysis of STAT3 with exogenous FLAG-SIRT2-H187Y in HEK293T cells. Data are representative of 3 independent experiments.
Extended Data Fig. 6 Dissecting the SIRT2-STAT3 axis in human pluripotent stem cell-derived cardiomyocytes.
a, Violin plot showing the transcriptional levels of STAT3 in SIRT2+/+ and SIRT2-/- human cardiomyocytes. The black lines represent the mean value. FPKM, Fragments Per Kilobase per Million mapped reads. b, Verification of STAT3 mRNA expression levels by RT-qPCR in SIRT2+/+ and SIRT2-/- human cardiomyocytes. c, Western blotting of STAT3 in SIRT2+/+ and SIRT2-/- human cardiomyocytes. d,e, Western blotting of SIRT2 (d), as well as RT-qPCR analysis of the relative expression levels of BNP (e) in male hESC-derived cardiomyocytes transduced with lentiviral shRNAs targeting SIRT2 (shSIRT2) or non-targeting control (shGL2). f,g, Western blotting of SIRT2 (f), as well as RT-qPCR analysis of the relative expression levels of BNP (g) in male human induced pluripotent stem cell (hiPSC)-derived cardiomyocytes transduced with lentiviral shSIRT2 or shGL2. h,i, Western blotting showing the acetylation level of STAT3-K685 in male hESC-derived (h) or hiPSC-derived (i) cardiomyocytes transduced with lentiviral shSIRT2 or shGL2. Two-tailed Student’s t-test was used for statistical analysis in a-i. Data are presented as the mean ± s.e.m. n = 3, 4, 3, 3 biological replicates in a, b, e, g, respectively. n = 3 independent experiments in c, d, f, h and i. For western blotting, GAPDH was used as the control. hCardiomyocyte, human cardiomyocyte.
Extended Data Fig. 7 Dissecting the SIRT2-STAT3-CDKN2B axis in monkey hearts.
a, Violin plot showing the transcriptional levels of STAT3 in young and aged monkey hearts. The black lines represent the mean value. b, Violin plot showing the protein level of STAT3 in young and aged monkey hearts. The black lines represent the mean value. c, Verification of STAT3 mRNA expression levels by RT-qPCR in the young and aged monkey hearts. d, Western blotting of STAT3 in the young and aged monkey hearts. e,f, The acetylation level of STAT3 in young and aged female (e) or male (f) monkey hearts. Lysates prepared from young and aged female or male monkey hearts were immunoprecipitated with antibodies to STAT3 and analyzed with antibodies to acetylated lysine by western blotting. g, Left, box plot showing the transcriptional level of CDKN2B in young and aged female monkey hearts; Right, box plot showing the transcriptional level of CDKN2B in young and aged male monkey hearts. The boxes show the median values (centre line) and interquartile range. The whiskers extend from the quartile to the minimum and maximum values. h, Western blotting of CDKN2B in young and aged female or male monkey hearts. Two-tailed Student’s t-test was used for statistical analysis in a-h. Data are presented as the mean ± s.e.m. n = 8 biological replicates in a, c and d. n = 6 replicates in b. n = 3 replicates in e and f, representative of 2 independent experiments, respectively. n = 4 biological replicates in g and h. For western blotting, GAPDH was used as the control. mkHeart, monkey heart.
Extended Data Fig. 8 Dissecting the SIRT2-STAT3-CDKN2B axis in human cardiomyocytes.
a,b, Western blotting of CDKN2B in male hESC-derived (a) or hiPSC-derived (b) cardiomyocytes transduced with lentiviral shSIRT2 or shGL2. c, Western blotting of CDKN2B in wildtype human cardiomyocytes transduced with lentiviruses expressing Luc, STAT3-WT, or STAT3-K685R. Two-tailed Student’s t-test was used for statistical analysis in a-c. Data are presented as the mean ± s.e.m. n = 4, 3, 3 independent experiments in a, b, c, respectively. For western blotting, GAPDH was used as the control. hCardiomyocyte, human cardiomyocyte.
Extended Data Fig. 9 CDKN2B mediates aging phenotype in human cardiomyocytes.
a-c, Schematic diagram (a), western blotting of CDKN2B (b), and immunofluorescence analysis of cTnT (c) in wildtype human cardiomyocytes transduced with lentiviruses expressing Luc or CDKN2B. Scale bars in c, 20 μm. d, SA-β-Gal staining of wildtype human cardiomyocytes transduced with lentiviruses expressing Luc or CDKN2B. Scale bars, 50 μm. e, Western blotting of CDKN2B in SIRT2-/- human cardiomyocytes transfected with vectors expressing Cas9 and sgNTC or sgCDKN2B. Two-tailed Student’s t-test was used for statistical analysis in b-e. Data are presented as the mean ± s.e.m. n = 4, 3 independent experiments in b, e, respectively. n = 100 cells examined in 3 independent experiments in c. n = 3 biological replicates in d. For western blotting, GAPDH was used as the control.
Supplementary information
Supplementary information
Supplementary Fig. 1
Supplementary Tables
Supplementary Tables 1–6. Supplementary Table 1: Information summary of the cynomolgus monkeys used in this study. Supplementary Table 2: DEPs between aged and young cynomolgus monkey hearts. Supplementary Table 3: DEGs between aged and young cynomolgus monkey hearts or between SIRT2−/− and SIRT2+/+ human cardiomyocytes. Supplementary Table 4: The candidate SIRT2-interacting proteins identified by mass spectrometry. Supplementary Table 5: List of antibodies used in this study. Supplementary Table 6: The sequences of sgRNAs and primers used for gene editing, plasmid construction, RT–qPCR and ChIP–qPCR.
Source data
Source Data Fig. 1
Unprocessed western blots.
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Unprocessed western blots.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Unprocessed western blots.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 5
Unprocessed western blots.
Source Data Fig. 5
Statistical source data.
Source Data Fig. 6
Unprocessed western blots.
Source Data Fig. 6
Statistical source data.
Source Data Fig. 7
Unprocessed western blots.
Source Data Fig. 7
Statistical source data.
Source Data Extended Data Fig. 1
Unprocessed western blots.
Source Data Extended Data Fig. 1
Statistical source data.
Source Data Extended Data Fig. 2
Unprocessed western blots.
Source Data Extended Data Fig. 2
Statistical source data.
Source Data Extended Data Fig. 5
Unprocessed western blots.
Source Data Extended Data Fig. 6
Unprocessed western blots.
Source Data Extended Data Fig. 6
Statistical source data.
Source Data Extended Data Fig. 7
Unprocessed western blots.
Source Data Extended Data Fig. 7
Statistical source data.
Source Data Extended Data Fig. 8
Unprocessed western blots.
Source Data Extended Data Fig. 8
Statistical source data.
Source Data Extended Data Fig. 9
Unprocessed western blots.
Source Data Extended Data Fig. 9
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ye, Y., Yang, K., Liu, H. et al. SIRT2 counteracts primate cardiac aging via deacetylation of STAT3 that silences CDKN2B. Nat Aging 3, 1269–1287 (2023). https://doi.org/10.1038/s43587-023-00486-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s43587-023-00486-y
This article is cited by
-
Sirtuin 2 protects against cardiac ageing
Nature Reviews Cardiology (2023)
-
SIRT2 counteracts primate cardiac aging
Nature Aging (2023)
-
CHIT1-positive microglia drive motor neuron ageing in the primate spinal cord
Nature (2023)