Abstract
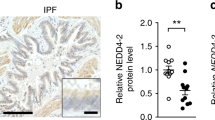
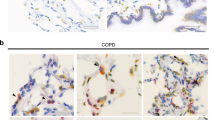
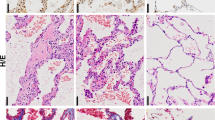
Aging is a risk factor for progressive fibrotic disorders involving diverse organ systems, including the lung. Idiopathic pulmonary fibrosis, an age-associated degenerative lung disorder, is characterized by persistence of apoptosis-resistant myofibroblasts. Here we demonstrate that sirtuin 3 (SIRT3), a mitochondrial deacetylase, is downregulated in the lungs of humans with idiopathic pulmonary fibrosis and in mice subjected to lung injury. Overexpression of Sirt3 cDNA via airway delivery restored the capacity for fibrosis resolution in aged mice, in association with activation of the forkhead box transcription factor FoxO3a in fibroblasts, upregulation of pro-apoptotic members of the Bcl2 family and recovery of apoptosis susceptibility. While transforming growth factor-β1 reduced levels of SIRT3 and FOXO3A in lung fibroblasts, cell non-autonomous effects involving macrophage-secreted products were necessary for SIRT3-mediated activation of FOXO3A. Together, these findings reveal a novel role of SIRT3 in pro-resolution macrophage functions that restore susceptibility to apoptosis in fibroblasts via a FOXO3A-dependent mechanism.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
Microarray data have been deposited in the Gene Expression Omnibus with the accession code number GSE17518. The authors declare that the main data supporting the findings of this study are available within the article and its Supplementary Information. Source data are provided with this paper. Extra data are available from the corresponding author upon request.
References
Thannickal, V. J., Zhou, Y., Gaggar, A. & Duncan, S. R. Fibrosis: ultimate and proximate causes. J. Clin. Invest.124, 4673–4677 (2014).
Thannickal, V. J., Toews, G. B., White, E. S., Lynch, J. P. 3rd & Martinez, F. J. Mechanisms of pulmonary fibrosis. Annu. Rev. Med.55, 395–417 (2004).
Wynn, T. A. & Ramalingam, T. R. Mechanisms of fibrosis: therapeutic translation for fibrotic disease. Nat. Med.18, 1028–1040 (2012).
Kapetanaki, M. G., Mora, A. L. & Rojas, M. Influence of age on wound healing and fibrosis. J. Pathol.229, 310–322 (2013).
Thannickal, V. J. Mechanistic links between aging and lung fibrosis. Biogerontology14, 609–615 (2013).
Newgard, C. B. & Sharpless, N. E. Coming of age: molecular drivers of aging and therapeutic opportunities. J. Clin. Invest.123, 946–950 (2013).
Ferrucci, L. et al. Measuring biological aging in humans: a quest. Aging Cell19, e13080 (2020).
Goodell, M. A. & Rando, T. A. Stem cells and healthy aging. Science350, 1199–1204 (2015).
Raghu, G., Chen, S. Y., Hou, Q., Yeh, W. S. & Collard, H. R. Incidence and prevalence of idiopathic pulmonary fibrosis in US adults 18–64 years old. Eur. Respir. J.48, 179–186 (2016).
Kennedy, B. K. et al. Geroscience: linking aging to chronic disease. Cell159, 709–713 (2014).
Thannickal, V. J. et al. Blue Journal Conference. Aging and susceptibility to lung disease. Am. J. Respir. Crit. Care Med.191, 261–269 (2015).
Lopez-Otin, C., Blasco, M. A., Partridge, L., Serrano, M. & Kroemer, G. The hallmarks of aging. Cell153, 1194–1217 (2013).
Willcox, B. J. et al. FOXO3A genotype is strongly associated with human longevity. Proc. Natl Acad. Sci. USA105, 13987–13992 (2008).
Broer, L. et al. GWAS of longevity in CHARGE consortium confirms APOE and FOXO3 candidacy. J. Gerontol. A70, 110–118 (2015).
Rose, G. et al. Variability of the SIRT3 gene, human silent information regulator Sir2 homologue, and survivorship in the elderly. Exp. Gerontol.38, 1065–1070 (2003).
van de Ven, R. A. H., Santos, D. & Haigis, M. C. Mitochondrial sirtuins and molecular mechanisms of aging. Trends Mol. Med.23, 320–331 (2017).
North, B. J. & Verdin, E. Sirtuins: Sir2-related NAD-dependent protein deacetylases. Genome Biol.5, 224 (2004).
Haigis, M. C. & Guarente, L. P. Mammalian sirtuins–emerging roles in physiology, aging, and calorie restriction. Genes Dev.20, 2913–2921 (2006).
Onyango, P., Celic, I., McCaffery, J. M., Boeke, J. D. & Feinberg, A. P. SIRT3, a human SIR2 homologue, is an NAD-dependent deacetylase localized to mitochondria. Proc. Natl Acad. Sci. USA99, 13653–13658 (2002).
Someya, S. et al. Sirt3 mediates reduction of oxidative damage and prevention of age-related hearing loss under caloric restriction. Cell143, 802–812 (2010).
Hirschey, M. D. et al. SIRT3 deficiency and mitochondrial protein hyperacetylation accelerate the development of the metabolic syndrome. Mol. Cell44, 177–190 (2011).
Yu, W. et al. Loss of SIRT3 provides growth advantage for b cell malignancies. J. Biol. Chem.291, 3268–3279 (2016).
Lam, E. W., Brosens, J. J., Gomes, A. R. & Koo, C. Y. Forkhead box proteins: tuning forks for transcriptional harmony. Nat. Rev. Cancer13, 482–495 (2013).
Paik, J. H. et al. FoxOs are lineage-restricted redundant tumor suppressors and regulate endothelial cell homeostasis. Cell128, 309–323 (2007).
Tseng, A. H., Shieh, S. S. & Wang, D. L. SIRT3 deacetylates FOXO3 to protect mitochondria against oxidative damage. Free Radic. Biol. Med.63, 222–234 (2013).
Kops, G. J. et al. Forkhead transcription factor FOXO3a protects quiescent cells from oxidative stress. Nature419, 316–321 (2002).
Al-Tamari, H. M. et al. FoxO3 an important player in fibrogenesis and therapeutic target for idiopathic pulmonary fibrosis. EMBO Mol. Med.10, 276–293 (2018).
Nho, R. S., Hergert, P., Kahm, J., Jessurun, J. & Henke, C. Pathological alteration of FoxO3a activity promotes idiopathic pulmonary fibrosis fibroblast proliferation on type I collagen matrix. Am. J. Pathol.179, 2420–2430 (2011).
Jablonski, R. P. et al. SIRT3 deficiency promotes lung fibrosis by augmenting alveolar epithelial cell mitochondrial DNA damage and apoptosis. FASEB J.31, 2520–2532 (2017).
Sosulski, M. L., Gongora, R., Feghali-Bostwick, C., Lasky, J. A. & Sanchez, C. G. Sirtuin 3 deregulation promotes pulmonary fibrosis. J. Gerontol. A72, 595–602 (2017).
Rangarajan, S. et al. Metformin reverses established lung fibrosis in a bleomycin model. Nat. Med.24, 1121–1127 (2018).
McGlynn, L. M. et al. SIRT3 & SIRT7: potential novel biomarkers for determining outcome in pancreatic cancer patients. PLoS ONE10, e0131344 (2015).
Hecker, L. et al. Reversal of persistent fibrosis in aging by targeting Nox4-Nrf2 redox imbalance. Sci. Transl. Med.6, 231ra247 (2014).
Hinz, B. & Lagares, D. Evasion of apoptosis by myofibroblasts: a hallmark of fibrotic diseases. Nat. Rev. Rheumatol.16, 11–31 (2020).
Sundaresan, N. R. et al. Sirt3 blocks the cardiac hypertrophic response by augmenting Foxo3a-dependent antioxidant defense mechanisms in mice. J. Clin. Invest.119, 2758–2771 (2009).
Kirkwood, T. B. Understanding the odd science of aging. Cell120, 437–447 (2005).
Horowitz, J. C. & Thannickal, V. J. Mechanisms for the resolution of organ fibrosis. Physiology34, 43–55 (2019).
Sundaresan, N. R. et al. SIRT3 blocks aging-associated tissue fibrosis in mice by deacetylating and activating glycogen synthase kinase 3β. Mol. Cell Biol.36, 678–692 (2015).
Srivastava, S. P. et al. SIRT3 deficiency leads to induction of abnormal glycolysis in diabetic kidney with fibrosis. Cell Death Dis.9, 997 (2018).
Ferber, E. C. et al. FOXO3a regulates reactive oxygen metabolism by inhibiting mitochondrial gene expression. Cell Death Differ.19, 968–979 (2012).
Zhou, Y. et al. Inhibition of mechanosensitive signaling in myofibroblasts ameliorates experimental pulmonary fibrosis. J. Clin. Invest.123, 1096–1108 (2013).
Lagares, D. et al. Targeted apoptosis of myofibroblasts with the BH3 mimetic ABT-263 reverses established fibrosis. Sci. Transl. Med.9, eaal3765 (2017).
Sanders, Y. Y. et al. Histone modifications in senescence-associated resistance to apoptosis by oxidative stress. Redox Biol.1, 8–16 (2013).
Kato, M. et al. Role of the Akt/FoxO3a pathway in TGF-beta1-mediated mesangial cell dysfunction: a novel mechanism related to diabetic kidney disease. J. Am. Soc. Nephrol.17, 3325–3335 (2006).
Wynn, T. A. & Vannella, K. M. Macrophages in tissue repair, regeneration, and fibrosis. Immunity44, 450–462 (2016).
Bitterman, P. B., Wewers, M. D., Rennard, S. I., Adelberg, S. & Crystal, R. G. Modulation of alveolar macrophage-driven fibroblast proliferation by alternative macrophage mediators. J. Clin. Invest.77, 700–708 (1986).
Cui, H. et al. Monocyte-derived alveolar macrophage apolipoprotein E participates in pulmonary fibrosis resolution. JCI Insight5, e134539 (2020).
Larson-Casey, J. L., Deshane, J. S., Ryan, A. J., Thannickal, V. J. & Carter, A. B. Macrophage Akt1 kinase-mediated mitophagy modulates apoptosis resistance and pulmonary fibrosis. Immunity44, 582–596 (2016).
Joshi, N. et al. A spatially restricted fibrotic niche in pulmonary fibrosis is sustained by M-CSF/M-CSFR signalling in monocyte-derived alveolar macrophages. Eur. Respir. J.55, 1900646 (2020).
Bao, L. et al. The pathogenicity of SARS-CoV-2 in hACE2 transgenic mice. Nature583, 830–833 (2020).
Hecker, L. et al. NADPH oxidase-4 mediates myofibroblast activation and fibrogenic responses to lung injury. Nat. Med.15, 1077–1081 (2009).
Kurundkar, D. et al. SIRT3 diminishes inflammation and mitigates endotoxin-induced acute lung injury. JCI Insight4, e120722 (2019).
Acknowledgements
This work was supported by the NIH grants P01 HL114470 (to V.J.T.), R01 AG046210 (to V.J.T.) and R01 HL139617 (to J.W.Z. and V.J.T.); the US Department of Defense grant W81XWH-17-1-0577 (to J.W.Z.); and the US Department of Veterans Affairs Merit Award I01BX003056 (to V.J.T.). We thank Y. Wang for her assistance in the FACS experiment.
Author information
Authors and Affiliations
Contributions
M.R. and V.J.T. conceived and designed the study. M.R., D.K., A.R.K., K.B., D.C., S.R.S. and N.J.L. performed experiments. M.R., D.K., A.R.K., S.R., Y.Y.S., J.S.D., K.G.D. and V.J.T. analyzed and interpreted the data. M.R., D.K., J.W.Z. and V.J.T. drafted the manuscript.
Corresponding author
Ethics declarations
Competing interests
V.J.T. has served as a consultant for Mistral Therapeutics, Inc., Boehringer Ingelheim, United Therapeutics, Blade Therapeutics, Versant Venture and Translate Bio. All other authors have no competing interests.
Additional information
Peer review informationNature Aging thanks David Lagares, David Sinclair and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 SIRT3 antibody specifically recognizes SIRT3 protein.
(a) Western blot showing over expression of HA tagged SIRT3 in human fibroblasts transfected with control or SIRT3 cDNA plasmid. (b) Western blot showing expression levels of SIRT3 in human fibroblasts transfected with non-targeting or SIRT3 siRNA. SIRT3 and HA antibodies were used to probe SIRT3 or SIRT3-HA expression levels.
Extended Data Fig. 2 Exogenous SIRT3 cDNA is preferentially expressed in lung macrophages.
(a) Schematic of experiment design. (b) RT-PCR analysis of total SIRT3 or vector derived SIRT3-HA mRNA levels in bronchoalveolar lavage (BAL) from mice subjected to bleomycin injury followed by treatment with control vector (n = 3) or SIRT3 cDNA plasmid (n = 5), as indicated in the Methods section. Data presented as means ± s.e.m., *P = 0.0357 (unpaired t-test, non-parametric, two-tailed). (c) Flow cytometric analysis of macrophages (CD45+ F4 80+ MerTK+) sorted from collagenase lung digests of naïve mice and mice injured with bleomycin followed by treatment with control vector or SIRT3 cDNA are shown. (d) Gating strategy: (i) cells were gated for FSC-A against SSC-A; (ii) doublets were excluded using FSC-H against FSC-A; (iii) singlets were gated for CD45 positive/negative population; (iv) within the CD45- population, epithelial cells were gated as EPCAM+CD31-; and (v) within the CD45+ population, macrophages were gated as F480+MerTK+. (e–g) RT-PCR analysis of total SIRT3 or vector derived SIRT3-HA mRNA levels in FACS sorted macrophages (e) and epithelial cells (f), and adherence-purified fibroblasts (g) from whole lung collagenase digest from mice subjected to bleomycin injury followed by treatment with control vector (n = 3) or SIRT3 cDNA plasmid (n = 5). Data presented as means ± s.e.m., *P = 0.0357 (macrophages;unpaired t-test, non-parametric, two-tailed), P = 0.0052 (fibroblasts; unpaired t-test, two-tailed statistical analysis), n.s. = not significant.
Extended Data Fig. 3 TGF-β1 downregulates SIRT3 and FoxO3a levels in human fibroblasts.
Top panel, schematic diagram showing experiment design. Bottom panel, western blots demonstrating early and late downregulation of SIRT3 and FoxO3a, respectively, and upregulation of α-SMA at indicated time points in TGF-β1 treatment of human fibroblasts.
Extended Data Fig. 4 FoxO3a overexpression in IPF fibroblasts induces Noxa and inhibits Col1a1.
Western blots and quantitative analysis of FoxO3a, cleaved caspase-3, cleaved PARP, Noxa and Col1a1 protein levels in IPF fibroblasts transfected with control plasmid or FoxO3a cDNA. n = 3 per group; Data presented as means ± s.e.m., P values as indicated by unpaired t-test, two-tailed statistical analysis.
Extended Data Fig. 5 The effects of SIRT3 overexpression on FoxO3a levels in control and IPF fibroblasts.
(a) Left panel, schematic of experiment design. Right panel, western blot showing levels of SIRT3 and FoxO3a in IMR-90 fibroblasts and IPF fibroblasts overexpressing control or SIRT3 plasmid. (b) Left panel, schematic diagram of experiment design. Right panel, western blot showing SIRT3 and FoxO3a levels in cytoplasmic and nuclear extracts of human fibroblasts overexpressing SIRT3 at 24 and 48 hours after transfection.
Extended Data Fig. 6 The effects of SIRT3 overexpression on FoxO3a recovery in TGF-β1 treated human lung fibroblasts.
(a) Left panel, schematic of the experiment design. Right panel, representative western blot showing levels of HA tag, SIRT3, FoxO3a and α-SMA in human fibroblasts cells transfected with SIRT3-HA plasmid followed by treatment with TGF-β1 (2.5 ng/ml) for 48 hours. (b) Left panel, schematic diagram of the experiment design. Right panel, representative western blot analyses of HA, SIRT3, FoxO3a and α-SMA in IMR-90 cells treated with TGF-β1 for 48 hours followed by SIRT3 overexpression.
Extended Data Fig. 7 Effects of conditioned media from SIRT3-overexpressing human and mouse lung epithelial cells on FoxO3a levels in human lung fibroblasts.
(a) Schematic of the experimental design. (b) Left panel, representative western blot showing overexpression of SIRT3 in A549 cells. Right panel, western blot showing levels of FoxO3a in human fibroblasts incubated with the conditioned media from SIRT3-overexpressing A549 cells. Cond. Med. = conditioned media (c) Left panel, western blot showing overexpression of SIRT3 in L2 mouse epithelial cells. Right panel, representative western blot showing levels of FoxO3a in IMR-90 fibroblasts incubated with conditioned media from SIRT3-overexpressing L2 cells at 48 hours. Cond. Med. = conditioned media.
Extended Data Fig. 8 Effects of conditioned media from SIRT3-overexpressing macrophages (THP1 or RAW264.7 cells) on FoxO3a levels in human fibroblasts.
(a) Schematic of the experimental design. (b) Left panel, representative western blot showing overexpression of SIRT3 in human macrophage line THP1 cells. Right panel, western blot showing expression of FoxO3a in human fibroblasts incubated with conditioned media of SIRT3 overexpressing THP1 cells. Cond. Med. = conditioned media. (c) Left panel, western blot showing overexpression of SIRT3 in RAW264.7 mouse macrophages. Right panel, western blot showing levels of FoxO3a in fibroblasts incubated with conditioned media of SIRT3 overexpressing RAW264.7 cells at 48 hours. Cond. Med. = conditioned media.
Extended Data Fig. 9 FoxO3a levels in young and aged mouse fibroblasts co-cultured with SIRT3 overexpressing L2 cells.
(a) Schematic of the experiment design; FB = fibroblasts. (b) Representative western blots indicate SIRT3 levels in whole cell lysates of L2 cells (b), FoxO3a levels in the cytoplasm (c), and in nuclear fractions (d) from young and old mouse fibroblasts. Fibroblasts were co-cultured with L2 cells that overexpress SIRT3 or control plasmid.
Extended Data Fig. 10 Cytokine array of secreted factors by SIRT3 overexpressing macrophages.
Mouse XL cytokine array and quantitative analysis performed on cell supernatants of co-cultured SIRT3-overexpressing macrophages and mouse fibroblasts.
Supplementary information
Supplementary Information
Supplementary Tables 1–3.
Source data
Source Data Fig. 1
Unprocessed western blots.
Source Data Fig. 2
Unprocessed western blots.
Source Data Fig. 3
Unprocessed western blots.
Source Data Fig. 4
Unprocessed western blots.
Source Data Fig. 5
Unprocessed western blots.
Source Data Fig. 6
Unprocessed western blots.
Source Data Fig. 7
Unprocessed western blots.
Source Data Extended Data Fig. 1
Unprocessed western blots.
Source Data Extended Data Fig. 3
Unprocessed western blots.
Source Data Extended Data Fig. 4
Unprocessed western blots.
Source Data Extended Data Fig. 5
Unprocessed western blots.
Source Data Extended Data Fig. 6
Unprocessed western blots.
Source Data Extended Data Fig. 7
Unprocessed western blots.
Source Data Extended Data Fig. 8
Unprocessed western blots.
Source Data Extended Data Fig. 9
Unprocessed western blots.
Rights and permissions
About this article
Cite this article
Rehan, M., Kurundkar, D., Kurundkar, A.R. et al. Restoration of SIRT3 gene expression by airway delivery resolves age-associated persistent lung fibrosis in mice. Nat Aging 1, 205–217 (2021). https://doi.org/10.1038/s43587-021-00027-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s43587-021-00027-5
This article is cited by
-
Mangiferin alleviates diabetic pulmonary fibrosis in mice via inhibiting endothelial-mesenchymal transition through AMPK/FoxO3/SIRT3 axis
Acta Pharmacologica Sinica (2024)
-
Sirtuins and Cellular Senescence in Patients with Idiopathic Pulmonary Fibrosis and Systemic Autoimmune Disorders
Drugs (2024)
-
Insights on the role of l-lactate as a signaling molecule in skin aging
Biogerontology (2023)
-
FOXO4-D-Retro-Inverso targets extracellular matrix production in fibroblasts and ameliorates bleomycin-induced pulmonary fibrosis in mice
Naunyn-Schmiedeberg's Archives of Pharmacology (2023)
-
Epigenetic Dysregulation in Autoimmune and Inflammatory Skin Diseases
Clinical Reviews in Allergy & Immunology (2022)