Abstract
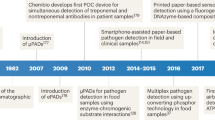
Fast and simultaneous identification of multiple viable pathogens on food is critical to public health. Here we report a pathogen identification system using a paper chromogenic array (PCA) enabled by machine learning. The PCA consists of a paper substrate impregnated with 23 chromogenic dyes and dye combinations, which undergo colour changes on exposure to volatile organic compounds emitted by pathogens of interest. These colour changes are digitized and used to train a multi-layer neural network (NN), endowing it with high-accuracy (91–95%) strain-specific pathogen identification and quantification capabilities. The trained PCA–NN system can distinguish between viable Escherichia coli, E. coli O157:H7 and other viable pathogens, and can simultaneously identify both E. coli O157:H7 and Listeria monocytogenes on fresh-cut romaine lettuce, which represents a realistic and complex environment. This approach has the potential to advance non-destructive pathogen detection and identification on food, without enrichment, culturing, incubation or other sample preparation steps.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding authors on reasonable request. The sample dataset is available in the GitHub repository (https://github.com/FoodSafetyResearch/Nature-Food---Machine-Learning-Enabled-Paper-Chromogenic-Array.git).
Code availability
The NN algorithm is available in the GitHub repository (https://github.com/FoodSafetyResearch/Nature-Food---Machine-Learning-Enabled-Paper-Chromogenic-Array.git).
References
WHO Estimates of the Global Burden of Foodborne Diseases: Foodborne Disease Burden Epidemiology Reference Group 2007–2015 (World Health Organization, 2015); https://www.who.int/foodsafety/publications/foodborne_disease/fergreport/en/
Surveillance for Foodborne Disease Outbreak, United States, 2017: Annual Report (Centers for Disease Control and Prevention, 2019); https://www.cdc.gov/fdoss/annual-reports/index.html
Scallan, E., Griffin, P. M., Angulo, F. J., Tauxe, R. V. & Hoekstra, R. M. Foodborne illness acquired in the United States–unspecified agents. Emerg. Infect. Dis. 17, 16–22 (2011).
Heiat, M., Ranjbar, R. & Alavian, S. M. Classical and modern approaches used for viral hepatitis diagnosis. Hepat. Mon. 14, e17632 (2014).
Chassy, B. M. Can –omics inform a food safety assessment? Regul. Toxicol. Pharmacol. 58, S62–S70 (2010).
Doyle, M. P. Food safety issued arising at food production in a global market. J. Agribus. 18, 129–133 (2000).
Jun, Z. et al. Culture-dependent and -independent analysis of bacterial community structure in Jiangshui, a traditional Chinese fermented vegetable food. LWT 96, 244–250 (2018).
Sun, Y.-M. & Ockerman, H. W. A review of the needs and current applications of hazard analysis and critical control point (HACCP) system in foodservice areas. Food Control 16, 325–332 (2005).
Kou, L. et al. Temperature abuse timing affects the rate of quality deterioration of commercially packaged ready-to-eat baby spinach. Part I: sensory analysis and selected quality attributes. Postharvest Biol. Technol. 91, 96–103 (2014).
Haugen, J.-E., Rudi, K., Langsrud, S. & Bredholt, S. Application of gas-sensor array technology for detection and monitoring of growth of spoilage bacteria in milk: a model study. Anal. Chim. Acta 565, 10–16 (2006).
Huang, X.-w et al. Determination of pork spoilage by colorimetric gas sensor array based on natural pigments. Food Chem. 145, 549–554 (2014).
Bunge, M. et al. On-line monitoring of microbial volatile metabolites by proton transfer reaction-mass spectrometry. Appl. Environ. Microbiol. 74, 2179–2186 (2008).
Tait, E., Perry, J. D., Stanforth, S. P. & Dean, J. R. Identification of volatile organic compounds produced by bacteria using HS-SPME-GC-MS. J. Chromatogr. Sci. 52, 363–373 (2014).
Favre, L. et al. Discrimination of four marine biofilm-forming bacteria by LC-MS metabolomics and influence of culture parameters. J. Proteome Res. 16, 1962–1975 (2017).
Carey, J. R. et al. Rapid identification of bacteria with a disposable colorimetric sensing array. J. Am. Chem. Soc. 133, 7571–7576 (2011).
Chen, Q., Li, H., Ouyang, Q. & Zhao, J. Identification of spoilage bacteria using a simple colorimetric sensor array. Sens. Actuators B 205, 1–8 (2014).
Feng, L., Musto, C. J. & Suslick, K. S. A simple and highly sensitive colorimetric detection method for gaseous formaldehyde. J. Am. Chem. Soc. 132, 4046–4047 (2010).
Guidelines for the Microbiological Examination of Ready - to - Eat Foods (Food Standards Australia and New Zealand, 2001); https://www.foodstandards.gov.au/code/microbiollimits/documents/Guidelines%20for%20Micro%20exam.pdf?
Statistical Aspects of Microbiological Criteria Related to Foods: a Risk Managers Guide (World Health Organization, 2016); https://apps.who.int/iris/handle/10665/249531
Feldsine, P., Abeyta, C. & Andrews, W. H. AOAC International Methods Committee guidelines for validation of qualitative and quantitative food microbiological official methods of analysis. J. AOAC Int. 85, 1187–1200 (2002).
National Advisory Committee on Microbiological Criteria for Foods. Response to questions posed by the food safety and inspection service regarding determination of the most appropriate technologies for the food safety and inspection service to adopt in performing routine and baseline microbiological analyses. J. Food Prot. 73, 1160–1200 (2010).
Downey, T. J., Meyer, D. J., Price, R. K. & Spitznagel, E. L. Using the receiver operating characteristic to assess the performance of neural classifiers. In Proc. International Joint Conference on Neural Networks (ed. Brown, D.) 5, 3642–3646 (IEEE Service Center, 1999).
Meistrell, M. L. Evaluation of neural network performance by receiver operating characteristic (ROC) analysis: examples from the biotechnology domain. Comput. Methods Programs Biomed. 32, 73–80 (1990).
Metz, C. E. Basic principles of ROC analysis. Semin. Nucl. Med. 8, 283–298 (1978).
Kainz, P., Pfeiffer, M. & Urschler, M. Segmentation and classification of colon glands with deep convolutional neural networks and total variation regularization. PeerJ 5, e3874–e3874 (2017).
Kline, D. M. & Berardi, V. L. Revisiting squared-error and cross-entropy functions for training neural network classifiers. Neural Comput. Appl. 14, 310–318 (2005).
Outbreak of E. coli Infections Linked to Romaine Lettuce (Centers for Disease Control and Prevention, 2020); https://www.cdc.gov/ecoli/2019/o157h7-11-19/index.html
Yu, D. & Deng, L. Deep learning and its applications to signal and information processing [Exploratory DSP]. IEEE Signal Process. Mag. 28, 145–154 (2011).
Wang, G., Zhang, Y., Ye, X. & Mou, X. Machine Learning for Tomographic Imaging (IOP Publishing, 2019).
An, J. H., Goo, E., Kim, H., Seo, Y.-S. & Hwang, I. Bacterial quorum sensing and metabolic slowing in a cooperative population. Proc. Natl Acad. Sci. USA 111, 14912 (2014).
Lu, H., Que, Y., Wu, X., Guan, T. & Guo, H. Metabolomics deciphered metabolic reprogramming required for biofilm formation. Sci. Rep. 9, 13160 (2019).
Wang, G. et al. Interactive medical image segmentation using deep learning with image-specific fine tuning. IEEE Trans. Med. Imaging 37, 1562–1573 (2018).
Tang, M., Djelouah, A., Perazzi, F., Boykov, Y. & Schroers, C. Normalized cut loss for weakly-supervised CNN segmentation. In Proc. IEEE/CVF Conference on Computer Vision and Pattern Recognition (eds Brown, M. S. et al.) 1818–1827 (IEEE Service Center, 2018).
Shi, J. & Malik, J. Normalized cuts and image segmentation. IEEE Trans. Pattern Anal. Mach. Intell. 22, 888–905 (2000).
Hui, T. & Ngan, K. N. Depth enhancement using RGB-D guided filtering. In Proc. IEEE International Conference on Image Processing (eds Pesquet-Popescu, B. & Fowler, J.) 3832–3836 (IEEE Service Center, 2014).
Papari, G., Idowu, N. & Varslot, T. Fast bilateral filtering for denoising large 3D images. IEEE Trans. Image Process. 26, 251–261 (2017).
Gay, M., Cerf, O. & Davey, K. R. Significance of pre-incubation temperature and inoculum concentration on subsequent growth of Listeria monocytogenes at 14 °C. J. Appl. Bacteriol. 81, 433–438 (1996).
Rodriguez-Caturla, M. Y., Valero Díaz, A., Vallejo, J. L. R., García-Gimeno, R. M. & Cosano, G. Z. Effect of pre-incubation conditions on growth and survival of Staphylococcus aureus in sliced cooked chicken breast. Meat Sci. 92, 409–416 (2012).
Xiao, Z., Nou, X., Luo, Y. & Wang, Q. Comparison of the growth of Escherichia coli O157: H7 and O104: H4 during sprouting and microgreen production from contaminated radish seeds. Food Microbiol. 44, 60–63 (2014).
Xu, Y., Nagy, A., Bauchan, G., Xia, X. & Nou, X. Enhanced biofilm formation in dual-species culture of Listeria monocytogenes and Ralstonia insidiosa. AIMS Microbiol. 3, 774–783 (2017).
Noh, H., You, T., Mun, J. & Han, B. Regularizing deep neural networks by noise: its interpretation and optimization. In Proc. 31st International Conference on Neural Information Processing Systems (eds Luxburg, U. et al.) 5115–5124 (Curran Associates, 2017).
Su, F., Yuan, P., Wang, Y. & Zhang, C. The superior fault tolerance of artificial neural network training with a fault/noise injection-based genetic algorithm. Protein Cell 7, 735–748 (2016).
An, G. The effects of adding noise during backpropagation training on a generalization performance. Neural Comput. 8, 643–674 (1996).
Bishop, C. M. Training with noise is equivalent to Tikhonov regularization. Neural Comput. 7, 108–116 (1995).
Wei, Y. et al. HCP: a flexible CNN framework for multi-label image classification. IEEE Trans. Pattern Anal. Mach. Intell. 38, 1901–1907 (2016).
Zhu, F. et al. Learning spatial regularization with image-level supervisions for multi-label image classification. In Proc. IEEE Conference on Computer Vision and Pattern Recognition (eds Chellappa, R. et al.) 5513–5522 (IEEE Service Center, 2017).
Ban, J.-C. & Chang, C.-H. The learning problem of multi-layer neural networks. Neural Netw. 46, 116–123 (2013).
Ban, J.-C., Hu, W.-G. & Lin, S.-S. Pattern generation problems arising in multiplicative integer systems. Ergod. Theory Dyn. Syst. 39, 1234–1260 (2017).
Fukushima, K. Training multi-layered neural network neocognitron. Neural Netw. 40, 18–31 (2013).
Svozil, D., Kvasnicka, V. & Pospichal, J. Introduction to multi-layer feed-forward neural networks. Chemom. Intell. Lab. Syst. 39, 43–62 (1997).
Acknowledgements
The project is supported by the US Department of Agriculture (S51600000035794). We are grateful to Z. Teng, E. Turner and T. Gu for their assistance in PCA fabrication. The contents are solely the responsibility of the authors and do not necessarily represent the official views of the US Department of Agriculture.
Author information
Authors and Affiliations
Contributions
X.L., Y.L., H.Y. and B. Zhang designed the research; M.Y., X.L., S.W., H.D., K.R., Z.J., A.S. and B. Zhou performed the experiments and analysed the data; Y.L., D.P., A.J.P., H.Y. and B. Zhang wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
US provisional patent and patent cooperation treaty (PCT) applications have been filed (B. Zhang, Y.L., H.Y. and X.L. Method and system for chromogenic array-based food testing. US provisional patent application no. 62/757,388 and PCT/ US2019/059816).
Additional information
Peer review information Nature Food thanks Byron Brehm-Stecher, Hyeran Noh, Jasmina Vidic and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Discussion, Figs. 1–5 and Tables 1–3.
Rights and permissions
About this article
Cite this article
Yang, M., Liu, X., Luo, Y. et al. Machine learning-enabled non-destructive paper chromogenic array detection of multiplexed viable pathogens on food. Nat Food 2, 110–117 (2021). https://doi.org/10.1038/s43016-021-00229-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s43016-021-00229-5