Abstract
Nuclear receptor corepressors (NCoRs) function in multiprotein complexes containing histone deacetylase 3 (HDAC3) to alter transcriptional output primarily through repressive chromatin remodelling at target loci1,2,3,4,5. In the liver, loss of HDAC3 causes a marked hepatosteatosis largely because of de-repression of genes involved in lipid metabolism6,7; however, the individual roles and contribution of other complex members to hepatic and systemic metabolic regulation are unclear. Here we show that adult loss of both NCoR1 and NCoR2 (double knockout (KO)) in hepatocytes phenocopied the hepatomegalic fatty liver phenotype of HDAC3 KO. In addition, double KO livers exhibited a dramatic reduction in glycogen storage and gluconeogenic gene expression that was not observed with hepatic KO of individual NCoRs or HDAC3, resulting in profound fasting hypoglycaemia. This surprising HDAC3-independent activation function of NCoR1 and NCoR2 is due to an unexpected loss of chromatin accessibility on deletion of NCoRs that prevented glucocorticoid receptor binding and stimulatory effect on gluconeogenic genes. These studies reveal an unanticipated, non-canonical activation function of NCoRs that is required for metabolic health.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The RNA-seq, ATAC–seq and ChIP–seq datasets have been deposited in the Gene Expression Omnibus under accession no. GSE234234. This paper also used publicly available ChIP–seq and RNA-seq data that include NCoR1 ChIP (GSM647027; ref. 6), NCoR2 ChIP (GSM1236494; ref. 7), GR ChIP (GSE45674; ref. 61), CREB ChIP (GSE45674; ref. 61), PPARα ChIP (GSE113157; ref. 47), GR ChIP in HNF4α KO (E-MTAB-10224; ref. 42) and GR KO RNA-seq (GSE173723, ZT10; ref. 37). Source data are provided with this paper.
Code availability
No custom code was used in this study.
References
Emmett, M. J. & Lazar, M. A. Integrative regulation of physiology by histone deacetylase 3. Nat. Rev. Mol. Cell Biol. 20, 102–115 (2019).
Lonard, D. M. & O’Malley, B. W. Nuclear receptor coregulators: modulators of pathology and therapeutic targets. Nat. Rev. Endocrinol. 8, 598–604 (2012).
Wen, Y. D. et al. The histone deacetylase-3 complex contains nuclear receptor corepressors. Proc. Natl Acad. Sci. USA 97, 7202–7207 (2000).
Li, J. et al. Both corepressor proteins SMRT and N-CoR exist in large protein complexes containing HDAC3. EMBO J. 19, 4342–4350 (2000).
Guenther, M. G. et al. A core SMRT corepressor complex containing HDAC3 and TBL1, a WD40-repeat protein linked to deafness. Genes Dev. 14, 1048–1057 (2000).
Feng, D. et al. A circadian rhythm orchestrated by histone deacetylase 3 controls hepatic lipid metabolism. Science 331, 1315–1319 (2011).
Sun, Z. et al. Deacetylase-independent function of HDAC3 in transcription and metabolism requires nuclear receptor corepressor. Mol. Cell 52, 769–782 (2013).
De Bosscher, K., Desmet, S. J., Clarisse, D., Estébanez-Perpiña, E. & Brunsveld, L. Nuclear receptor crosstalk—defining the mechanisms for therapeutic innovation. Nat. Rev. Endocrinol. 16, 363–377 (2020).
Xiao, Y., Kim, M. & Lazar, M. A. Nuclear receptors and transcriptional regulation in non-alcoholic fatty liver disease. Mol. Metab. 50, 101119 (2021).
Schulman, I. G. Nuclear receptors as drug targets for metabolic disease. Adv. Drug Deliv. Rev. 62, 1307–1315 (2010).
Ahmadian, M. et al. PPARγ signaling and metabolism: the good, the bad and the future. Nat. Med. 19, 557–566 (2013).
Li, J.-X. & Cummins, C. L. Fresh insights into glucocorticoid-induced diabetes mellitus and new therapeutic directions. Nat. Rev. Endocrinol. 18, 540–557 (2022).
Park, E. J. et al. SMRTe, a silencing mediator for retinoid and thyroid hormone receptors-extended isoform that is more related to the nuclear receptor corepressor. Proc. Natl Acad. Sci. USA 96, 3519–3524 (1999).
Ordentlich, P. et al. Unique forms of human and mouse nuclear receptor corepressor SMRT. Proc. Natl Acad. Sci. USA 96, 2639–2644 (1999).
Jepsen, K. et al. Combinatorial roles of the nuclear receptor corepressor in transcription and development. Cell 102, 753–763 (2000).
Jepsen, K., Gleiberman, A. S., Shi, C., Simon, D. I. & Rosenfeld, M. G. Cooperative regulation in development by SMRT and FOXP1. Genes Dev. 22, 740–745 (2008).
Astapova, I. et al. The nuclear corepressor, NCoR, regulates thyroid hormone action in vivo. Proc. Natl Acad. Sci. USA 105, 19544–19549 (2008).
Astapova, I. et al. The nuclear receptor corepressor (NCoR) controls thyroid hormone sensitivity and the set point of the hypothalamic–pituitary–thyroid axis. Mol. Endocrinol. 25, 212–224 (2011).
Fozzatti, L. et al. Resistance to thyroid hormone is modulated in vivo by the nuclear receptor corepressor (NCOR1). Proc. Natl Acad. Sci. USA 108, 17462–17467 (2011).
Shimizu, H. et al. NCoR1 and SMRT play unique roles in thyroid hormone action in vivo. Mol. Cell. Biol. 35, 555–565 (2015).
Nofsinger, R. R. et al. SMRT repression of nuclear receptors controls the adipogenic set point and metabolic homeostasis. Proc. Natl Acad. Sci. USA 105, 20021–20026 (2008).
Reilly, S. M. et al. Nuclear receptor corepressor SMRT regulates mitochondrial oxidative metabolism and mediates aging-related metabolic deterioration. Cell Metab. 12, 643–653 (2010).
Zhang, J., Kalkum, M., Chait, B. T. & Roeder, R. G. The N-CoR-HDAC3 nuclear receptor corepressor complex inhibits the JNK pathway through the integral subunit GPS2. Mol. Cell 9, 611–623 (2002).
Sun, Z. et al. Hepatic Hdac3 promotes gluconeogenesis by repressing lipid synthesis and sequestration. Nat. Med. 18, 934–942 (2012).
Jo, Y. S. et al. Phosphorylation of the nuclear receptor corepressor 1 by protein kinase B switches its corepressor targets in the liver in mice. Hepatology 62, 1606–1618 (2015).
Han, H.-S., Choi, B. H., Kim, J. S., Kang, G. & Koo, S.-H. Hepatic Crtc2 controls whole body energy metabolism via a miR-34a-Fgf21 axis. Nat. Commun. 8, 1878 (2017).
Selen, E. S. et al. Requirement of hepatic pyruvate carboxylase during fasting, high fat, and ketogenic diet. J. Biol. Chem. 298, 102648 (2022).
Bose, S. K., Hutson, I. & Harris, C. A. Hepatic glucocorticoid receptor plays a greater role than adipose GR in metabolic syndrome despite renal compensation. Endocrinology 157, 4943–4960 (2016).
Estall, J. L. et al. PGC-1α negatively regulates hepatic FGF21 expression by modulating the heme/Rev-Erbα axis. Proc. Natl Acad. Sci. USA 106, 22510–22515 (2009).
You, S.-H. et al. Nuclear receptor co-repressors are required for the histone-deacetylase activity of HDAC3 in vivo. Nat. Struct. Mol. Biol. 20, 182–187 (2013).
Zhang, X., Yang, S., Chen, J. & Su, Z. Unraveling the regulation of hepatic gluconeogenesis. Front. Endocrinol. 9, 802 (2019).
Rui, L. Energy metabolism in the liver. Compr. Physiol. 4, 177–197 (2014).
Arden, C. et al. Elevated glucose represses liver glucokinase and induces its regulatory protein to safeguard hepatic phosphate homeostasis. Diabetes 60, 3110–3120 (2011).
Bideyan, L., Nagari, R. & Tontonoz, P. Hepatic transcriptional responses to fasting and feeding. Genes Dev. 35, 635–657 (2021).
Felig, P. The glucose-alanine cycle. Metabolism 22, 179–207 (1973).
Kuo, T., McQueen, A., Chen, T.-C. & Wang, J.-C. Glucocorticoid signaling, from molecules to mice to man. Adv. Exp. Med. Biol. 872, 99–126 (2015).
Præstholm, S. M. et al. Impaired glucocorticoid receptor expression in liver disrupts feeding-induced gene expression, glucose uptake, and glycogen storage. Cell Rep. 37, 109938 (2021).
Opherk, C. et al. Inactivation of the glucocorticoid receptor in hepatocytes leads to fasting hypoglycemia and ameliorates hyperglycemia in streptozotocin-induced diabetes mellitus. Mol. Endocrinol. 18, 1346–1353 (2004).
Tronche, F. et al. Glucocorticoid receptor function in hepatocytes is essential to promote postnatal body growth. Genes Dev. 18, 492–497 (2004).
Quagliarini, F. et al. Cistromic reprogramming of the diurnal glucocorticoid hormone response by high-fat diet. Mol. Cell 76, 531–545 (2019).
Armour, S. M. et al. An HDAC3-PROX1 corepressor module acts on HNF4α to control hepatic triglycerides. Nat. Commun. 8, 549 (2017).
Hunter, A. L. et al. HNF4A modulates glucocorticoid action in the liver. Cell Rep. 39, 110697 (2022).
Hörlein, A. J. et al. Ligand-independent repression by the thyroid hormone receptor mediated by a nuclear receptor co-repressor. Nature 377, 397–404 (1995).
Sande, S. & Privalsky, M. L. Identification of TRACs (T3 receptor-associating cofactors), a family of cofactors that associate with, and modulate the activity of, nuclear hormone receptors. Mol. Endocrinol. 10, 813–825 (1996).
Chen, J. D. & Evans, R. M. A transcriptional co-repressor that interacts with nuclear hormone receptors. Nature 377, 454–457 (1995).
Ritter, M. J. et al. Nuclear receptor corepressors, NCOR1 and SMRT, are required for maintaining systemic metabolic homeostasis. Mol. Metab. 53, 101315 (2021).
Liang, N. Hepatocyte-specific loss of GPS2 in mice reduces non-alcoholic steatohepatitis via activation of PPARα. Nat. Commun. 10, 1684 (2019).
Kulozik, P. et al. Hepatic deficiency in transcriptional cofactor TBL1 promotes liver steatosis and hypertriglyceridemia. Cell Metab. 13, 389–400 (2011).
John, S. et al. Chromatin accessibility pre-determines glucocorticoid receptor binding patterns. Nat. Genet. 43, 264–268 (2011).
Emmett, M. J. et al. Histone deacetylase 3 prepares brown adipose tissue for acute thermogenic challenge. Nature 17, 544–548 (2017).
Abe, Y. et al. RANK ligand converts the NCoR/HDAC3 co-repressor to a PGC1β- and RNA-dependent co-activator of osteoclast gene expression. Mol. Cell 83, 3421–3437 (2023).
Nguyen, H. C. B., Adlanmerini, M., Hauck, A. K. & Lazar, M. A. Dichotomous engagement of HDAC3 activity governs inflammatory responses. Nature 584, 286–290 (2020).
Mullican, S. E. et al. Histone deacetylase 3 is an epigenomic brake in macrophage alternative activation. Genes Dev. 25, 2480–2488 (2011).
Xie, Z. et al. Gene set knowledge discovery with Enrichr. Curr. Protoc. 1, e90 (2021).
Kuleshov, M. V. et al. Enrichr: a comprehensive gene set enrichment analysis web server 2016 update. Nucleic Acids Res. 44, W90–W97 (2016).
Chen, E. Y. et al. Enrichr: interactive and collaborative HTML5 gene list enrichment analysis tool. BMC Bioinformatics 14, 128 (2013).
Corces, M. R. et al. An improved ATAC-seq protocol reduces background and enables interrogation of frozen tissues. Nat. Methods 14, 959–962 (2017).
Ackermann, A. M., Wang, Z., Schug, J., Naji, A. & Kaestner, K. H. Integration of ATAC-seq and RNA-seq identifies human alpha cell and beta cell signature genes. Mol. Metab. 5, 233–244 (2016).
Zheng, R. et al. Cistrome Data Browser: expanded datasets and new tools for gene regulatory analysis. Nucleic Acids Res. 47, D729–D735 (2019).
Mei, S. et al. Cistrome Data Browser: a data portal for ChIP–Seq and chromatin accessibility data in human and mouse. Nucleic Acids Res. 45, D658–D662 (2017).
Everett, L. J. et al. Integrative genomic analysis of CREB defines a critical role for transcription factor networks in mediating the fed/fasted switch in liver. BMC Genomics 14, 337 (2013).
Guan, D. et al. The hepatocyte clock and feeding control chronophysiology of multiple liver cell types. Science 369, 1388–1394 (2020).
Papazyan, R. et al. Physiological suppression of lipotoxic liver damage by complementary actions of HDAC3 and SCAP/SREBP. Cell Metab. 24, 863–874 (2016).
Acknowledgements
We thank H. C. B. Nguyen, K. Zhu, L. Woodie and T. R. Matsuura for valuable discussions, and B. M. Krusen for assistance with mouse husbandry. We thank Y. Xiao for technical advice on ATAC–seq and R. N. Cohen (University of Chicago) for providing the NCoR2 loxP mice. We thank the Functional Genomics Core of the Penn Diabetes Research Center (DK19525) for assistance with bioinformatics and next-generation sequencing and L. Cheng and the Institute for Diabetes, Obesity, and Metabolism Histology Core for assistance with slide preparation. Experimental schematics were created with BioRender.com. This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases grant nos. R01DK43806 (to M.A.L.), T32 DK007314 (to A.K.H.) and F32 DK122684 (to A.K.H.), the Osamu Hayaishi Memorial Scholarship for Study Abroad (to S.I.), the Uehara Memorial Foundation (to S.I.), the Leading Young Researcher Overseas Visit Program and the Cell Science Research Foundation (to S.I.), the Cox Medical Institute and the JPB Foundation.
Author information
Authors and Affiliations
Contributions
A.K.H. and M.A.L. devised the research project, interpreted the data and wrote the manuscript. A.K.H. performed the mouse experiments, animal husbandry, ChIP–seq, RNA-seq, tissue processing, biochemical assays and bioinformatic analysis. R.M. assisted with the bioinformatic analysis. B.J.C. assisted with animal husbandry, tissue collection, RT–qPCR and immunoblotting. M.T.F. constructed the ATAC–seq libraries. M.C.T. assisted with RNA-seq and the feeding behaviour analysis. S.I. performed the histological analysis. M.K.K. assisted with animal husbandry, tissue collection, RT–qPCR and immunoblotting. F.N.C.B. performed the RT–qPCR assays. M.K.M. assisted with the bioinformatic analysis. D.M.Z. assisted with tissue collection. K.B. performed and analysed the bioinformatic analysis.
Corresponding author
Ethics declarations
Competing interests
M.A.L. is a member of the scientific advisory board for Pfizer, has consulted for Madrigal Pharmaceuticals and is a cofounder and scientific advisory board member for Flare Therapeutics. The other authors declare no competing interests.
Peer review
Peer review information
Nature Metabolism thanks N. Henriette Uhlenhaut, David Moore and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Yanina-Yasmin Pesch, in collaboration with the Nature Metabolism team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
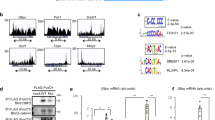
Extended Data Fig. 1 Adult mice with depletion of hepatic NCoR1 and 2 rapidly develop liver failure.
a, Model for hepatocyte-specific knockout of Ncor1/2 in adult mice (Created with BioRender.com). Ncor1loxP/loxPNcor2loxP/loxPmice were injected with AAV8-TBG-eGFP (control) or AAV8-TBG-Cre (dKO) virus by tail vein at 8–10 weeks of age. Mice were sacrificed 5 days or 15 days post AAV injection. b, RT-qPCR of Ncor1 and Ncor2 relative to 36b4 5 or 15 days after AAV injection. Data represented as mean ±s.e.m, with P-values calculated by two-tailed students t-test. Biological replicates representing individual mice are plotted (n = 3-4 animals) c, Western blots of NCoR1, NCoR2 and Vinculin (VINC) in whole cell lysates from Control and dKO livers at day 5 and 15 post AAV injection. NCOR1 and 2 blots are from the same samples but separate membranes. Equal loading was confirmed by Vinculin blots on each membrane. d, Body weight and e, liver weight in grams 5 and 15 days post AAV injection. Biological replicates are plotted (n = 3–5 animals) and data represented as mean ±s.e.m, with P-values calculated by 2-way ANOVA. f, Representative liver sections at 5 and 15 days. HE (Hematoxylin and Eosin), SR (Sirius Red), αSMA (alpha-Smooth Muscle Actin), F4/80 antigen. Scale bar indicates 20 μM in all panels. g, Activity of ALT in the serum collected serially from mice on Day 0, 5,10, and 15 post AAV injection (n = 5 mice). Data represented as mean ±s.e.m. P-values calculated by two-way ANOVA. h, Survival curve of control and dKO mice with day 0 indicating time of AAV administration. p-value was calculated by Log rank Mantel-Cox test. i, Model for single KO of Ncor1, Ncor2, and Hdac3 (Created with BioRender.com). j, RT-qPCR of, Ncor1, Ncor2, and Hdac3 in each respective single knockout model at 5 and 15 days after AAV administration. Biological replicates are plotted (n = 3–5 animals) and data represented as mean ±s.e.m with P-values calculated by unpaired two-tailed students t-test. Ncor1 mRNA: Ncor1 KO (Day 5: p = 0.0039, Day15: p = 0.0025), Ncor2 mRNA:Ncor2 KO (Day 5: p = 0.0008, Day15: p = 0.0001), Hdac3 mRNA:Hdac3 KO (Day 5: p < 0.0001, Day15: p = 0.0015). k, Western blot of NCoR1, NCoR2, HDAC3, and VINCULIN in whole cell lysates from control floxed (loxP/loxP) livers and single knockout (KO) livers at day 15. Superscript denotes which membrane (1 or 2) was used to blot for each protein. l, Liver to body weight ratio in loxP/loxP control animals (gray) or knockout animals, 15 days post AAV injection. Biological replicates are plotted (n = 3–5 animals) and data is represented as mean ±s.e.m. P-values were calculated by two-way ANOVA. m, Serum ALT levels in floxed (fl/fl) control animals (gray) or knockout (KO) animals 15 days post AAV injection. Biological replicates are plotted (n = 3–5 animals) and data is represented as mean ±s.e.m. P-values were calculated by calculated by two-way ANOVA. n, Expression represented as Log2 Fold Change relative to each floxed control for example fibrosis genes and pro-inflammatory genes (Adj P-value: * <0.01, **<0.001,*** < 0.001, #<0.000001).
Extended Data Fig. 2 Lipogenic gene induction in both NCoR1/2 dKO and HDAC3 knockout livers.
a, Upset plot showing overlap of upregulated genes (fold change>1.5 relative to floxed control, adj P-value <0.05) between each knockout model: (dKO-5 day, purple dots), Hdac3 KO (blue dots), Ncor2 KO (yellow dots), and Ncor1 KO (green dots). Each linkage represents common differentially expressed genes between the indicated groups. b, Z-score normalized heatmap of all genes upregulated (Adj P-value <0.05, Fold Change >1.5) in both Hdac3 KO and dKO models. c, Top ontology terms (GO:Biological Process) for all genes upregulated in both Hdac3 KO and dKO models. d, RT-qPCR of a panel of lipogenic genes relative to 36b4. Data represented as fold change relative to loxP/loxP controls and plotted as mean ±s.e.m. P-values were calculated by unpaired two-tailed students t-test with correction for multiple comparisons using the Holm-Šídák method. Biological replicates are plotted (n = 3–5 animals) e,Western blot of lipogenic factors and day 5 and 15 post dKO. The same samples were loaded into 4 separate gels. Equal loading was confirmed by Vinculin blotting on each membrane. f, Liver triglyceride relative to loxP/loxP controls for each model. 5 days and 15 days post AAV injection for (dKO) and 15 days post AAV injection (Hdac3 KO, Ncor1 KO, Ncor2 KO). Data represented as fold change relative to mean of loxP/loxP control. Biological replicates are plotted (n = 3–5 animals) as mean ±s.e.m. P-values were calculated by unpaired two-tailed students t-test.
Extended Data Fig. 3 Impaired glucose metabolism in hepatic dKO animals.
a, Upset plot showing overlap of downregulated genes (FC > 1.5, p-value <0.05) between each knockout model: (dKO-5day, purple dots), Hdac3 KO (blue dots), Ncor2 KO (yellow dots), and Ncor1 KO (green dots). Each linkage represents common differentially expressed genes between the indicated groups. b, Z-score normalized heatmap of all genes downregulated only in the dKO model (Adj P-value <0.05, Fold Change >1.5). c, Top ontology terms (GO:Biological Process) for all genes downregulated only in the dKO model. d, Experimental setup for food monitoring experiment in the BioDAQ (Created with BioRender.com). e, IP-GTT of control and dKO mice (Ncor1/2loxP/loxP, day 5 (n = 4 mice), dKO day 5 (n = 5 mice), Ncor1/2loxP/loxP day 15 (n = 4 mice), dKO day 15 (n = 5 mice). and g, IP-ITT of control and dKO mice at day 5. n = 4 mice for all groups. Data in e and g are plotted as mean value ±s.e.m. and statistical significance calculated by RM two-way ANOVA. P-values in panel represent genotype effect: dKO GTT Day 5 (Interaction p = 0.0129, Genotype p = 0.0002, Time p < 0.0001), dKO GTT Day 15 (Interaction p < 0.0001, Genotype p = <0.0079, Time p < 0.0001), f, h, Body weight of GTT (n = 4 for controls and n = 5 for dKO mice) and ITT (n = 4 mice for all groups) cohorts. i, Blood glucose in ad-libitum fed mice at 4 timepoints, 15 days post AAV. The mean ±s.e.m of the biological replicates is plotted: Ncor1loxP/loxP (n = 5 mice), Ncor1 KO (n = 5 mice), Ncor2loxP/loxP (n = 3 mice), Ncor2 KO (n = 5 mice), Hdac3loxP/loxP (n = 4 mice), Hdac3 KO (n = 4 mice), Ncor1/2loxP/loxP (n = 4 mice), dKO (n-4 mice).p-values were calculated by RM two-way ANOVA with Šídák’s multiple comparisons test (ZT4 p = 0.0023, ZT10 p = 0.0076).
Extended Data Fig. 4 Inhibition of HDAC3 activity does not have a major effect on hepatic glucose metabolism.
a, Schematic of NSDAD mouse model. b, HDAC activity in HDAC3 immunoprecipitates from Control (WT) (n = 4 mice) and NSDAD (n = 3 mice) mice. P-values calculated by one-way ANOVA with Turkey’s multiple comparisons test. IP with normal rabbit IgG (IgG) in control mice is a negative control (n = 3 mice). c, Body weight and d, liver weight of control (n = 5 mice) and NSDAD (n = 5 mice) mice. e, Serum ALT Activity (n = 3 control and 5 NSDAD mice). f, RT-qPCR of Lipogenic Gene Expression relative to 36b4 (n = 4 mice). P values calculated by unpaired two-tailed student’s t-test with correction for multiple comparisons using the Holm-Šídák method. g, Hepatic triglyceride levels (n = 4 mice) P-value calculated by unpaired two-tailed student’s t-test. h, RT-qPCR of hepatic glucose output genes relative to 36b4 (n = 4 mice). i, Hepatic glycogen levels (n = 4 mice). j, Glucose levels during a 24 hour fast: WT (n = 6 mice), NSDAD (n = 5 mice). For all panels, the mean ±s.e.m. is plotted.
Extended Data Fig. 5 NCoRs are required for the hepatic fasting response.
a, Liver glycogen levels in animals either fasted for 24 hours (Fasted) or fasted for 24 hours followed by a 3-hour Re-feed (Refed). Refed Control vs Refed dKO (p = 0.0302) b, Serum Beta-hydroxy Butyrate levels (βHB). c, RT-qPCR of Ketogenic markers relative to 36b4. d, Hepatic free fatty acids (FFA) e, RT-qpcr of Fatty Acid Oxidation markers relative to 36b4. f,g, RT-qPCR of glucoregulatory genes relative to 36b4. RT-qPCR data represented as fold change relative to Control-Refed animals. For all panels, biological replicates are plotted (n = 3-4 mice) as mean ±s.e.m. and P-values were calculated by two-way ANOVA with Turkey’s multiple comparisons test. Panel e: Pdk4 (fasted p = 0.003), Panel f: Ldha (fasted p < 0.0001, fed p = 0.0041), Slc38a2 (fasted p = 0.0007), Slc38a3 (fasted p < 0.0001), Gpt (fasted p = 0.0007, fed p = 0.0366), Got2 (fasted p = 0.0003, fed p = 0.0178), Gls2 (fasted p < 0.0001, fed p = 0.0003), Slc2a2 (fasted p < 0.0001, fed p = 0.0008), Slc37a4 (fasted p = 0.0245). p < 0.05*, p < 0.01**, p < 0.001***, p < 0.0001****.
Extended Data Fig. 6 Hepatic dKO of NCoR1 and 2 in female mice.
a, Body weight and b, liver weight of female control and dKO mice 5 and 15 days post dKO. Biological replicates include: Control day 5 (n = 4 mice), dKO day 5 (n = 5 mice), Control day 15 (n = 5 mice), dKO day 15 (n = 5 mice). P-values calculated by two-tailed unpaired students t-test: day 5 p = 0.0048, day 15 p < 0.0001. c, RT-qPCR of lipogenic genes (n = 4 mice). P-values calculated by unpaired, two-tailed students t-test. d, Hepatic TG levels (n = 4-5 mice). P-values calculated by unpaired, two-tailed students t-test e, Glucose metabolism genes in female dKO mice (n = 4 mice). P-values calculated by unpaired, two-tailed students t-test. f, Glucose levels throughout a 24 hour fast in female control and dKO mice (n = 5 mice per group). P-values calculated by RM two-way ANOVA with Šídák’s multiple comparisons test: ZT8 p = 0.0004, ZT12 p = 0.0001. g, IP-Alanine Tolerance Test in Vehicle Treated (-Saline n = 3) or Alanine Treated (-Ala) female mice, (n = 8 control mice and 4 dKO mice). P-values were calculated by RM two-way ANOVA (Control:dKO p = 0.0135, Control:Saline p = 0.0001). For all panels, biological replicates are plotted as mean ±s.e.m. p < 0.05*, p < 0.01**, p < 0.001***, p < 0.0001****.
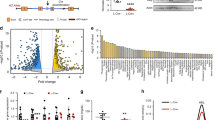
Extended Data Fig. 7 Loss of hepatic NCoRs results in decreased chromatin accessibility at enhancers of glucoregulatory genes.
a, Correlation scatter plots of ATACseq biological replicates. b, Scatter plot of ATACseq normalized counts from hepatocytes isolated from control and dKO livers, 5 days post AAV injection. Sites of increased accessibility relative to control livers are shown in red and decreased accessibility shown in blue. Differential sites exhibit a fold change>1.5 in dKO compared to control, with FDR < 0.05 (n = 3). c, Average profiles of NCoR1 (GSM647027) and NCoR2 (GSM1236494) ChIPseq from control livers (ZT10) at sites exhibiting increased accessibility in dKO hepatocytes (blue track) vs. sites exhibiting decreased accessibility (orange track). Shading indicates 95% confidence interval. d, Heatmap showing Log2 Fold Change in gene expression (RNAseq, Day 5 dKO vs Ncor1/2 floxed contols) of SWI/SNF factors. * indicates FDR < 0.05 and fold change >1.5. e, FDR of Top significant terms (GO: Biological Process) of upregulated genes nearest to the 273 enhancers bound by NCoR1/2 with increased accessibility. f, Example tracks of loci with enhancers exhibiting increased accessibility and increased gene expression. RNAseq tracks (control and dKO day 5 livers, group autoscaled), ATACseq tracks (hepatocytes isolated from control and dKO day 5 livers, group autoscaled) and NCoR1 ChIPseq (control livers, ZT10, autoscaled) are shown. g, Top 12 factors, ranked by Giggle Score, with overlapping cistromes compared to 273 NCoR1/2-bound ATAC peaks near upregulated genes. h, Venn diagrams showing overlap of peaks between Liver CREB ChIPseq (GSE45674, Fasted) and PPARα (GSE113157) with differential ATAC peaks. i, Scatter plot of differentially expressed genes in GR-liver specific knockouts (Præsthom et al: GSE173723, ZT10) and 5-day dKO livers. Differentially expressed genes were filtered based on fold change greater than 1.5 relative to controls with FDR < 0.05. Inset indicates Top 5 Reactome Pathway terms for genes downregulated in both GR-KO and NCoR1/2 dKO livers.
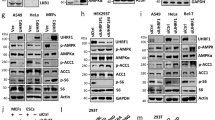
Extended Data Fig. 8 NCoRs are required for GR binding and transcriptional activity at NCoR1/GR cobound sites.
a, Experimental design (Created with BioRender.com). 5 days after AAV injection, mice were injected intraperitoneally with 1 mg/kg Dexamethasone (Dex) or vehicle (sterile saline). Mice were euthanized 2 hours after Dex administration (ZT10). b, Serum Corticosterone levels in control and dKO mice, measured serially over 24 hours (Control (n = 6 mice) dKO (n = 8 mice) and plotted as mean +/- S.E.M. c, Representative western blot of nuclear and cytoplasmic extracts (total of 4 samples were assayed). Samples were loaded into 3 separate gels and equal loading was confirmed by TBP and GAPDH blots on each membrane. d, Heatmap of all GR ChIP peaks induced by Dex treatment in control mice. Lower panel shows average ChIP profile of peaks with lost Dex response vs. retained Dex response GR binding. e, GR and NCoR1 ChIP-qPCR in animals fasted for 24 hours (fasted) and animals fasted for 24 hours and refed for 3 hours (Fed) at example loci exhibiting a blunted response to Dex treatment. Data shown as percent enrichment relative to input. Biological replicates are plotted (n = 4 mice per group) as mean ±s.e.m. and P-values for Genotype effect were calculated by two-way ANOVA: GR ChIP Got1 p = 0.0071, Pgc1α p = 0.0051, Pck1 p = 0.0137 and NCoR1 ChIP Got1 p = 0.0013, Pgc1α p < 0.0001, Pck1 p = 0.0174) f, Motif analysis at sites with lost Dex-induced GR binding and those with retained GR binding. X-axis indicates the fold enrichment relative to either retained or lost peaks, y-axis indicates % of peaks with that motif.
Supplementary information
Supplementary Information
Supplementary Table 1.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Extended Data Fig. 1
Statistical source data.
Source Data Extended Data Fig. 1
Uncropped immunoblots, ED_FIG1c and ED_FIG1k.
Source Data Extended Data Fig. 2
Statistical source data.
Source Data Extended Data Fig. 2
Uncropped immunoblots, ED_FIG2e.
Source Data Extended Data Fig. 3
Statistical source data.
Source Data Extended Data Fig. 4
Statistical source data.
Source Data Extended Data Fig. 5
Statistical source data.
Source Data Extended Data Fig. 6
Statistical source data.
Source Data Extended Data Fig. 7
Statistical source data.
Source Data Extended Data Fig. 8
Statistical source data.
Source Data Extended Data Fig. 8
Uncropped immuno blots, ED_FIG8c.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hauck, A.K., Mehmood, R., Carpenter, B.J. et al. Nuclear receptor corepressors non-canonically drive glucocorticoid receptor-dependent activation of hepatic gluconeogenesis. Nat Metab (2024). https://doi.org/10.1038/s42255-024-01029-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s42255-024-01029-4