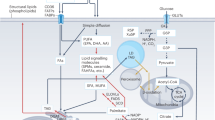
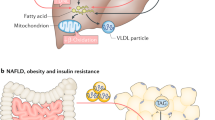
Abstract
In the healthy state, the fat stored in our body isn’t just inert. Rather, it is dynamically mobilized to maintain an adequate concentration of fatty acids (FAs) in our bloodstream. Our body tends to produce excess FAs to ensure that the FA availability is not limiting. The surplus FAs are actively re-esterified into glycerides, initiating a cycle of breakdown and resynthesis of glycerides. This cycle consumes energy without generating a new product and is commonly referred to as the ‘futile lipid cycle’ or the glyceride/FA cycle. Contrary to the notion that it’s a wasteful process, it turns out this cycle is crucial for systemic metabolic homeostasis. It acts as a control point in intra-adipocyte and inter-organ cross-talk, a metabolic rheostat, an energy sensor and a lipid diversifying mechanism. In this Review, we discuss the metabolic regulation and physiological implications of the glyceride/FA cycle and its mechanistic underpinnings.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Brownstein, A. J., Veliova, M., Acin-Perez, R., Liesa, M. & Shirihai, O. S. ATP-consuming futile cycles as energy dissipating mechanisms to counteract obesity. Rev. Endocr. Metab. Disord. 23, 121–131 (2022).
Greenhill, C. Unravelling the molecular basis of futile creatine cycling. Nat. Rev. Endocrinol. 17, 381–381 (2021).
Katz, J. & Rognstad, R. Futile cycling in glucose metabolism. Trends Biochem. Sci. 3, 171–174 (1978).
Sharma, A. K. & Wolfrum, C. Lipid cycling isn’t all futile. Nat. Metab. 5, 540–541 (2023).
Prentki, M. & Madiraju, S. R. M. Glycerolipid metabolism and signaling in health and disease. Endocr. Rev. 29, 647–676 (2008).
Prentki, M. & Madiraju, S. R. M. Glycerolipid/freefatty acid cycle and islet β-cell function in health, obesity and diabetes. Mol. Cell. Endocrinol. 353, 88–100 (2012).
Wunderling, K., Zurkovic, J., Zink, F., Kuerschner, L. & Thiele, C. Triglyceride cycling enables modification of stored fatty acids. Nat. Metab. 5, 699–709 (2023). In this paper, the authors used an advanced mass-spectrometry tracing approach to quantify the rate of futile lipid cycling in 3T3-L1 adipocytes and calculated the half-life of the TG turnover.
Grabner, G. F., Xie, H., Schweiger, M. & Zechner, R. Lipolysis: cellular mechanisms for lipid mobilization from fat stores. Nat. Metab. 3, 1445–1465 (2021).
Zechner, R. et al. FAT SIGNALS - lipases and lipolysis in lipid metabolism and signaling. Cell Metab. 15, 279–291 (2012).
Harayama, T. & Antonny, B. Beyond fluidity: the role of lipid unsaturation in membrane function. Cold Spring Harb. Perspect. Biol. https://doi.org/10.1101/cshperspect.a041409 (2023).
Harayama, T. & Riezman, H. Understanding the diversity of membrane lipid composition. Nat. Rev. Mol. Cell Biol. 19, 281–296 (2018).
Cao, H. et al. Identification of a lipokine, a lipid hormone linking adipose tissue to systemic metabolism. Cell 134, 933–944 (2008).
Lynes, M. D., Kodani, S. D. & Tseng, Y.-H. Lipokines and thermogenesis. Endocrinology 160, 2314–2325 (2019).
Resh, M. D. Chapter 13 - Lipid modification of proteins. In Biochemistry of Lipids, Lipoproteins and Membranes (Sixth Edition) (eds. Ridgway, N. D. & McLeod, R. S.) 391–414 (Elsevier, 2016). https://doi.org/10.1016/B978-0-444-63438-2.00013-4
Resh, M. D. Covalent lipid modifications of proteins. Curr. Biol. 23, R431–R435 (2013).
Varga, T., Czimmerer, Z. & Nagy, L. PPARs are a unique set of fatty acid regulated transcription factors controlling both lipid metabolism and inflammation. Biochim. Biophys. Acta 1812, 1007–1022 (2011).
Shi, H. et al. PPARγ regulates genes involved in triacylglycerol synthesis and secretion in mammary gland epithelial cells of dairy goats. PPAR Res. 2013, 310948 (2013).
Cook, H. W. & McMaster, C. R. in New Comprehensive Biochemistry Vol. 36, Ch. 7 pp. 181–204 (Elsevier, 2002).
Jacquemyn, J., Cascalho, A. & Goodchild, R. E. The ins and outs of endoplasmic reticulum-controlled lipid biosynthesis. EMBO Rep. 18, 1905–1921 (2017).
Newsholme, E. A., Challiss, R. A. J. & Crabtree, B. Substrate cycles: their role in improving sensitivity in metabolic control. Trends Biochem. Sci. 9, 277–280 (1984). In this classic review, pioneering authors discussed how substrate cycles can improve metabolic sensitivity and improve metabolic control.
Nye, C., Kim, J., Kalhan, S. C. & Hanson, R. W. Reassessing triglyceride synthesis in adipose tissue. Trends Endocrinol. Metab. 19, 356–361 (2008).
Vaughan, M. The production and release of glycerol by adipose tissue incubated in vitro. J. Biol. Chem. 237, 3354–3358 (1962). In this pioneering study, Vaughan showed evidence of recycling of FAs and glycerol from adipose tissue explant.
Vaughan, M. & Steinberg, D. Effect of hormones on lipolysis and esterification of free fatty acids during incubation of adipose tissue in vitro. J. Lipid Res. 4, 193–199 (1963). This study revealed how various metabolic hormones affect the rate of lipolysis and FA recycling.
Prusiner, S. & Poe, M. Thermodynamic considerations of mammalian thermogenesis. Nature 220, 235–237 (1968). This is one of the earliest studies that speculated a thermogenic role of the glyceride/FA cycle.
Ball, E. G. & Jungas, R. L. On the action of hormones which accelerate the rate of oxygen consumption and fatty acid release in rat adipose tissue in vitro. Proc. Natl Acad. Sci. USA. 47, 932–941 (1961).
Cahill, G. F., Leboeuf, B. & Flinn, R. B. Studies on rat adipose tissue in vitro: VI. effect of epinephrine on glucose metabolism. J. Biol. Chem. 235, 1246–1250 (1960).
Hammond, V. A. & Johnston, D. G. Substrate cycling between triglyceride and fatty acid in human adipocytes. Metabolism 36, 308–313 (1987).
Oeckl, J. et al. Loss of UCP1 function augments recruitment of futile lipid cycling for thermogenesis in murine brown fat. Mol. Metab. 61, 101499 (2022). This study reported that the brown adipocytes from Ucp1 KO mice utilize DGAT1-dependent futile lipid cycling to increase oxygen consumption.
Veliova, M. et al. Blocking mitochondrial pyruvate import in brown adipocytes induces energy wasting via lipid cycling. EMBO Rep. 21, e49634 (2020).
Edens, N. K., Leibel, R. L. & Hirsch, J. Mechanism of free fatty acid re-esterification in human adipocytes in vitro. J. Lipid Res. 31, 1423–1431 (1990).
Elia, M., Zed, C., Neale, G. & Livesey, G. The energy cost of triglyceride-fatty acid recycling in nonobese subjects after an overnight fast and four days of starvation. Metabolism 36, 251–255 (1987). In this insightful human study, the authors estimated that the futile lipid cycle may account for ~2.5% REE in fasted participants.
Reshef, L. et al. Glyceroneogenesis and the triglyceride/fatty acid cycle. J. Biol. Chem. 278, 30413–30416 (2003).
Jensen, M. D., Ekberg, K. & Landau, B. R. Lipid metabolism during fasting. Am. J. Physiol. 281, E789–E793 (2001).
Klein, S. & Wolfe, R. R. Whole-body lipolysis and triglyceride-fatty acid cycling in cachectic patients with esophageal cancer. J. Clin. Invest. 86, 1403–1408 (1990).
Sharma, A. K. et al. Basal re-esterification finetunes mitochondrial fatty acid utilization. Mol. Metab. 71, 101701 (2023). This paper showed that in basal conditions, DGAT1 and DGAT2 redundantly re-esterify the majority of the FAs, and a combined inhibition blocks re-esterification and leads to the diversion of FAs to mitochondrial oxidation.
Chitraju, C. et al. Triglyceride synthesis by DGAT1 protects adipocytes from lipid-induced ER stress during lipolysis. Cell Metab. 26, 407–418 (2017). This paper demonstrated that on lipolysis stimulation, DGAT1 prominently re-esterifies lipolysis-derived FAs to prevent mitochondrial lipotoxicity.
Cohen, P. & Kajimura, S. The cellular and functional complexity of thermogenic fat. Nat. Rev. Mol. Cell Biol. 22, 393–409 (2021).
Zechner, R., Kienesberger, P. C., Haemmerle, G., Zimmermann, R. & Lass, A. Adipose triglyceride lipase and the lipolytic catabolism of cellular fat stores. J. Lipid Res. 50, 3–21 (2009).
Herzer, S., Meldner, S., Gröne, H. -J. & Nordström, V. Fasting-induced lipolysis and hypothalamic insulin signaling are regulated by neuronal glucosylceramide synthase. Diabetes 64, 3363–3376 (2015).
Mottillo, E. P. et al. Coupling of lipolysis and de novo lipogenesis in brown, beige, and white adipose tissues during chronic β3-adrenergic receptor activation. J. Lipid Res. 55, 2276–2286 (2014).
Gyamfi, D., Ofori Awuah, E. & Owusu, S. Chapter 2 - Lpid metabolism: an overview. in The Molecular Nutrition of Fats (ed. Patel, V. B.) 17–32 (Academic Press, 2019). https://doi.org/10.1016/B978-0-12-811297-7.00002-0
Zimmermann, R. et al. Fat mobilization in adipose tissue is promoted by adipose triglyceride lipase. Science 306, 1383–1386 (2004). This study reported the discovery of ATGL, the key lipolytic enzyme of adipocytes.
Poursharifi, P. et al. Adipose ABHD6 regulates tolerance to cold and thermogenic programs. JCI Insight 5, e140294 (2020).
Chandramohan, C. et al. Mice lacking triglyceride synthesis enzymes in adipose tissue are resistant to diet-induced obesity. eLife 12, RP88049 (2023). This paper reported that Dgat1/Dgat2 DKO mice show improved metabolic health and may utilize Ucp1 upregulation for thermogenic compensation.
McLelland, G.-L. et al. Identification of an alternative triglyceride biosynthesis pathway. Nature 621, 171–178 (2023). This study identified a terminal FA transferase that synthesises TG independent of DGAT1/DGAT2.
Wilfling, F. et al. Triacylglycerol synthesis enzymes mediate lipid droplet growth by relocalizing from the ER to lipid droplets. Dev. Cell 24, 384–399 (2013).
Stone, S. J. et al. The endoplasmic reticulum enzyme DGAT2 is found in mitochondria-associated membranes and has a mitochondrial targeting signal that promotes its association with mitochondria. J. Biol. Chem. 284, 5352–5361 (2009).
Nguyen, T. B. et al. DGAT1-dependent lipid droplet biogenesis protects mitochondrial function during starvation-induced autophagy. Dev. Cell 42, 9–21 (2017).
Kuerschner, L., Moessinger, C. & Thiele, C. Imaging of lipid biosynthesis: how a neutral lipid enters lipid droplets. Traffic 9, 338–352 (2008).
Poppelreuther, M. et al. The N-terminal region of acyl-CoA synthetase 3 is essential for both the localization on lipid droplets and the function in fatty acid uptake. J. Lipid Res. 53, 888–900 (2012).
Anderson, C. M. & Stahl, A. SLC27 fatty acid transport proteins. Mol. Aspects Med. 34, 516–528 (2013).
Wood, F. C., Leboeuf, B. & Cahill, G. F. Metabolic role of glucose. A source of glyceride-glycerol in controlling the release of fatty acids by adipose tissue. Diabetes 9, 261–263 (1960).
Gauthier, M.-S. et al. AMP-activated protein kinase is activated as a consequence of lipolysis in the adipocyte: potential mechanism and physiological relevance. J. Biol. Chem. 283, 16514–16524 (2008).
Muers, M. Futile protein cycle keeps mice thin. Nature https://doi.org/10.1038/news070903-4 (2007).
Brooks, B., Arch, J. R. & Newsholme, E. A. Effects of hormones on the rate of the triacylglycerol/fatty acid substrate cycle in adipocytes and epididymal fat pads. FEBS Lett. 146, 327–330 (1982).
Shapiro, B., Chowers, I. & Rose, G. Fatty acid uptake and esterification in adipose tissue. Biochim. Biophys. Acta 23, 115–120 (1957).
Irshad, Z., Dimitri, F., Christian, M. & Zammit, V. A. Diacylglycerol acyltransferase 2 links glucose utilization to fatty acid oxidation in the brown adipocytes. J. Lipid Res. 58, 15–30 (2017).
Lundgren, P. et al. A subpopulation of lipogenic brown adipocytes drives thermogenic memory. Nat. Metab. 5, 1691–1705 (2023). This study identified a subpopulation of the brown adipocytes that are enriched in lipogenic machinery and may be important to recall and adapt according to previous cold exposure episodes.
Bornstein, M. R. et al. Comprehensive quantification of metabolic flux during acute cold stress in mice. Cell Metab. https://doi.org/10.1016/j.cmet.2023.09.002 (2023). This paper described the metabolite flux in mice following cold exposure and demonstrated significant inter-organ cross-talk required for cold adaptation.
Samoilov, M., Plyasunov, S. & Arkin, A. P. Stochastic amplification and signaling in enzymatic futile cycles through noise-induced bistability with oscillations. Proc. Natl Acad. Sci. USA 102, 2310–2315 (2005).
Shahrezaei, V. & Swain, P. S. The stochastic nature of biochemical networks. Curr. Opin. Biotechnol. 19, 369–374 (2008).
Tonn, M. K., Thomas, P., Barahona, M. & Oyarzún, D. A. Stochastic modelling reveals mechanisms of metabolic heterogeneity. Commun. Biol. 2108 (2019).
Chomicki, G., Werner, G. D. A., West, S. A. & Kiers, E. T. Compartmentalization drives the evolution of symbiotic cooperation. Philos. Trans. R. Soc. B Biol. Sci. 375, 20190602 (2020).
Martin, W. Evolutionary origins of metabolic compartmentalization in eukaryotes. Philos. Trans. R. Soc. B Biol. Sci. 365, 847–855 (2010).
Prinz, W. A., Toulmay, A. & Balla, T. The functional universe of membrane contact sites. Nat. Rev. Mol. Cell Biol. 21, 7–24 (2020).
Faergeman, N. J. & Knudsen, J. Role of long-chain fatty acyl-CoA esters in the regulation of metabolism and in cell signalling. Biochem. J. 323, 1–12 (1997).
Li, L. O., Klett, E. L. & Coleman, R. A. Acyl-CoA synthesis, lipid metabolism and lipotoxicity. Biochim. Biophys. Acta 1801, 246–251 (2010).
Yan, S. et al. Long-chain acyl-CoA synthetase in fatty acid metabolism involved in liver and other diseases: an update. World J. Gastroenterol. 21, 3492–3498 (2015).
Trefely, S., Lovell, C. D., Snyder, N. W. & Wellen, K. E. Compartmentalised acyl-CoA metabolism and roles in chromatin regulation. Mol. Metab. 38, 100941 (2020).
Yang, Y. et al. ACSL3 and ACSL4, distinct roles in ferroptosis and cancers. Cancers 14, 5896 (2022).
Huh, J. Y. et al. TANK-binding kinase 1 regulates the localization of Acyl-CoA synthetase ACSL1 to control hepatic fatty acid oxidation. Cell Metab. 32, 1012–1027 (2020).
Ellis, J. M. et al. Adipose Acyl-CoA synthetase-1 directs fatty acids toward β-oxidation and is required for cold thermogenesis. Cell Metab. 12, 53–64 (2010).
Liu, J. & Waugh, M. G. The regulation and functions of ACSL3 and ACSL4 in the liver and hepatocellular carcinoma. Liver Cancer Int. 4, 28–41 (2023).
Klasson, T. D. et al. ACSL3 regulates lipid droplet biogenesis and ferroptosis sensitivity in clear cell renal cell carcinoma. Cancer Metab. 10, 14 (2022).
Rohm, M., Zeigerer, A., Machado, J. & Herzig, S. Energy metabolism in cachexia. EMBO Rep. 20, e47258 (2019).
Wolfe, R. R., Herndon, D. N., Jahoor, F., Miyoshi, H. & Wolfe, M. Effect of severe burn injury on substrate cycling by glucose and fatty acids. N. Engl. J. Med. 317, 403–408 (1987). This human study reported induction of pronounced futile lipid cycling and glucose cycling after severe burn injury.
Hunt, M. C., Siponen, M. I. & Alexson, S. E. H. The emerging role of acyl-CoA thioesterases and acyltransferases in regulating peroxisomal lipid metabolism. Biochim. Biophys. Acta. 1822, 1397–1410 (2012).
Tillander, V., Alexson, S. E. H. & Cohen, D. E. Deactivating fatty acids: Acyl-CoA thioesterase-mediated control of lipid metabolism. Trends Endocrinol. Metab. 28, 473–484 (2017).
Heden, T. D. et al. ACOT1 deficiency attenuates high-fat diet induced fat mass gain by increasing energy expenditure. JCI Insight https://doi.org/10.1172/jci.insight.160987 (2023).
Okada, K. et al. Thioesterase superfamily member 1 suppresses cold thermogenesis by limiting the oxidation of lipid droplet-derived fatty acids in brown adipose tissue. Mol. Metab. 5, 340–351 (2016).
Zhang, Y. et al. Targeted deletion of thioesterase superfamily member 1 promotes energy expenditure and protects against obesity and insulin resistance. Proc. Natl Acad. Sci. USA 109, 5417–5422 (2012).
Neess, D., Bek, S., Engelsby, H., Gallego, S. F. & Færgeman, N. J. Long-chain acyl-CoA esters in metabolism and signaling: role of acyl-CoA binding proteins. Prog. Lipid Res. 59, 1–25 (2015).
Udupa, P., Kumar, A., Parit, R. & Ghosh, D. K. Acyl-CoA binding protein regulates nutrient-dependent autophagy. Metabolism 145, 155338 (2023).
Randle, P. J., Garland, P. B., Hales, C. N. & Newsholme, E. A. The glucose fatty-acid cycle. Its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet Lond. Engl. 1, 785–789 (1963). This study made an early observation of FA toxicity and highlighted the conversion of glucose into FAs, its involvement in TG cycling and its physiological implication.
Hue, L. & Taegtmeyer, H. The Randle cycle revisited: a new head for an old hat. Am. J. Physiol. 297, E578–E591 (2009).
May, J. M. Triacylglycerol turnover in large and small rat adipocytes: effects of lipolytic stimulation, glucose, and insulin. J. Lipid Res. 23, 428–436 (1982).
Toker, A. The biology and biochemistry of diacylglycerol signalling. EMBO Rep. 6, 310–314 (2005).
Itoh, T. et al. Structural basis for the activation of PPARgamma by oxidized fatty acids. Nat. Struct. Mol. Biol. 15, 924–931 (2008).
Liberato, M. V. et al. Medium chain fatty acids are selective peroxisome proliferator activated receptor (PPAR) γ activators and pan-PPAR partial agonists. PLoS ONE 7, e36297 (2012).
Lasar, D. et al. Peroxisome proliferator activated receptor gamma controls mature brown adipocyte inducibility through glycerol kinase. Cell Rep. 22, 760–773 (2018).
Eichmann, T. O. et al. Studies on the substrate and stereo/regioselectivity of adipose triglyceride lipase, hormone-sensitive lipase, and diacylglycerol-O-acyltransferases. J. Biol. Chem. 287, 41446–41457 (2012).
Guan, H. -P. et al. A futile metabolic cycle activated in adipocytes by antidiabetic agents. Nat. Med. 8, 1122–1128 (2002). This paper reported that anti-diabetic agents, glitazones, induce glycerol kinase expression in adipocytes thereby enabling a FA re-esterification cycle.
Baggelaar, M. P., Maccarrone, M. & van der Stelt, M. 2-arachidonoylglycerol: a signaling lipid with manifold actions in the brain. Prog. Lipid Res. 71, 1–17 (2018).
Lynes, M. D. et al. The cold-induced lipokine 12,13-diHOME promotes fatty acid transport into brown adipose tissue. Nat. Med. 23, 631–637 (2017). This paper reported a potent brown adipose tissue-derived lipokine and its implications in systemic health.
Ortiz, G. U. & de Freitas, E. C. Physical activity and batokines. Am. J. Physiol. https://doi.org/10.1152/ajpendo.00160.2023 (2023).
Fasshauer, M. & Blüher, M. Adipokines in health and disease. Trends Pharmacol. Sci. 36, 461–470 (2015).
Tang, S., Wan, M., Huang, W., Stanton, R. C. & Xu, Y. Maresins: specialized proresolving lipid mediators and their potential role in inflammatory-related diseases. Mediators Inflamm. 2018, 2380319 (2018).
Reidy, S. P. & Weber, J.-M. Accelerated substrate cycling: a new energy-wasting role for leptin in vivo. Am. J. Physiol. 282, E312–E317 (2002).
Qiao, L., Kinney, B., Schaack, J. & Shao, J. Adiponectin inhibits lipolysis in mouse adipocytes. Diabetes 60, 1519–1527 (2011).
Harris, R. B. S. Direct and indirect effects of leptin on adipocyte metabolism. Biochim. Biophys. Acta 1842, 414–423 (2014).
Han, M. S. et al. Regulation of adipose tissue inflammation by interleukin 6. Proc. Natl Acad. Sci. USA 117, 2751–2760 (2020).
Van Hall, G. et al. Interleukin-6 stimulates lipolysis and fat oxidation in humans. J. Clin. Endocrinol. Metab. 88, 3005–3010 (2003).
Zhang, H. H., Halbleib, M., Ahmad, F., Manganiello, V. C. & Greenberg, A. S. Tumor necrosis factor-α stimulates lipolysis in differentiated human adipocytes through activation of extracellular signal-related kinase and elevation of intracellular camp. Diabetes 51, 2929–2935 (2002).
Weber, B. Z. C., Arabaci, D. H. & Kir, S. Metabolic reprogramming in adipose tissue during cancer cachexia. Front. Oncol. 12, 848394 (2022).
Kliewer, K. L. et al. Adipose tissue lipolysis and energy metabolism in early cancer cachexia in mice. Cancer Biol. Ther. 16, 886–897 (2014).
Arner, P. & Langin, D. Lipolysis in lipid turnover, cancer cachexia, and obesity-induced insulin resistance. Trends Endocrinol. Metab. 25, 255–262 (2014).
Narsale, A. A. & Carson, J. A. Role of IL-6 in cachexia – therapeutic implications. Curr. Opin. Support. Palliat. Care 8, 321–327 (2014).
Beaudry, J. L. et al. The brown adipose tissue glucagon receptor is functional but not essential for control of energy homeostasis in mice. Mol. Metab. 22, 37–48 (2019).
Longuet, C. et al. The glucagon receptor is required for the adaptive metabolic response to fasting. Cell Metab. 8, 359–371 (2008).
Pedersen, C., Bouman, S. D., Porsgaard, T., Rosenkilde, M. M. & Roed, N. K. Dual treatment with a fixed ratio of glucagon and insulin increases the therapeutic window of insulin in diabetic rats. Physiol. Rep. 6, e13657 (2018).
Hägele, F. A. et al. Impact of one-day fasting, ketogenic diet or exogenous ketones on control of energy balance in healthy participants. Clin. Nutr. ESPEN 55, 292–299 (2023).
Wolfe, R. R., Klein, S., Carraro, F. & Weber, J. M. Role of triglyceride-fatty acid cycle in controlling fat metabolism in humans during and after exercise. Am. J. Physiol. 258, E382–E389 (1990).
Arner, P. et al. Adipose lipid turnover and long-term changes in body weight. Nat. Med. 25, 1385–1389 (2019). Findings in this paper suggest a positive impact of lipid turnover in weight homeostasis.
Arner, P., Andersson, D. P., Bäckdahl, J., Dahlman, I. & Rydén, M. Weight gain and impaired glucose metabolism in women are predicted by inefficient subcutaneous fat cell lipolysis. Cell Metab. 28, 45–54 (2018).
Arner, P. et al. Dynamics of human adipose lipid turnover in health and metabolic disease. Nature 478, 110–113 (2011). This paper highlighted the positive association of lipid turnover in glucose homeostasis and body weight regulation.
Hsieh, C. -W., DeSantis, D. & M, C. Role of triglyceride/fatty acid cycle in development of type 2 diabetes. in Role of the Adipocyte in Development of Type 2 Diabetes (ed. Croniger, C.) (InTech, 2011). https://doi.org/10.5772/24033
Guillou, H., Zadravec, D., Martin, P. G. P. & Jacobsson, A. The key roles of elongases and desaturases in mammalian fatty acid metabolism: insights from transgenic mice. Prog. Lipid Res. 49, 186–199 (2010).
Los, D. A. & Murata, N. Membrane fluidity and its roles in the perception of environmental signals. Biochim. Biophys. Acta 1666, 142–157 (2004).
Tong, P. et al. Cell membrane dynamics and insulin resistance in non-insulin-dependent diabetes mellitus. Lancet Lond. Engl. 345, 357–358 (1995).
Pilon, M. Revisiting the membrane-centric view of diabetes. Lipids Health Dis. 15, 167 (2016).
Perona, J. S. Membrane lipid alterations in the metabolic syndrome and the role of dietary oils. Biochim. Biophys. Acta 1859, 1690–1703 (2017).
He, M. et al. Inhibiting phosphatidylcholine remodeling in adipose tissue increases insulin sensitivity. Diabetes 72, 1547–1559 (2023).
Calzada, E., Onguka, O. & Claypool, S. M. Phosphatidylethanolamine metabolism in health and disease. Int. Rev. Cell Mol. Biol. 321, 29–88 (2016).
Schütter, M., Giavalisco, P., Brodesser, S. & Graef, M. Local fatty acid channeling into phospholipid synthesis drives phagophore expansion during autophagy. Cell 180, 135–149 (2020).
Vance, J. E. Phospholipid synthesis and transport in mammalian cells. Traffic 16, 1–18 (2015).
von Essen, G. et al. Highly recruited brown adipose tissue does not in itself protect against obesity. Mol. Metab. 76, 101782 (2023). This paper demonstrated that UCP1 expression is not correlated with UCP1 activity; thus, conclusions drawn from expression may need reconsideration.
Qian, H. & Beard, D. A. Metabolic futile cycles and their functions: a systems analysis of energy and control. Syst. Biol. 153, 192–200 (2006).
Chen, H. C. Enhancing energy and glucose metabolism by disrupting triglyceride synthesis: lessons from mice lacking DGAT1. Nutr. Metab. 3, 10 (2006).
Unger, R. H. Lipotoxic diseases. Annu. Rev. Med. 53, 319–336 (2002).
Yang, W. S. et al. Peroxidation of polyunsaturated fatty acids by lipoxygenases drives ferroptosis. Proc. Natl Acad. Sci. USA 113, E4966–E4975 (2016).
Chitraju, C., Walther, T. C. & Farese, R. V. The triglyceride synthesis enzymes DGAT1 and DGAT2 have distinct and overlapping functions in adipocytes. J. Lipid Res. 60, 1112–1120 (2019).
Cahill, G. F., Ashmore, J., Renold, A. E. & Hastings, A. B. Blood glucose and the liver. Am. J. Med. 26, 264–282 (1959).
Willems, P. H. G. M., Rossignol, R., Dieteren, C. E. J., Murphy, M. P. & Koopman, W. J. H. Redox homeostasis and mitochondrial dynamics. Cell Metab. 22, 207–218 (2015).
Garbarino, J. & Sturley, S. L. Saturated with fat: new perspectives on lipotoxicity. Curr. Opin. Clin. Nutr. Metab. Care 12, 110–116 (2009).
Santoleri, D. & Titchenell, P. M. Resolving the paradox of hepatic insulin resistance. Cell. Mol. Gastroenterol. Hepatol. 7, 447–456 (2019).
Kumashiro, N. et al. Cellular mechanism of insulin resistance in nonalcoholic fatty liver disease. Proc. Natl Acad. Sci. USA 108, 16381–16385 (2011).
Sharma, A. K. & Wolfrum, C. DGAT inhibition at the post-absorptive phase reduces plasma FA by increasing FA oxidation. EMBO Mol. Med. 15, e18209 (2023).
Steinberg, G. R. & Hardie, D. G. New insights into activation and function of the AMPK. Nat. Rev. Mol. Cell Biol. 24, 255–272 (2023).
Pinkosky, S. L. et al. Long-chain fatty acyl-CoA esters regulate metabolism via allosteric control of AMPK β1 isoforms. Nat. Metab. 2, 873–881 (2020).
Glorian, M. et al. A single element in the phosphoenolpyruvate carboxykinase gene mediates thiazolidinedione action specifically in adipocytes. Biochimie 83, 933–943 (2001).
Leroyer, S. et al. Rosiglitazone controls fatty acid cycling by means of glyceroneogenesis and glycerol phosphorylation. FASEB J. 20, A957 (2006).
Haluzík, M. M. & Haluzík, M. PPAR-alpha and insulin sensitivity. Physiol. Res. 55, 115–122 (2006).
Lefebvre, P., Chinetti, G., Fruchart, J. -C. & Staels, B. Sorting out the roles of PPAR alpha in energy metabolism and vascular homeostasis. J. Clin. Invest. 116, 571–580 (2006).
Kalliora, C. & Drosatos, K. The glitazars paradox: cardiotoxicity of the metabolically beneficial dual PPARα and PPARγ activation. J. Cardiovasc. Pharmacol. 76, 514–526 (2020).
Dumont, L. et al. Targeting adrenergic receptors to activate brown fat without cardiovascular effects. Physiology 38, 5764490 (2023).
O’Mara, A. E. et al. Chronic mirabegron treatment increases human brown fat, HDL cholesterol, and insulin sensitivity. J. Clin. Invest. 130, 2209–2219 (2020).
Straat, M. E. et al. Stimulation of the beta-2-adrenergic receptor with salbutamol activates human brown adipose tissue. Cell Rep. Med. 4, 100942 (2023).
Harper, J. A., Dickinson, K. & Brand, M. D. Mitochondrial uncoupling as a target for drug development for the treatment of obesity. Obes. Rev. 2, 255–265 (2001).
Ukropec, J., Anunciado, R. P., Ravussin, Y., Hulver, M. W. & Kozak, L. P. UCP1-independent thermogenesis in white adipose tissue of cold-acclimated Ucp1−/− mice. J. Biol. Chem. 281, 31894–31908 (2006).
Chouchani, E. T., Kazak, L. & Spiegelman, B. M. New advances in adaptive thermogenesis: UCP1 and beyond. Cell Metab. 29, 27–37 (2019).
Cox, A. R. et al. The rheumatoid arthritis drug auranofin lowers leptin levels and exerts antidiabetic effects in obese mice. Cell Metab. 34, 1932–1946 (2022).
Zeng, W. et al. Sympathetic neuro-adipose connections mediate leptin-driven lipolysis. Cell 163, 84–94 (2015).
Blondin, D. P. et al. Inhibition of intracellular triglyceride lipolysis suppresses cold-induced brown adipose tissue metabolism and increases shivering in humans. Cell Metab. 25, 438–447 (2017). This paper showed that mobilization of TG from adipose tissue is crucial for thermogenesis in humans.
Crane, J. D. et al. Inhibiting peripheral serotonin synthesis reduces obesity and metabolic dysfunction by promoting brown adipose tissue thermogenesis. Nat. Med. 21, 166–172 (2015).
Suchacki, K. J. et al. The serotonin transporter sustains human brown adipose tissue thermogenesis. Nat. Metab. 5, 1319–1336 (2023).
Argilés, J. M., Fontes-Oliveira, C. C., Toledo, M., López-Soriano, F. J. & Busquets, S. Cachexia: a problem of energetic inefficiency. J. Cachexia Sarcopenia Muscle 5, 279–286 (2014).
Torosian, M. H., Bartlett, D. L., Chatzidakis, C. & Stein, T. P. Effect of tumor burden on futile glucose and lipid cycling in tumor-bearing animals. J. Surg. Res. 55, 68–73 (1993).
Maniyadath, B., Zhang, Q., Gupta, R. K. & Mandrup, S. Adipose tissue at single-cell resolution. Cell Metab. 35, 386–413 (2023).
Sun, W. et al. snRNA-seq reveals a subpopulation of adipocytes that regulates thermogenesis. Nature 587, 98–102 (2020).
Fedorenko, A., Lishko, P. V. & Kirichok, Y. Mechanism of fatty-acid-dependent UCP1 uncoupling in brown fat mitochondria. Cell 151, 400–413 (2012).
Enerbäck, S. et al. Mice lacking mitochondrial uncoupling protein are cold-sensitive but not obese. Nature 387, 90–94 (1997). This paper highlighted that at room temperature housing, loss of UCP1 does not cause obesity as previously speculated.
Townsend, L. K., Wang, D., Wright, D. C. & Blondin, D. P. Skeletal muscle, not adipose tissue, mediates cold-induced metabolic benefits. Nat. Metab. 5, 1074–1077 (2023).
Zhang, Y. et al. miR-378 activates the pyruvate-pep futile cycle and enhances lipolysis to ameliorate obesity in mice. EBioMedicine 5, 93–104 (2016).
Zhu, A., Romero, R. & Petty, H. R. A sensitive fluorimetric assay for pyruvate. Anal. Biochem. 396, 146–151 (2010).
Ikeda, K. et al. UCP1-independent signaling involving SERCA2b-mediated calcium cycling regulates beige fat thermogenesis and systemic glucose homeostasis. Nat. Med. 23, 1454–1465 (2017).
Bal, N. C. et al. Sarcolipin is a newly identified regulator of muscle-based thermogenesis in mammals. Nat. Med. 18, 1575–1579 (2012).
Ikeda, K. & Yamada, T. Adipose tissue thermogenesis by calcium futile cycling. J. Biochem. 172, 197–203 (2022).
Soeters, P. B. et al. The anabolic role of the Warburg, Cori-cycle and Crabtree effects in health and disease. Clin. Nutr. 40, 2988–2998 (2021).
Karpatkin, S., Helmreich, E. & Cori, C. F. Regulation of glycolysis in muscle. J. Biol. Chem. 239, 3139–3145 (1964).
Staehr, C. et al. Migraine‐associated mutation in the Na,K‐ATPase leads to disturbances in cardiac metabolism and reduced cardiac function. J. Am. Heart Assoc. 11, e021814 (2022).
Williams, C. H. Malignant hyperthermia: a runaway thermogenic futile cycle at the sodium channel level. Adv. Biosci. Biotechnol. 5, 197–200 (2014).
Kazak, L. et al. A creatine-driven substrate cycle enhances energy expenditure and thermogenesis in beige fat. Cell 163, 643–655 (2015).
Nicholls, D. G. & Brand, M. D. A critical assessment of the role of creatine in brown adipose tissue thermogenesis. Nat. Metab. 5, 21–28 (2023).
Rahbani, J. F. et al. Creatine kinase B controls futile creatine cycling in thermogenic fat. Nature 590, 480–485 (2021).
Sun, Y. et al. Mitochondrial TNAP controls thermogenesis by hydrolysis of phosphocreatine. Nature 593, 580–585 (2021).
Rotondo, F. et al. Glycerol is synthesized and secreted by adipocytes to dispose of excess glucose, via glycerogenesis and increased acyl-glycerol turnover. Sci. Rep. 7, 8983 (2017).
Mugabo, Y. et al. Identification of a mammalian glycerol-3-phosphate phosphatase: role in metabolism and signaling in pancreatic β-cells and hepatocytes. Proc. Natl Acad. Sci. USA 113, E430–E439 (2016). This paper reported the identification of a phosphatase that could hydrolyse G3P.
Singh, R. et al. Autophagy regulates lipid metabolism. Nature 458, 1131–1135 (2009).
Ward, C. et al. Autophagy, lipophagy and lysosomal lipid storage disorders. Biochim. Biophys. Acta 1861, 269–284 (2016).
Schulze, R. J., Sathyanarayan, A. & Mashek, D. G. Breaking fat: the regulation and mechanisms of lipophagy. Biochim. Biophys. Acta 1862, 1178–1187 (2017).
O’Rourke, E. J. & Ruvkun, G. MXL-3 and HLH-30 transcriptionally link lipolysis and autophagy to nutrient availability. Nat. Cell Biol. 15, 668–676 (2013).
Fu, Y. et al. Degradation of lipid droplets by chimeric autophagy-tethering compounds. Cell Res. 31, 965–979 (2021).
Chen, N., Lu, B. & Fu, Y. Autophagic clearance of lipid droplets alters metabolic phenotypes in a genetic obesity–diabetes mouse model. Phenomics 3, 119–129 (2023).
Song, W. et al. Lipid kinase PIK3C3 maintains healthy brown and white adipose tissues to prevent metabolic diseases. Proc. Natl Acad. Sci. USA 120, e2214874120 (2023).
Gomaraschi, M., Bonacina, F. & Norata, G. D. Lysosomal acid lipase: from cellular lipid handler to immunometabolic target. Trends Pharmacol. Sci. 40, 104–115 (2019).
Zhao, Z. et al. Tip60-mediated Rheb acetylation links palmitic acid with mTORC1 activation and insulin resistance. Preprint at bioRxiv https://doi.org/10.1101/2023.08.18.553816 (2023).
Angel, A. & Farkas, J. Regulation of cholesterol storage in adipose tissue. J. Lipid Res. 15, 491–499 (1974).
Gonen, A. & Miller, Y. I. From inert storage to biological activity—in search of identity for oxidized cholesteryl esters. Front. Endocrinol. 11, 602252 (2020).
Acknowledgements
We express our appreciation for many excellent studies that contributed to concept refining, but we could not cite them due to space limitations. We thank R. S. Dewal for very helpful inputs on content and presentation. We also thank M. Geiger and S. Turner for excellent management support. A.K.S. is grateful to previous mentors Y. Sharma and A. Prakash. Figures were created using BioRender.com.
Author information
Authors and Affiliations
Contributions
Conceptualization: A.K.S.; idea/content refinement: A.K.S., R.K. and C.W.; manuscript writing (first draft): A.K.S.; manuscript editing: A.K.S., R.K. and C.W.; figures were prepared by A.K.S.; funds, administration and supervision: C.W.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Metabolism thanks the anonymous reviewers for their contribution to the peer review of this work. Primary Handling Editors: Alfredo Gimenez-Cassina and Isabella Samuelson, in collaboration with the Nature Metabolism team
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sharma, A.K., Khandelwal, R. & Wolfrum, C. Futile lipid cycling: from biochemistry to physiology. Nat Metab (2024). https://doi.org/10.1038/s42255-024-01003-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s42255-024-01003-0