Abstract
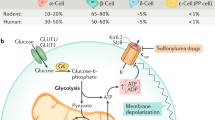
While pancreatic β and α cells are considered the main drivers of blood glucose homeostasis through insulin and glucagon secretion, the contribution of δ cells and somatostatin (SST) secretion to glucose homeostasis remains unresolved. Here we provide a quantitative assessment of the physiological contribution of δ cells to the glycaemic set point in mice. Employing three orthogonal mouse models to remove SST signalling within the pancreas or transplanted islets, we demonstrate that ablating δ cells or SST leads to a sustained decrease in the glycaemic set point. This reduction coincides with a decreased glucose threshold for insulin response from β cells, leading to increased insulin secretion to the same glucose challenge. Our data demonstrate that β cells are sufficient to maintain stable glycaemia and reveal that the physiological role of δ cells is to provide tonic feedback inhibition that reduces the β cell glucose threshold and consequently lowers the glycaemic set point in vivo.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this published article and its supplementary information files. Source data are available with this paper. Data for relevant images are available at Figshare at https://doi.org/10.6084/m9.figshare.24082434 (ref. 80). Source data are provided with this paper.
Code availability
The code used to perform glucose threshold analysis and neuron quantification is available on GitHub at github.com/Huising-Lab/Paracrine-signaling-by-pancreatic-mouse-cells-determines-the-glycemic-set-point.
References
Matschinsky, F. M. & Davis, E. A. The distinction between ‘glucose setpoint’, ‘glucose threshold’ and ‘glucose sensor’ is critical for understanding the role of the pancreatic β-cell in glucose homeostasis. Mol. Cell Biol. 14, 14–29 (1998).
Gerich, J. E. Control of glycaemia. Baillieres Clin. Endocrinol. Metab. 10.1016/s0950-351x(05)80207-1 (1993).
Blum, B. et al. Functional β-cell maturation is marked by an increased glucose threshold and by expression of urocortin 3. Nat. Biotechnol. 30, 261–264 (2012).
Rodriguez-Diaz, R. et al. Paracrine interactions within the pancreatic islet determine the glycemic set point. Cell Metab. 27, 549–558 (2018).
Ewing, K. L. & Tauber, O. E. Blood chemistry changes in mice fed high levels of polyoxyethylene sorbitan derivatives. Toxicol. Appl. Pharmacol. 6, 442–446 (1964).
Chambers, A. P. et al. The role of pancreatic preproglucagon in glucose homeostasis in mice. Cell Metab. 25, 927–934 (2017).
Svendsen, B. et al. Insulin secretion depends on intra-islet glucagon signaling. Cell Rep. 25, 1127–1134 (2018).
Capozzi, M. E. et al. Glucagon lowers glycemia when β cells are active. JCI Insight https://doi.org/10.1172/jci.insight.129954 (2019).
Zhu, L. et al. Intra-islet glucagon signaling is critical for maintaining glucose homeostasis. JCI Insight https://doi.org/10.1172/jci.insight.127994 (2019).
Tellez, K. et al. In vivo studies of glucagon secretion by human islets transplanted in mice. Nat. Metab. 2, 547–557 (2020).
Capozzi, M. E. et al. β Cell tone is defined by proglucagon peptides through cAMP signaling. JCI Insight 4, e126742 (2019).
Liu, L. et al. Gq signaling in α cells is critical for maintaining euglycemia. JCI Insight 6, 1–17 (2021).
Huising, M. O. Paracrine regulation of insulin secretion. Diabetologia 63, 2057–2063 (2020).
Pagliara, A. S., Stillings, S. N., Hover, B., Martin, D. M. & Matschinsky, F. M. Glucose modulation of amino acid induced glucagon and insulin release in the isolated perfused rat pancreas. J. Clin. Invest. 54, 819–832 (1974).
Alonge, K. M., D’Alessio, D. A. & Schwartz, M. W. Brain control of blood glucose levels: implications for the pathogenesis of type 2 diabetes. Diabetologia 64, 5–14 (2021).
Cheng, W. et al. Hindbrain circuits in the control of eating behaviour and energy balance. Nat. Metab. 4, 826–835 (2022).
Thorens, B. Sensing of glucose in the brain. Handb. Exp. Pharmacol. 209, 277–294 (2012).
German, J. P. et al. Leptin activates a novel CNS mechanism for insulin-independent normalization of severe diabetic hyperglycemia. Endocrinology 152, 394–404 (2011).
Ryan, K. K. et al. Fibroblast growth factor-19 action in the brain reduces food intake and body weight and improves glucose tolerance in male rats. Endocrinology 154, 9–15 (2013).
Morton, G. J. et al. FGF19 action in the brain induces insulin-independent glucose lowering. J. Clin. Invest. 123, 4799–4808 (2013).
Carroll, P. B., Zeng, Y., Alejandro, R., Starzl, T. E. & Ricordi, C. Glucose homeostasis is regulated by donor islets in xenografts. Transplant. Proc. 24, 2980–2981 (1992).
Vieira, E., Salehi, A. & Gylfe, E. Glucose inhibits glucagon secretion by a direct effect on mouse pancreatic α cells. Diabetologia 50, 370–379 (2007).
Lai, B. K. et al. Somatostatin is only partly required for the glucagonostatic effect of glucose but is necessary for the glucagonostatic effect of KATP channel blockers. Diabetes 67, 2239–2253 (2018).
van der Meulen, T. et al. Urocortin3 mediates somatostatin-dependent negative feedback control of insulin secretion. Nat. Med. 21, 769–776 (2015).
Shuai, H., Xu, Y., Yu, Q., Gylfe, E. & Tengholm, A. Fluorescent protein vectors for pancreatic islet cell identification in live-cell imaging. Pflug. Arch. Eur. J. Physiol. 468, 1765–1777 (2016).
Xu, S. F. S., Andersen, D. B., Izarzugaza, J. M. G., Kuhre, R. E. & Holst, J. J. In the rat pancreas, somatostatin tonically inhibits glucagon secretion and is required for glucose-induced inhibition of glucagon secretion. Acta Physiol. https://doi.org/10.1111/apha.13464 (2020).
Mandarino, L., Stenner, D., Blanchard, W., Nissen, S. & Gerich, J. Selective effects of somatostatin-14, -25 and -28 on in vitro insulin and glucagon secretion. Nature 291, 76–77 (1981).
Strowski, M. Z., Parmar, R. M., Blake, A. D. & Schaeffer, J. M. Somatostatin inhibits insulin and glucagon secretion via two receptor subtypes: an in vitro study of pancreatic islets from somatostatin receptor 2 knockout mice. Endocrinology 141, 111–117 (2000).
Hauge-Evans, A. C. et al. Somatostatin secreted by islet δ-cells fulfills multiple roles as a paracrine regulator of islet function. Diabetes 58, 403–411 (2009).
Singh, B. et al. KATP channel blockers control glucagon secretion by distinct mechanisms: A direct stimulation of α-cells involving a [Ca2+]c rise and an indirect inhibition mediated by somatostatin. Mol. Metab. 53, 101268 (2021).
van der Meulen, T. et al. Urocortin 3 marks mature human primary and embryonic stem cell-derived pancreatic α and β cells. PLoS ONE 7, 1–12 (2012).
Blum, B. et al. Reversal of β cell de-differentiation by a small molecule inhibitor of the TGFβ pathway. eLife 3, e02809 (2014).
Kavalakatt, S. et al. Urocortin 3 levels are impaired in overweight humans with and without type 2 diabetes and modulated by exercise. Front. Endocrinol. 10, 1–11 (2019).
Huising, M. O., van der Meulen, T., Huang, J. L., Pourhosseinzadeh, M. S. & Noguchi, G. M. The difference δ-cells make in glucose control. Physiology 33, 403–411 (2018).
Thorel, F. et al. Normal glucagon signaling and β-cell function after near-total α-cell ablation in adult mice. Diabetes 60, 2872–2882 (2011).
Pedersen, J. et al. Glucose metabolism is altered after loss of L cells and α-cells but not influenced by loss of K cells. Am. J. Physiol. Endocrinol. Metab. 304, 60–73 (2013).
Shiota, C. et al. α-cells are dispensable in postnatal morphogenesis and maturation of mouse pancreatic islets. Am. J. Physiol. Endocrinol. Metab. 305, 1030–1040 (2013).
Viollet, C. et al. Somatostatin-IRES-cre mice: between knockout and wild-type? Front. Endocrinol. 8, 1–8 (2017).
Arrojo e Drigo, R. et al. Structural basis for δ cell paracrine regulation in pancreatic islets. Nat. Commun. 10, 3700 (2019).
Barker, N. Adult intestinal stem cells: critical drivers of epithelial homeostasis and regeneration. Nat. Rev. Mol. Cell Biol. 15, 19–33 (2014).
Luo, S. X. et al. Regulation of feeding by somatostatin neurons in the tuberal nucleus. Science 361, 76–81 (2018).
Chen, Y. C. et al. Elevated islet prohormone ratios as indicators of insulin dependency in auto-islet transplant recipients. Am. J. Transplant. 22, 1992–2005 (2022).
Ackermann, A. M., Zhang, J., Heller, A., Briker, A. & Kaestner, K. H. High-fidelity glucagon-CreER mouse line generated by CRISPR-Cas9 assisted gene targeting. Mol. Metab. 6, 236–244 (2016).
Brazeau, P. et al. Hypothalamic polypeptide that inhibits the secretion of immunoreactive pituitary growth hormone. Science. 179, 77–79 (1973).
Dubois, M. P. Immunoreactive somatostatin is present in discrete cells of the endocrine pancreas. Proc. Natl Acad. Sci. USA 72, 1340–1343 (1975).
Koerker, D. J. et al. Somatostatin: hypothalamic inhibitor of the endocrine pancreas. Science 184, 482–484 (1974).
De Paoli, M., Zakharia, A. & Werstuck, G. H. The role of estrogen in insulin resistance: a review of clinical and preclinical data. Am. J. Pathol. 191, 1490–1498 (2021).
Hevener, A. L., Zhou, Z., Moore, T. M., Drew, B. G. & Ribas, V. The impact of ERα action on muscle metabolism and insulin sensitivity – Strong enough for a man, made for a woman. Mol. Metab. 15, 20–34 (2018).
Richardson, C. C. et al. Increased perinatal remodelling of the pancreas in somatostatin-deficient mice: potential role of transforming growth factor-β signalling in regulating β cell growth in early life. Horm. Metab. Res. 47, 56–63 (2015).
Luque, R. M. & Kineman, R. D. Gender-dependent role of endogenous somatostatin in regulating growth hormone-axis function in mice. Endocrinology 148, 5998–6006 (2007).
Luque, R. M., Cordoba-Chacon, J. & Pozo-Salas, A. I. Obesity- and gender-dependent role of endogenous somatostatin and cortistatin in the regulation of endocrine and metabolic homeostasis in mice. Nat. Publ. Gr. https://doi.org/10.1038/srep37992 (2016).
Gahete, M. D. et al. Cortistatin is not a somatostatin analogue but stimulates prolactin release and inhibits GH and ACTH in a gender-dependent fashion: potential role of ghrelin. Endocrinology 152, 4800–4812 (2011).
Zaborska, K. E. et al. Liraglutide increases islet Ca2+ oscillation frequency and insulin secretion by activating hyperpolarization-activated cyclic nucleotide-gated channels. Diabetes Obes. Metab. https://doi.org/10.1111/dom.14747 (2022).
Bloom, S. R. et al. Inhibition of gastrin and gastric-acid secretion by growth-hormone release-inhibiting hormone. Lancet 304, 1106–1109 (1974).
Holst, J. J., Orskov, C. & Seier-Poulsen, S. Somatostatin is an essential paracrine link in acid inhibition of gastrin secretion. Digestion 51, 95–102 (1992).
Johansson, C., Wisen, O., Efendic, S. & Uvnäs-Wallensten, K. Effects of somatostatin on gastrointestinal propagation and absorption of oral glucose in man. Digestion 22, 126–137 (1981).
Holst, J. J., Gribble, F., Horowitz, M. & Rayner, C. K. Roles of the gut in glucose homeostasis. Diabetes Care 39, 884–892 (2016).
Karasawa, H. et al. Brain somatostatin receptor 2 mediates the dipsogenic effect of central somatostatin and cortistatin in rats: Role in drinking behavior. Am. J. Physiol. Regul. Integr. Comp. Physiol. 307, R793–R801 (2014).
Stengel, A. & Taché, Y. Central somatostatin signaling and regulation of food intake. Ann. NY Acad. Sci. 1455, 98–104 (2019).
Huang, C. et al. Depleting hypothalamic somatostatinergic neurons recapitulates diabetic phenotypes in mouse brain, bone marrow, adipose and retina. Diabetologia 64, 2575–2588 (2021).
Li, N. et al. Ablation of somatostatin cells leads to impaired pancreatic islet function and neonatal death in rodents article. Cell Death Dis. 9, 682 (2018).
Ivanova, A. et al. In vivo genetic ablation by Cre-mediated expression of diphtheria toxin fragment A. Genesis 43, 129–135 (2005).
Zhang, J., Mckenna, L. B., Bogue, C. W. & Kaestner, K. H. The diabetes gene Hhex maintains δ -cell differentiation and islet function. Genes Dev. https://doi.org/10.1101/gad.235499.113 (2014).
Noguchi, G. M. & Huising, M. O. Integrating the inputs that shape pancreatic islet hormone release. Nat. Metab. 1, 1189–1201 (2019).
Caicedo, A. Paracrine and autocrine interactions in the human islet: more than meets the eye. Semin. Cell Dev. Biol. 24, 11–21 (2013).
Steiner, D. J., Kim, A., Miller, K. & Hara, M. Pancreatic islet plasticity: interspecies comparison of islet architecture and composition. Islets 2, 135–145 (2010).
Walker, J. T., Saunders, Di. C., Brissova, M. & Powers, A. C. The human islet: mini-organ with mega-impact. Endocr. Rev. 42, 605–657 (2021).
Kellard, J. A. et al. Reduced somatostatin signalling leads to hypersecretion of glucagon in mice fed a high-fat diet. Mol. Metab. 40, 101021 (2020).
Rozzo, A., Meneghel-Rozzo, T., Delakorda, S. L., Yang, S. B. & Rupnik, M. Exocytosis of insulin: in vivo maturation of mouse endocrine pancreas. Ann. NY Acad. Sci. 1152, 53–62 (2009).
Taniguchi, H. et al. A resource of Cre driver lines for genetic targeting of GABAergic neurons in cerebral cortex. Neuron 71, 995–1013 (2011).
Srinivas, S. et al. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev. Biol. 1, 1–8 (2001).
Buch, T. et al. A Cre-inducible diphtheria toxin receptor mediates cell lineage ablation after toxin administration. Nat. Methods 2, 419–426 (2005).
Madisen, L. et al. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat. Neurosci. 13, 133–140 (2010).
Wicksteed, B. et al. Conditional gene targeting in mouse pancreatic β-cells: analysis of ectopic cre transgene expression in the brain. Diabetes 59, 3090–3098 (2010).
Madisen, L. et al. Transgenic mice for intersectional targeting of neural sensors and effectors with high specificity and performance. Neuron 85, 942–958 (2015).
Zhu, H. et al. Cre-dependent DREADD (designer receptors exclusively activated by designer drugs) mice. Genesis 54, 439–446 (2016).
Sofroniew, N. et al. napari: a multi-dimensional image viewer for Python. Zenodo https://doi.org/10.5281/ZENODO.7276432 (2022).
Postić, S. et al. High-resolution analysis of the cytosolic Ca2+ events in β cell collectives in situ. Am. J. Physiol. Endocrinol. Metab. 324, E42–E55 (2023).
Szot, G. L., Koudria, P. & Bluestone, J. A. Transplantation of pancreatic islets into the kidney capsule of diabetic mice. J. Vis. Exp. https://doi.org/10.3791/404 (2007).
Huang, J. L., Pourhosseinzadeh, M. S. & Huising, M. O. Paracrine signaling by pancreatic mouse δ cells determines the glycemic set point. Dataset. Figshare https://doi.org/10.6084/m9.figshare.24082434 (2023).
Acknowledgements
This work was supported by the National Institute of Diabetes and Digestive and Kidney Disease (NIDDK-110276; M.O.H.). J.L.H. was supported by a National Institute of General Medical Sciences-funded Pharmacology Training Program (T32 GM-099608). S.L. was supported by the NSF Graduate Research Fellowship (1650042), the UC Davis Training Program in Molecular and Cellular Biology (T32 GM-007377) and the UC Davis NSF Bridge to Doctorate Program (1612490). M.S.P. is supported by the UC Davis Training Program in Molecular and Cellular Biology (T32 GM-007377).
Author information
Authors and Affiliations
Contributions
Conceptualization was the responsibility of J.L.H. and M.O.H. Methodology was the responsibility of J.L.H., S.L. and M.O.H. Software was the responsibility of M.S.P. Validation was carried out by J.L.H., S.L. and M.S.P. Formal analysis was conducted by J.L.H., S.L. and M.S.P. Investigation was the responsibility of J.L.H., M.S.P., S.L., N.K., J.V.G., N.C., P.A., A.T.M. and S.K. Writing of the original draft was carried out by J.L.H. and M.O.H. Review and editing was carried out by J.L.H., M.S.P. and M.O.H. Visualization was the responsibility of J.L.H., M.S.P. and M.O.H. Supervision was carried out by M.O.H.
Corresponding author
Ethics declarations
Competing interests
M.O.H. received grant support from Crinetics to evaluate proprietary somatostatin-related compounds. None of this work is discussed in this paper. All other authors declare no competing interests.
Peer review
Peer review information
Nature Metabolism thanks Marcela Brissova and the other, anonymous, reviewers for their contribution to the peer review of this work. Primary Handling Editor: Christoph Schmitt, in collaboration with the Nature Metabolism team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Body weight in Sst-Cre+/TG and Sst-Cre TG/TG males and females.
a) Body weight measurements of male Sst-Cre+/TG and Sst-CreTG/TG mice from Fig. 1d (n = 6 Sst-Cre+/TG, n = 9 Sst-CreTG/TG). b) Body weight measurements of female Sst-Cre+/TG and Sst-CreTG/TG mice from Fig. 1e (n = 7 Sst-Cre+/TG, n = 7 Sst-CreTG/TG). Significance was determined by two-way ANOVA or mixed modeling for genotype and age followed by Holm-Sidak’s correction for multiple comparisons. Error bars represent SEM.
Extended Data Fig. 2 Glucose measurements in SAL/DT-treated Sst-Cre only, DTR only, and Sst-Cre x lsl-DTR mice.
a) Blood glucose measurements of SAL and DT-treated male Sst-Cre only (n = 3 and n = 4, respectively) and SAL and DT-treated Sst-Cre x lsl-DTR (n = 5 and n = 6, respectively) male mice. Black arrows represent IP administration of DT. Significance was determined by three-way ANOVA for genotype and DT administration, followed by multiple comparisons of every mean to every other mean and Holm-Sidak’s correction. Error bars represent SEM. † represents statistically significant difference (p = 0.029) between DT-treated Sst-Cre only and Sst-Cre x lsl-DTR mice. b) Blood glucose measurements of male DT-treated lsl-DTR only (n = 6) and Sst-Cre x lsl-DTR (n = 5) mice. Black arrows represent IP administration of DT. Significance was determined by two-way ANOVA for ablation followed by Holm-Sidak’s correction for multiple comparisons (A; B, *p = 0.022, *p = 0.022, ****p < 0.0001). Error bars represent SEM.
Extended Data Fig. 3 Body weight and feeding measurements in Sst-Cre x lsl-DTR mice.
a) Body weight measurements of male CTRL and DT mice in Fig. 3a (n = 8 CTRL, n = 6 DT). Black arrows represent IP administration of SAL or DT. b) Body weight measurements of female CTRL and DT mice in Fig. 3b (n = 6 CTRL, n = 6 DT). Black arrows represent IP administration of SAL or DT. c) Feeding measurements in DT-treated Sst-Cre x lsl-DTR (n = 3) and Sst-Cre only (n = 3) mice. Black arrows represent IP administration of DT. d) Glucose tolerance test of male mice 3 months after ablation (n = 4 CTRL, n = 6 DT). e) Glucose tolerance test of female mice 3 months after ablation (n = 3 CTRL, n = 3 DT). Significance was determined by two-way ANOVA for ablation (A, B, C) or ablation and glucose (D, E) followed by Holm-Sidak’s correction for multiple comparisons (A: *p = 0.039; D: *p = 0.045, *p = 0.032) or two-tailed unpaired t-test (baseline-corrected AUC for D, *p = 0.040; baseline-corrected AUC for E, *p = 0.021). Error bars represent SEM.
Extended Data Fig. 4 Glucagon secretion in islets isolated from Sst-Cre x lsl-DTR mice.
a) Static glucagon secretion assay performed on islets isolated from SAL- or DT-treated Sst-Cre x lsl-DTR mice (n = 4 each). Islets were stimulated with 100 nM epinephrine to stimulate glucagon secretion. b) Static glucagon secretion assay performed on the same islets from Fig. 3m (n = 5 replicates per group, 10 islets each, pooled from 3 CTRL or 3 DT mice). c) Static glucagon secretion performed on the same islets from Fig. 3n (n = 5 replicates per group, 10 islets each, pooled from 3 CTRL or 3 DT mice). Significance was determined by two-way ANOVA followed by Holm-Sidak’s correction for multiple comparisons (A, *p = 0.015; B, *p = 0.042; C). Error bars represent SEM.
Extended Data Fig. 5 β and δ cell traces from Pair 1 of non-ablated and ablated mice.
a) Non-ablated and b) ablated islet calcium responses. Each box represents an islet. Each line represents calcium activity of a single β (green) or δ (red) cell. Dashed lines represent points at which glucose levels were changed. Ghrelin was used to functionally distinguish δ cells. 30 mM KCl was used to confirm viability of the cells. c and d) Traces from the same c) non-ablated and d) ablated mice in which islets were perfused with 5 mM glucose between each glucose step to confirm that the responses are not due to time or a delayed response to previous glucose levels.
Extended Data Fig. 6 β and δ cell traces from Pair 2 of non-ablated and ablated mice.
a) Non-ablated and b) ablated islet calcium responses. Each box represents an islet. Each line represents calcium activity of a single β cell. Dashed lines represent points at which glucose levels were changed. 30 mM KCl was used to confirm viability of the cells. c and d) Traces from the same c) non-ablated and d) ablated mice in which islets were perfused with 5 mM glucose between each glucose step to confirm that the responses are not due to time or a delayed response to previous glucose levels. 30 mM KCl was used to confirm viability of the cells.
Extended Data Fig. 7 β and δ cell traces from Pair 3 of non-ablated and ablated mice.
a) Non-ablated and b) ablated islet calcium responses. Each box represents an islet. Each line represents calcium activity of a single β cell. Dashed lines represent points at which glucose levels were changed. 30 mM KCl was used to confirm viability of the cells.
Extended Data Fig. 8 Simultaneous collection of calcium dynamics in islets and insulin secretion.
The top graph shows insulin secretion over time from control (blue) and δ cell-ablated (green) islets as glucose is raised from 4 mM to 11 mM glucose. Below are the respective calcium traces of whole islets (n = 60 each) from the control (middle) and δ cell-ablated (bottom) mouse imaged at 4x. Each row represents the response of an individual islet.
Supplementary information
Supplementary Video 1
Supplementary video accompanying Fig. 7.
Source data
Source Data Fig. 1
Statistical Source Data for Fig. 1.
Source Data Fig. 2
Statistical Source Data for Fig. 2.
Source Data Fig. 3
Statistical Source Data for Fig. 3.
Source Data Fig. 4
Statistical Source Data for Fig. 4.
Source Data Fig. 5
Statistical Source Data for Fig. 5.
Source Data Fig. 6
Statistical Source Data for Fig. 6.
Source Data Fig. 7
Statistical Source Data for Fig. 7.
Source Data Extended Data Fig. 1
Statistical Source Data for Extended Data Fig. 1.
Source Data Extended Data Fig. 2
Statistical Source Data for Extended Data Fig. 2.
Source Data Extended Data Fig. 3
Statistical Source Data for Extended Data Fig. 3.
Source Data Extended Data Fig. 4
Statistical Source Data for Extended Data Fig. 4.
Source Data Extended Data Fig. 5
Statistical Source Data for Extended Data Fig. 5.
Source Data Extended Data Fig. 6
Statistical Source Data for Extended Data Fig. 6.
Source Data Extended Data Fig. 7
Statistical Source Data for Extended Data Fig. 7.
Source Data Extended Data Fig. 8
Statistical Source Data for Extended Data Fig. 8.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Huang, J.L., Pourhosseinzadeh, M.S., Lee, S. et al. Paracrine signalling by pancreatic δ cells determines the glycaemic set point in mice. Nat Metab 6, 61–77 (2024). https://doi.org/10.1038/s42255-023-00944-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s42255-023-00944-2
This article is cited by
-
Pancreatic δ-cells influence the glycaemic set point
Nature Reviews Endocrinology (2024)