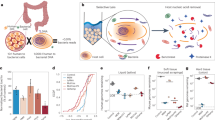
Abstract
The intestinal epithelium is replaced every few days1. Enterocytes are shed into the gut lumen predominantly from the tips of villi2,3 and have been believed to rapidly die upon their dissociation from the tissue4,5. However, technical limitations prohibited studying the cellular states and fates of shed intestinal cells. Here we show that shed epithelial cells remain viable and upregulate distinct anti-microbial programmes upon shedding, using bulk and single-cell RNA sequencing of male mouse intestinal faecal washes. We further identify abundant shedding of immune cells, which is elevated in mice with dextran sulfate sodium-induced colitis. We find that faecal host transcriptomics reflect changes in the intestinal tissue following perturbations. Our study suggests potential functions of shed cells in the intestinal lumen and demonstrates that host cell transcriptomes in intestinal washes can be used to probe tissue states.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
All data generated in this study are available at the Zenodo repository at https://doi.org/10.5281/zenodo.7137319. GRCm38 genome (release 84; Ensembl) was used in this study (http://www.ensembl.org/Mus_musculus/Info/Index).
Code availability
Codes for unique analysis done in this study were uploaded to Zenodo (https://doi.org/10.5281/zenodo.7137319). All other common codes used in this study will be available upon request.
References
Sender, R. & Milo, R. The distribution of cellular turnover in the human body. Nat. Med. 27, 45–48 (2021).
Radtke, F. & Clevers, H. Self-renewal and cancer of the gut: two sides of a coin. Science 307, 1904–1909 (2005).
Eastwood, G. L. Gastrointestinal epithelial renewal. Gastroenterology 72, 962–975 (1977).
Gudipaty, S. A. & Rosenblatt, J. Epithelial cell extrusion: pathways and pathologies. Semin. Cell Dev. Biol. 67, 132–140 (2017).
Patankar, J. V. & Becker, C. Cell death in the gut epithelium and implications for chronic inflammation. Nat. Rev. Gastroenterol. Hepatol. 17, 543–556 (2020).
Flier, L. G. & Clevers, H. Stem cells, self-renewal, and differentiation in the intestinal epithelium. Annu. Rev. Physiol. 71, 241–260 (2009).
Krndija, D. et al. Active cell migration is critical for steady-state epithelial turnover in the gut. Science 365, 705–710 (2019).
Parker, A. et al. Cell proliferation within small intestinal crypts is the principal driving force for cell migration on villi. FASEB J. 31, 636–649 (2017).
Madara, J. L. Maintenance of the macromolecular barrier at cell extrusion sites in intestinal epithelium: physiological rearrangement of tight junctions. J. Membr. Biol. 116, 177–184 (1990).
Guan, Y. et al. Redistribution of the tight junction protein ZO-1 during physiological shedding of mouse intestinal epithelial cells. Am. J. Physiol. Cell Physiol. 300, C1404–C1414 (2011).
Beauséjour, M., Boutin, A. & Vachon, P. H. in Anoikis: How the Extracellular Matrix Regulates Life-or-Death Decisions (ed. Frisch, S. M.) 95–126 (Springer, 2021).
Eisenhoffer, G. T. et al. Crowding induces live cell extrusion to maintain homeostatic cell numbers in epithelia. Nature 484, 546–549 (2012).
Chapkin, R. S. et al. Noninvasive stool-based detection of infant gastrointestinal development using gene expression profiles from exfoliated epithelial cells. Am. J. Physiol. Gastrointest. Liver Physiol. 298, G582–G589 (2010).
Schlaberg, R. et al. Fecal host transcriptomics for non-invasive human mucosal immune profiling: proof of concept in Clostridium difficile infection. Pathog. Immun. 3, 164–180 (2018).
Whitfield-Cargile, C. M. et al. The non-invasive exfoliated transcriptome (exfoliome) reflects the tissue-level transcriptome in a mouse model of NSAID enteropathy. Sci. Rep. 7, 14687 (2017).
Ungar, B. et al. Host transcriptome signatures in human faecal-washes predict histological remission in patients with IBD. Gut https://doi.org/10.1136/gutjnl-2021-325516 (2022).
Casteleyn, C., Rekecki, A., Van Der Aa, A., Simoens, P. & Van Den Broeck, W. Surface area assessment of the murine intestinal tract as a prerequisite for oral dose translation from mouse to man. Lab. Anim. 44, 176–183 (2010).
Bagnoli, J. W. et al. Sensitive and powerful single-cell RNA sequencing using mcSCRB-seq. Nat. Commun. 9, 2937 (2018).
Haber, A. L. et al. A single-cell survey of the small intestinal epithelium. Nature 551, 333–339 (2017).
Kashyap, P. C. et al. Complex interactions among diet, gastrointestinal transit, and gut microbiota in humanized mice. Gastroenterology 144, 967–977 (2013).
Padmanabhan, P., Grosse, J., Asad, A. B. M. A., Radda, G. K. & Golay, X. Gastrointestinal transit measurements in mice with 99mTc-DTPA-labeled activated charcoal using NanoSPECT-CT. EJNMMI Res. 3, 60 (2013).
Woting, A. & Blaut, M. Small intestinal permeability and gut-transit time determined with low and high molecular weight fluorescein isothiocyanate-dextrans in C3H mice. Nutrients 10, 685 (2018).
Subramanian, A. et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl Acad. Sci. USA 102, 15545–15550 (2005).
Moor, A. E. et al. Spatial reconstruction of single enterocytes uncovers broad zonation along the intestinal villus axis. Cell https://doi.org/10.1016/j.cell.2018.08.063 (2018).
Buchauer, L. & Itzkovitz, S. cellanneal: a user-friendly deconvolution software for omics data. Preprint at https://arxiv.org/abs/2110.08209 (2021).
Ngo, P. A., Neurath, M. F. & López-Posadas, R. Impact of epithelial cell shedding on intestinal homeostasis. Int. J. Mol. Sci. 23, 4160 (2022).
Keren-Shaul, H. et al. MARS-seq2.0: an experimental and analytical pipeline for indexed sorting combined with single-cell RNA sequencing. Nat. Protoc. 14, 1841–1862 (2019).
Kumar, S. et al. Intra-tumoral metabolic zonation and resultant phenotypic diversification are dictated by blood vessel proximity. Cell Metab. 30, 201–211.e6 (2019).
Bahar Halpern, K. et al. Lgr5+ telocytes are a signaling source at the intestinal villus tip. Nat. Commun. 11, 1936 (2020).
Czarnewski, P. et al. Conserved transcriptomic profile between mouse and human colitis allows unsupervised patient stratification. Nat. Commun 10, 2892 (2019).
Khaki-Khatibi, F. et al. Calprotectin in inflammatory bowel disease. Clin. Chim. Acta 510, 556–565 (2020).
Reikvam, D. H. et al. Depletion of murine intestinal microbiota: effects on gut mucosa and epithelial gene expression. PLoS ONE 6, e17996 (2011).
Hooper, L. V., Stappenbeck, T. S., Hong, C. V. & Gordon, J. I. Angiogenins: a new class of microbicidal proteins involved in innate immunity. Nat. Immun. https://doi.org/10.1038/ni888 (2003).
Propheter, D. C., Chara, A. L., Harris, T. A., Ruhn, K. A. & Hooper, L. V. Resistin-like molecule β is a bactericidal protein that promotes spatial segregation of the microbiota and the colonic epithelium. Proc. Natl Acad. Sci. USA 114, 11027–11033 (2017).
Cordes, F., Foell, D., Ding, J. N., Varga, G. & Bettenworth, D. Differential regulation of JAK/STAT-signaling in patients with ulcerative colitis and Crohn’s disease. World J. Gastroenterol. 26, 4055–4075 (2020).
Salas, A. et al. JAK–STAT pathway targeting for the treatment of inflammatory bowel disease. Nat. Rev. Gastroenterol. Hepatol. 17, 323–337 (2020).
Loetscher, Y. et al. Salmonella transiently reside in luminal neutrophils in the inflamed gut. PLoS ONE 7, e34812 (2012).
Brazil, J. C. & Parkos, C. A. Pathobiology of neutrophil–epithelial interactions. Immunol. Rev. 273, 94–111 (2016).
Lawrence, A.-L. E. et al. Human neutrophil IL1β directs intestinal epithelial cell extrusion during Salmonella infection. PLoS Pathog. 18, e1010855 (2022).
Niess, J. H. et al. CX3CR1-mediated dendritic cell access to the intestinal lumen and bacterial clearance. Science 307, 254–258 (2005).
Dan, S. et al. Distal fecal wash host transcriptomics identifies inflammation throughout the colon and terminal ileum. Cell. Mol. Gastroenterol. Hepatol. https://doi.org/10.1016/j.jcmgh.2023.02.002 (2023).
Vorperian, S. K., Moufarrej, M. N., Tabula Sapiens Consortium & Quake, S. R. Cell types of origin of the cell-free transcriptome. Nat. Biotechnol. 40, 855–861 (2022).
Rakoff-Nahoum, S., Paglino, J., Eslami-Varzaneh, F., Edberg, S. & Medzhitov, R. Recognition of commensal microflora by Toll-like receptors is required for intestinal homeostasis. Cell 118, 229–241 (2004).
Ichinohe, T. et al. Microbiota regulates immune defense against respiratory tract influenza A virus infection. Proc. Natl Acad. Sci. USA 108, 5354–5359 (2011).
Fagarasan, S. et al. Critical roles of activation-induced cytidine deaminase in the homeostasis of gut flora. Science 298, 1424–1427 (2002).
Parekh, S., Ziegenhain, C., Vieth, B., Enard, W. & Hellmann, I. zUMIs—a fast and flexible pipeline to process RNA sequencing data with UMIs. GigaScience 7, giy059 (2018).
Biton, M. et al. T helper cell cytokines modulate intestinal stem cell renewal and differentiation. Cell 175, 1307–1320.e22 (2018).
Širvinskas, D. et al. Single-cell atlas of the aging mouse colon. iScience 25, 104202 (2022).
Schwanhäusser, B. et al. Global quantification of mammalian gene expression control. Nature 473, 337–342 (2011).
Butler, A., Hoffman, P., Smibert, P., Papalexi, E. & Satija, R. Integrating single-cell transcriptomic data across different conditions, technologies, and species. Nat. Biotechnol. 36, 411–420 (2018).
Devroye, L. in Handbooks in Operations Research and Management Science Vol. 13 (eds Henderson, S. G. & Nelson, B. L.) Ch. 4 (Elsevier, 2006).
Lyubimova, A. et al. Single-molecule mRNA detection and counting in mammalian tissue. Nat. Protoc. 8, 1743–1758 (2013).
Acknowledgements
We thank H. Shapiro and E. Elinav from the department of Systems Immunology, Weizmann Institute of Science, for help with experimental procedures of mice perturbations. We thank Y. Levin and A. Savidor from the INCPM proteomics unit, Weizmann Institute of Science, for help with the proteomics experiment. Y.K.K is supported by the JSMF Postdoctoral Fellowship in Understanding Dynamic and Multi-scale Systems (Award #https://doi.org/10.37717/2020-1428). S.I. is supported by the Moross Integrated Cancer Center, the Yad Abraham Research Center for Cancer Diagnostics and Therapy, the Helen and Martin Kimmel Institute for Stem Cell Research, the Minerva Stiftung grant, the Israel Science Foundation grants no. 908/21 and no. 3663/21, the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation programme grant no. 768956, a Weizmann-Sheba joint research grant and a research grant from the Ministry of Innovation, Science and Technology, Israel.
Author information
Authors and Affiliations
Contributions
S.I. and K.B.H. conceived the study. K.B.H. performed all bulk, scRNA-seq, smFISH, mice perturbation experiments, cell viability and proteomics assays. A.B. and Z.S. performed intra-vital imaging. K.B.H. and Y.H. performed immunofluorescence experiments. K.B.H. and O.Y. performed Hoechst in vivo assay. K.B.H., A.E., Y.K.K. and S.I. performed all data analysis. S.I. and K.B.H. wrote the paper. All authors discussed the results and commented on the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Metabolism thanks Jose Ordovas-Montanes and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Yanina-Yasmin Pesch, in collaboration with the Nature Metabolism team. Research briefing related to this: CSFV23069202A.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Differential gene expression between faecal washed to tissues.
a. Examples of genes that are only expressed in the small intestine but not in the colon tissue, yet are still observed in colonic faecal washes. These genes demonstrate a slow decline in gene expression of shed intestinal cells. Y axis is log10(Normalized RNA Expression), n = 10 mice. Medians are marked with white dot. Means are marked with x and connected with a dashed line. b, c Volcano plot showing differentially expressed genes between faecal washes and tissues in small (b) or large (c) intestine, each dot is a gene, x-axis is the log2- ratio of expression between faecal wash and tissue, y axis is –log10 (q value), where p value is computed using 2-sided Wilcoxon rank-sum tests and q- values computed using Benjamini- Hochberg FDR correction. Genes with corresponding q-values below 0.05 and fold change greater than 1.5 are marked in red or blue for faecal wash or tissue elevated genes respectively. Names of representative up-regulated genes are shown in red or blue for faecal wash or tissue respectively.
Extended Data Fig. 2 Protein and mRNA levels in small intestine faecal washes are correlated.
Correlation between protein levels and faecal wash mRNA levels of small intestine samples. Y axis shows the log10 relative protein expression (4 mice), X axis shows log10 relative mRNA expression (27 samples from 10 mice). Shown are names of cell type marker genes extracted from Fig. 3b in addition to the pancreatic genes AMY2A, PRSS2, CELA1 and CELA3B. Spearman correlation 2-sided test was used.
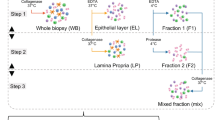
Extended Data Fig. 3 Sorting strategy for isolation of mouse intestinal cells from faecal washes.
a. FSC-A and SSC-A are used to select cells based on size. b. PI staining is used to gate out dead cells. C. Hoechst fluorescense is used to select eukaryotic cells while avoiding low DNA amount of bacteria cells. Numbers in A-C represent the percent of gated cells.
Extended Data Fig. 4 Faecal wash flushing preserves tissue cells and lead to minor cell shedding.
a. Schematic outline of the procedure. Created with BioRender.com b. Tissue cells after in-vivo Hoechst staining showing robust labeling of tissue cells. Scale bar 100 μm. image is representative of 3 independent experiments. c-d. FACS analysis of cells depicting gating strategy to exclude debris, dead cells, and fractionation according to the level of Hoechst 33342 uptake (dubbed as Hoechst positive [H+] and Hoechst negative [H−]) in tissue dissociated cells and in two faecal wash samples. e. Quantification of fraction of Hoechst positive and Hoechst negative cells in the samples indicated in d. f. Preservation of mucus and tissue morphology by Alcian blue and H&E staining respectively. Scale bar 100 μm. images are representative of 2 independent experiments.
Extended Data Fig. 5 Validation of cell viability of shed cells.
a. Fluorescence analysis showing representative faecal wash cells stained for apoptotic markers. White is CytoCalcein Violet 450, representative of live cells, magenta is Apopxin green Indicator, representative of apoptotic cells. b. Quantification of FACS analysis showing the percentage of live cells as measured by CytoCalcein Violet 450 levels and the percentage of apoptotic cells as measured by Apopxin Green in faecal wash samples from three mice. c. Mitochondrial health assessment demonstrate the existence of live host shed cells. Fluorescence analysis showing faecal wash host cells (white arrows) stained with marker of mitochondrial membrane potential (white, MitoHealth) and with non-viability stain (magenta Image-iT DEAD Green). Cells are also stained with Hoechst to mark the DNA (blue). White arrow-heads show auto-fluorescence of bacterial colonies. images are representative of 3 independent experiments.
Extended Data Fig. 6 Wide field view of a representative smFISH image.
Cells are stained for the epithelial markers Reg3g and Ada and the monocyte/macrophage marker Lyz1. Cells are Dapi positive and marked by white dashed line. In each panel, cells positive for the respective genes are outlined in red. Scale bar, 20 μm. Images are representative of 5 independent experiments.
Extended Data Fig. 7 Protein polarity of shed cells.
a-b. Intact mouse Jejunum tissue stained with an antibody against CD73 (green) and Dapi (grey), show apical polarity of CD73 at the villi tip. Other CD73 positive cells are monocytes/macrophages. Scale bar in a and b are 25μm and 10μm respectively. c. Dissociated mouse Jejunum tissue stained with anti EpCAM (magenta), anti CD73 (green) and Hoechst (grey). Both EpCAM and CD73 show cell polarity. d. Faecal wash cells stained with anti EpCAM (magenta), anti CD73 (green) and Hoechst (grey). Both EpCAM and CD73 show loss of cell polarity. Images are representative of at least 3 independent experiments.
Extended Data Fig. 8 Differential gene expression of faecal washes between control and mice pertubation.
Volcano plot showing differentially expressed genes between large intestine faecal wash in control (CT) and DSS (a) or ABX and CT (b), each dot is a gene, x-axis is the log2- ratio of expression between faecal wash and tissue, y axis is –log10 (q value), where p value is computed using 2-sided Wilcoxon rank-sum tests and q- values computed using Benjamini- Hochberg FDR correction. Genes with corresponding q-values below 0.05 and fold change garter than 1.5 are marked in red or blue for treatment or control elevated genes respectively. Names of representative up-regulated genes are shown in red or blue for treatment or control respectively.
Extended Data Fig. 9 Faecal washes mirror perturbed tissue states.
a. Scatter plot of log2 gene expression ratio between DSS and control mice in colon tissue (x-axis) or colon faecal washes (y-axis). Each dot is a gene. Red dots are inflammatory-related genes previously shown to be upregulated in faecal washes collected during lower endoscopies of ulcerative colitis patients16. Red dots mark the genes with AUC (area under the curve) value above 0.9 in Ungar et al.16 n = 16 biologically independent samples. Shown are the gene names of selected upregulated inflammatory-related genes. Spearman correlation 2-sided test was used correlation = 0.29, p-value = 3.1e-308. b. Violin plot showing the distribution of genes in a. Medians are marked with white circles. Two-sided kruskalwallis test was used.
Supplementary information
Supplementary Table 1
Control bulk RNA raw data.
Supplementary Table 2
Differential gene expression between faecal wash and tissue of small or large intestine.
Supplementary Table 3
Control mice SI proteomics of faecal washes.
Supplementary Table 4
Computational deconvolution.
Supplementary Table 5
GSEA results colon faecal wash of mice perturbation.
Supplementary Table 6
smFISH probes.
Supplementary Video 1
Active monocyte/macrophage shedding.
Source data
Source Data Fig. 1
Numeric source data.
Source Data Fig. 2
Numeric source data.
Source Data Fig. 3
Numeric source data.
Source Data Fig. 4
Numeric source data.
Source Data Extended Data Fig. 1
Numeric source data.
Source Data Extended Data Fig. 2
Numeric source data.
Source Data Extended Data Fig. 3
Numeric source data.
Source Data Extended Data Fig. 4
Numeric source data.
Source Data Extended Data Fig. 8
Numeric source data.
Source Data Extended Data Fig. 9
Numeric source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bahar Halpern, K., Korem Kohanim, Y., Biram, A. et al. The cellular states and fates of shed intestinal cells. Nat Metab 5, 1858–1869 (2023). https://doi.org/10.1038/s42255-023-00905-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s42255-023-00905-9