Abstract
Neuronal activity creates an intense energy demand that must be met by rapid metabolic responses. To investigate metabolic adaptations in the neuron-enriched dentate granule cell (DGC) layer within its native tissue environment, we employed murine acute hippocampal brain slices, coupled with fast metabolite preservation and followed by mass spectrometry (MS) imaging, to generate spatially resolved metabolomics and isotope-tracing data. Here we show that membrane depolarization induces broad metabolic changes, including increased glycolytic activity in DGCs. Increased glucose metabolism in response to stimulation is accompanied by mobilization of endogenous inosine into pentose phosphates via the action of purine nucleotide phosphorylase (PNP). The PNP reaction is an integral part of the neuronal response to stimulation, because inhibition of PNP leaves DGCs energetically impaired during recovery from strong activation. Performing MS imaging on brain slices bridges the gap between live-cell physiology and the deep chemical analysis enabled by MS.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
Key MSI data are deposited at the NIH Common Fund’s National Metabolomics Data Repository Metabolomics Workbench (study ID ST002699; https://doi.org/10.21228/M8Q716)72. Source data are provided with this paper.
Code availability
The custom MATLAB code for thermal monitoring and control, using the hardware described, is available at github.com/gyellen/ThermoD.
References
Shin, T. H. et al. Metabolome changes in cerebral ischemia. Cells 9, E1630 (2020).
Puig, B., Brenna, S. & Magnus, T. Molecular communication of a dying neuron in stroke. Int. J. Mol. Sci. 19, E2834 (2018).
Taoufik, E. & Probert, L. Ischemic neuronal damage. Curr. Pharmacol. Des. 14, 3565–3573 (2008).
Stopka, S. A. et al. Spatially resolved characterization of tissue metabolic compartments in fasted and high-fat diet livers. PLoS ONE 17, e0261803 (2022).
Cohen, L. H. & Gusev, A. I. Small molecule analysis by MALDI mass spectrometry. Anal. Bioanal. Chem. 373, 571–586 (2002).
Tsai, Y.-H., Garrett, T. J., Carter, C. S. & Yost, R. A. Metabolomic analysis of oxidative and glycolytic skeletal muscles by Matrix-Assisted Laser Desorption/Ionization Mass Spectrometric Imaging (MALDI MSI). J. Am. Soc. Mass Spectrom. 26, 915–923 (2015).
Sugiura, Y. et al. Visualization of in vivo metabolic flows reveals accelerated utilization of glucose and lactate in penumbra of ischemic heart. Sci. Rep. 6, 32361 (2016).
Kleinridders, A. et al. Regional differences in brain glucose metabolism determined by imaging mass spectrometry. Mol. Metab. 12, 113–121 (2018).
Wang, Z. et al. Spatial-resolved metabolomics reveals tissue-specific metabolic reprogramming in diabetic nephropathy by using mass spectrometry imaging. Acta Pharmacol. Sin. B 11, 3665–3677 (2021).
Wang, L. et al. Spatially resolved isotope tracing reveals tissue metabolic activity. Nat. Methods 19, 223–230 (2022).
Wang, G. et al. Analyzing cell-type-specific dynamics of metabolism in kidney repair. Nat. Metab. 4, 1109–1118 (2022).
Dienel, G. A. Stop the rot. Enzyme inactivation at brain harvest prevents artifacts: a guide for preservation of the in vivo concentrations of brain constituents. J. Neurochem. 158, 1007–1031 (2021).
Lu, W. et al. Metabolite measurement: pitfalls to avoid and practices to follow. Annu. Rev. Biochem. 86, 277–304 (2017).
Blatherwick, E. Q., Svensson, C. I., Frenguelli, B. G. & Scrivens, J. H. Localisation of adenine nucleotides in heat-stabilised mouse brains using ion mobility enabled MALDI imaging. Int. J. Mass Spectrom. 345–347, 19–27 (2013).
Fox, P. T., Raichle, M. E., Mintun, M. A. & Dence, C. Nonoxidative glucose consumption during focal physiologic neural activity. Science 241, 462–464 (1988).
Madsen, P. L., Cruz, N. F., Sokoloff, L. & Dienel, G. A. Cerebral oxygen/glucose ratio is low during sensory stimulation and rises above normal during recovery: excess glucose consumption during stimulation is not accounted for by lactate efflux from or accumulation in brain tissue. J. Cereb. Blood Flow Metab. 19, 393–400 (1999).
Bak, L. K. et al. Neuronal glucose but not lactate utilization is positively correlated with NMDA-induced neurotransmission and fluctuations in cytosolic Ca2+ levels. J. Neurochem. 109, 87–93 (2009).
Hyder, F., Fulbright, R. K., Shulman, R. G. & Rothman, D. L. Glutamatergic function in the resting awake human brain is supported by uniformly high oxidative energy. J. Cereb. Blood Flow Metab. 33, 339–347 (2013).
Díaz-García, C. M. et al. Neuronal stimulation triggers neuronal glycolysis and not lactate uptake. Cell Metab. 26, 361–374 (2017).
Ghergurovich, J. M. et al. A small molecule G6PD inhibitor reveals immune dependence on pentose phosphate pathway. Nat. Chem. Biol. 16, 731–739 (2020).
Tozzi, M. G., Camici, M., Mascia, L., Sgarrella, F. & Ipata, P. L. Pentose phosphates in nucleoside interconversion and catabolism. FEBS J. 273, 1089–1101 (2006).
Ipata, P. L., Camici, M., Micheli, V. & Tozz, M. G. Metabolic network of nucleosides in the brain. Curr. Top. Med. Chem. 11, 909–922 (2011).
Ipata, P. L. & Balestri, F. The functional logic of cytosolic 5′-nucleotidases. Curr. Med. Chem. 20, 4205–4216 (2013).
Zhang, Y. et al. An RNA-sequencing transcriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex. J. Neurosci. 34, 11929–11947 (2014).
Pastor-Anglada, M. & Pérez-Torras, S. Emerging roles of nucleoside transporters. Front. Pharmacol. 9, 606 (2018).
Lin, W. & Buolamwini, J. K. Synthesis, flow cytometric evaluation, and identification of highly potent dipyridamole analogues as equilibrative nucleoside transporter 1 inhibitors. J. Med. Chem. 50, 3906–3920 (2007).
Wu, Z. et al. Neuronal activity-induced, equilibrative nucleoside transporter-dependent, somatodendritic adenosine release revealed by a GRAB sensor. Proc. Natl. Acad. Sci. USA 120, e2212387120 (2023).
Admyre, T. et al. Inhibition of AMP deaminase activity does not improve glucose control in rodent models of insulin resistance or diabetes. Chem. Biol. 21, 1486–1496 (2014).
Li, G., Nakagome, I., Hirono, S., Itoh, T. & Fujiwara, R. Inhibition of adenosine deaminase (ADA)-mediated metabolism of cordycepin by natural substances. Pharmacol. Res. Perspect. 3, e00121 (2015).
Mohlin, C., Säve, S., Nilsson, M. & Persson, K. Studies of the extracellular ATP-adenosine pathway in human urinary tract epithelial cells. Pharmacology 84, 196–202 (2009).
Martínez-François, J. R. et al. BAD and KATP channels regulate neuron excitability and epileptiform activity. eLife 7, e32721 (2018).
Barry, J. A., Groseclose, M. R. & Castellino, S. Quantification and assessment of detection capability in imaging mass spectrometry using a revised mimetic tissue model. Bioanalysis 11, 1099–1116 (2019).
Källback, P. et al. Cross-validated matrix-assisted laser desorption/ionization mass spectrometry imaging quantitation protocol for a pharmaceutical drug and its drug-target effects in the brain using time-of-flight and Fourier transform ion cyclotron resonance analyzers. Anal. Chem. 92, 14676–14684 (2020).
Woods, A. S. et al. Lipid/peptide/nucleotide separation with MALDI-ion mobility-TOF MS. Anal. Chem. 76, 2187–2195 (2004).
Fernandez-Lima, F., Kaplan, D. A., Suetering, J. & Park, M. A. Gas-phase separation using a trapped ion mobility spectrometer. Int. J. Ion Mobil. Spectrom. 14, 93–98 (2011).
Tian, H. et al. Multi-modal mass spectrometry imaging reveals single-cell metabolic states in mammalian liver. Preprint at bioRxiv https://doi.org/10.1101/2022.09.26.508878 (2022).
Harriott, A. M., Takizawa, T., Chung, D. Y. & Chen, S.-P. Spreading depression as a preclinical model of migraine. J. Headache Pain. 20, 45 (2019).
Charles, A. C. & Baca, S. M. Cortical spreading depression and migraine. Nat. Rev. Neurol. 9, 637–644 (2013).
Aiba, I. & Noebels, J. L. Spreading depolarization in the brainstem mediates sudden cardiorespiratory arrest in mouse SUDEP models. Sci. Transl. Med. 7, 282ra46 (2015).
Gupta, S. et al. KL1 domain of longevity factor klotho mimics the metabolome of cognitive stimulation and enhances cognition in young and aging mice. J. Neurosci. 42, 4016–4025 (2022).
Lewin, E. & Bleck, V. Electroshock seizures in mice: effect on brain adenosine and its metabolites. Epilepsia 22, 577–581 (1981).
Barsotti, C. & Ipata, P. L. Metabolic regulation of ATP breakdown and of adenosine production in rat brain extracts. Int. J. Biochem. Cell Biol. 36, 2214–2225 (2004).
Sahlin, K. & Broberg, S. Adenine nucleotide depletion in human muscle during exercise: causality and significance of AMP deamination. Int. J. Sports Med. 11, S62–S67 (1990).
Idström, J. P., Soussi, B., Elander, A. & Bylund-Fellenius, A. C. Purine metabolism after in vivo ischemia and reperfusion in rat skeletal muscle. Am. J. Physiol. 258, H1668–H1673 (1990).
Gerlach, E., Deuticke, B. & Dreisbach, R. H. Der Nucleotid-Abbau im Herzmuskel bei Sauerstoffmangel und seine mögliche Bedeutung für die Coronardurchblutung. Naturwissenschaften 50, 228–229 (1963).
Carlson, J. D. & Fischer, A. G. Thyroid purine nucleoside phosphorylase. II. Kinetic model by alternate substrate and inhibition studies. Biochim. Biophys. Acta 566, 259–265 (1979).
Erion, M. D., Stoeckler, J. D., Guida, W. C., Walter, R. L. & Ealick, S. E. Purine nucleoside phosphorylase. 2. Catalytic mechanism. Biochemistry 36, 11735–11748 (1997).
Barsotti, C., Pesi, R., Felice, F. & Ipata, P. L. The purine nucleoside cycle in cell-free extracts of rat brain: evidence for the occurrence of an inosine and a guanosine cycle with distinct metabolic roles. Cell. Mol. Life Sci. 60, 786–793 (2003).
Abt, E. R. et al. Purine nucleoside phosphorylase enables dual metabolic checkpoints that prevent T cell immunodeficiency and TLR7-associated autoimmunity. J. Clin. Invest. 132, e160852 (2022).
Wang, T. et al. Inosine is an alternative carbon source for CD8+-T-cell function under glucose restriction. Nat. Metab. 2, 635–647 (2020).
Markert, M. L. Purine nucleoside phosphorylase deficiency. Immunodefic. Rev. 3, 45–81 (1991).
Alangari, A., Al-Harbi, A., Al-Ghonaium, A., Santisteban, I. & Hershfield, M. Purine nucleoside phosphorylase deficiency in two unrelated Saudi patients. Ann. Saudi Med. 29, 309–312 (2009).
Toro, A. & Grunebaum, E. TAT-mediated intracellular delivery of purine nucleoside phosphorylase corrects its deficiency in mice. J. Clin. Invest. 116, 2717–2726 (2006).
Nascimento, F. P., Macedo-Júnior, S. J., Lapa-Costa, F. R., Cezar-Dos-Santos, F. & Santos, A. R. S. Inosine as a tool to understand and treat central nervous system disorders: a neglected actor? Front. Neurosci. 15, 703783 (2021).
Liu, F. et al. Secondary degeneration reduced by inosine after spinal cord injury in rats. Spinal Cord. 44, 421–426 (2006).
Chen, P., Goldberg, D. E., Kolb, B., Lanser, M. & Benowitz, L. I. Inosine induces axonal rewiring and improves behavioral outcome after stroke. Proc. Natl Acad. Sci. USA 99, 9031–9036 (2002).
Soares Dos Santos Cardoso, F., Blanco Martinez, A. M. & Martins de Almeida, F. Inosine: a novel treatment for sciatic nerve injury. Neural Regen. Res. 16, 127–128 (2021).
Chen, T.-W. et al. Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature 499, 295–300 (2013).
Díaz-García, C. M., Nathwani, N., Martínez-François, J. R. & Yellen, G. Delivery of AAV for expression of fluorescent biosensors in juvenile mouse hippocampus. Bio Protoc. 11, e4259 (2021).
Miller, A. et al. Exploring metabolic configurations of single cells within complex tissue microenvironments. Cell Metab. 26, 788–800 (2017).
Van Noorden, C. J. & Frederiks, W. M. Enzyme Histochemistry: A Laboratory Manual of Current Methods Vol. 26 (Oxford Univ. Press, 1992).
Wishart, D. S. et al. HMDB 5.0: the Human Metabolome Database for 2022. Nucleic Acids Res. 50, D622–D631 (2022).
Bittremieux, W. et al. Comparison of cosine, modified cosine, and neutral loss based spectrum alignment for discovery of structurally related molecules. J. Am. Soc. Mass Spectrom. 33, 1733–1744 (2022).
Ross, D. H., Cho, J. H. & Xu, L. Breaking down structural diversity for comprehensive prediction of ion-neutral collision cross sections. Anal. Chem. 92, 4548–4557 (2020).
Gabelica, V. et al. Recommendations for reporting ion mobility mass spectrometry measurements. Mass Spectrom. Rev. 38, 291–320 (2019).
Schwaiger-Haber, M. et al. Using mass spectrometry imaging to map fluxes quantitatively in the tumor ecosystem. Nat. Commun. 14, 2876 (2023).
Andersen, J. V. et al. Astrocyte metabolism of the medium-chain fatty acids octanoic acid and decanoic acid promotes GABA synthesis in neurons via elevated glutamine supply. Mol. Brain 14, 132 (2021).
Millard, P. et al. IsoCor: isotope correction for high-resolution MS labeling experiments. Bioinformatics 35, 4484–4487 (2019).
Mackay, G. M., Zheng, L., van den Broek, N. J. F. & Gottlieb, E. Analysis of cell metabolism using LC-MS and isotope tracers. Methods Enzymol. 561, 171–196 (2015).
Packer, M. R. et al. Raf promotes dimerization of the Ras G-domain with increased allosteric connections. Proc. Natl Acad. Sci. USA 118, e2015648118 (2021).
Harrison, J. A., Kelso, C., Pukala, T. L. & Beck, J. L. Conditions for analysis of native protein structures using uniform field drift tube ion mobility mass spectrometry and characterization of stable calibrants for TWIM-MS. J. Am. Soc. Mass Spectrom. 30, 256–267 (2019).
Sud, M. et al. Metabolomics Workbench: an international repository for metabolomics data and metadata, metabolite standards, protocols, tutorials and training, and analysis tools. Nucleic Acids Res. 44, D463–D470 (2016).
Acknowledgements
We thank members of the Yellen laboratory for comments on the manuscript. We also thank the Neurobiology Machine Shop (supported by NIH grant no. P30 EY012196) for construction of the custom chamber assembly, and N. Nathwani for her expert assistance with mouse surgery. We thank LioChem for sharing samples of the thermal interface material. Figure 1c was created with BioRender.com. Instrumentation and MS imaging in the Agar laboratory has been supported by the Massachusetts Life Sciences Center and by NIH grant no. U54 CA210180. This work was supported by a Dean’s Innovation Award from Harvard Medical School (to G.Y. and N.Y.R.A.), an EMBO Postdoctoral Fellowship (to A.M.), an Alice and Joseph Brooks Fund Postdoctoral Fellowship (to A.M.), a Lefler Small Grant award (to G.Y.), a Hearst Fellowship (to E.M.Y.), an NIH Postdoctoral Training Grant position (no. T32 EB025823, to S.A.S.) and NIH grant nos. R01 NS102586 (to G.Y.) and R01 NS126248 (to G.Y. and N.Y.R.A.). The National Metabolomics Data Repository is supported by NIH grant nos. U2C DK119886 and OT2 OD030544.
Author information
Authors and Affiliations
Contributions
G.Y., N.Y.R.A., A.M., E.M.Y. and J.R.M.-F. designed the study. S.A.S., N.Y.R.A. and M.S.R. designed MS acquisition protocols. G.Y. designed the thermal preservation device. A.M. and E.M.Y. performed brain slice experiments. A.M., E.M.Y., S.A.S. and M.A.H. acquired MSI data. A.M., E.M.Y., S.A.S., J.R.M.-F., G.B., N.Y.R.A. and G.Y. analysed the data and wrote the manuscript. G.Y., N.Y.R.A., A.M. and E.M.Y. acquired funding. G.Y. and N.Y.R.A. oversaw the project. All authors discussed results and edited the manuscript.
Corresponding authors
Ethics declarations
Competing interests
N.Y.R.A. is a key opinion leader to Bruker. The other authors declare no competing interests.
Peer review
Peer review information
Nature Metabolism thanks Jorgina Satrústegui, Ruoning Wang and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Ashley Castellanos-Jankiewicz, in collaboration with the Nature Metabolism team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Details of thermal preservation, antibody characterization of the DGC layer, and electrical stimulation controls.
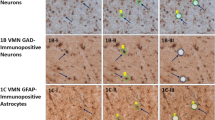
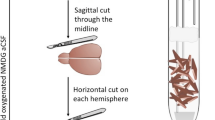
(a) Schematic of the thermal control assembly to perform the heat-freezing of brain slices. Details of the construction and operation are given in the Methods. (b) Effect of flash heating on enzyme activities detected by histochemical assays. Flash heating reduces the purple staining indicating glyceraldehyde-3-phosphate dehydrogenase (GAPDH) or lactate dehydrogenase (LDH) activity to an undetectable background level. Insets show the location of the DGC layer in the larger image indicated by a nuclear DAPI staining. These are representative images from 2 independent experiments. (c) ATP:AMP and phosphocreatine:creatine ratios in frozen and heat-frozen brain slices (n = 3 slices, values are mean ± SD). Raw ion count ratios are not calibrated to metabolite concentrations. Hypothesis testing was done by two-tailed, unpaired Student′s t-tests (p-values = 0.039 and 0.022). (d) The levels of various metabolites in a polar fraction of a brain homogenate (n = 4 mice; values are mean ± SEM) or (e) yeast extract (n = 1 extract, values are mean ± SD). (f) Scatter plot for all detected ion peaks in yeast extracts with coefficient of variation < 0.2, x-position = no-heat, y-position = flash-heat. (g) Consecutive cryosections of the same brain slice were used for H&E staining, MSI and immunofluorescence of nuclei (DAPI), a neuronal marker (NeuN) and an astrocyte marker (GFAP). (h) Quantification of the area in the DG that is covered by NeuN and GFAP staining (n = 3 mice, values are mean ± SEM). (i) Placement of a stimulation electrode without stimulation (sham) (j) and the ion images of different metabolites. Ino = inosine; Cr = creatine, PCr = phosphocreatine; GAP = glyceraldehyde 3-phosphate (and dihydroxyacetone phosphate); BPG = bisphosphoglycerates, PG = phosphoglycerates; PEP = phosphoenolpyruvate; 6PG = 6-phosphogluconate; PP/PentoseP = pentose phosphates.
Extended Data Fig. 2 Ion images with KCl stimulation, glutamate/glutamine time courses, and pathway schematic.
(a) Ion images of hippocampal slices that were unstimulated (control) or stimulated with 50 mM KCl for 5 min. (b) Levels of glutamate and glutamine in the DGC layer in response to 50 mM KCl for 30 s, 1 min, 2 min, 3 min, or 5 min. Values are mean ± SEM; n = 7 mice. (c) Pathway overview indicating inosine metabolism as a link between energy metabolism and glucose metabolism. G6P = glucose 6-phosphate; GAP/DHAP = glyceraldehyde 3-phosphate/dihydroxyacetone phosphate; BPG = bisphosphoglycerates, PG = phosphoglycerates; PEP = phosphoenolpyruvate; 6PG = 6-phosphogluconate; PentoseP = pentose phosphates; S7P = sedoheptulose phosphates; E4P = erythrose 4-phosphate; PCr = Phosphocreatine, Cr = Creatine; IMP = inosine monophosphate .
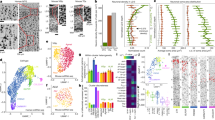
Extended Data Fig. 3 Details of 13C-glucose tracing experiments, inhibitors, depth profiling, and R1P fragmentation.
(a) Zoomed-in mass spectra obtained from 13C-labeled and control hippocampal slices. (b) Ion images of hippocampal slices that were unlabeled, labeled with 13C6 glucose (control) or labeled with 13C6 glucose and stimulated with 50 mM KCl for 5 min. (c) The relative contribution of each isotopologue in control or 5-min-KCl-stimulated DGs after 10 min (I) or 35 min (II) of 13C6 glucose labeling (n = 6 mice). Hypothesis testing was done by two-tailed, paired Student′s t-tests. P-values from left to right = 0.028, 0.036, 0.031, 0.004, 0.040. (d) The 6-phosphogluconate (6PG) levels in control and KCl stimulated DGs in absence or presence of G6PDi (50 µM) (n = 6 mice). (e) Inosine levels in control and KCl stimulated DGs in absence or presence of forodesine (Foro, 20 µM). (n = 6 mice). A two-sided t-test with multiple comparison correction was performed using the two-stage linear step-up procedure with Q = 1%. P-values from left to right bracket = 0.01, 0.049 (d); (top) 0.049, <0.001 (bottom), 0.003 (bottom). (f) Example spectrum of ribose 5-phosphate (R5P) and ribose 1-phosphate (R1P) and (g) chemical structure of R1P ([M-H]− m/z: 229.01) and its specific fragment ([M-H]− m/z: 211.00) detected in the multiple reaction monitoring strategy. (h) Average carbon atom labeling (atom%) of lower glycolytic intermediates throughout the depth of the hippocampal slice (n = 3 mice). All values are mean ± SEM. Significance is indicated as p < 0.001 (***), p < 0.01 (**), or p < 0.05 (*). GAP/DHAP = glyceraldehyde 3-phosphate/dihydroxyacetone phosphate; BPG = bisphosphoglycerates, PG = phosphoglycerates; PEP = phosphoenolpyruvate; 6PG = 6-phosphogluconate; PentoseP = pentose phosphates; S7P = sedoheptulose phosphates; E4P = erythrose 4-phosphate; PCr = Phosphocreatine, Cr = Creatine.
Extended Data Fig. 4 Purine transporters and inhibitor effects, and details of 13C-inosine tracing.
(a) Expression of purine transporting Solute Carrier (SLC) family members in neurons and astrocytes. (b) Inosine and pentose phosphate (PentoseP) levels in the DG upon treatment with the SLC29A1 and A2 inhibitors 8MDP (1 µM) or DIPY (10 µM) in combination with NBMPR (5 µM) in absence or presence of 100 µM inosine (n = 6 mice). P-values = <0.001, 0.026, 0.035. (left), 0.033, 0.032 (right). (c) Inosine and PentoseP levels in the DG upon treatment with cpd3 or pentostatin (pento) (20 µM) (n = 5–12 mice).P-values = 0.003, 0.007, 0.048 (left), 0.02, (right). (d) Schematic of the experimental setups for 10 min of 13C5 inosine tracing. (e) Average carbon atom labeling (atom%) of each indicated metabolite after 10 minutes of labeling with 13C5 inosine (prelabeled condition in panel d) (n = 6 mice). (f) The relative levels of pentoseP isotopologues in control or KCl-stimulated DGs after 10 minutes of labeling with 13C5 inosine (n = 6 mice). P-value = 0.010. (g) The contribution of each isotopologue to S7P after labeling with 13C5 inosine (n = 6 mice). # indicates source of labeling pattern. A two-sided t-test with multiple comparison correction was performed using the two-stage linear step-up procedure with Q = 1%, p- values = <0.001 (left), 0.014 (top). (h) Schematic of PPP reactions indicating the observed labeling pattern. Empty circles = unlabeled carbons, full circles = labeled carbons. TKT = Transketolase. # indicates generation of labeling pattern. (i) Ion images of hippocampal slices that were labeled with 13C5 inosine in absence (control) or presence of 50 mM KCl for 5 min. All values are mean ± SEM and hypothesis testing was done by two-tailed, paired Student′s t-tests unless otherwise stated. Significance is indicated as p<0.001 (***), p<0.01 (**), or p<0.05 (*). Data in (e), (f) and (g) were corrected for natural isotope abundance. GAP/DHAP = glyceraldehyde 3-phosphate/dihydroxyacetone phosphate; PG = phosphoglycerates; PEP = phosphoenolpyruvate; PentoseP = pentose phosphates; S7P = sedoheptulose phosphates; E4P = erythrose 4-phosphate.
Extended Data Fig. 5 Details of metabolite behavior and inhibitor sensitivity in recovery from stimulation.
(a) Schematic for the area under the curve (AUC) calculations. (b) Mean intensity of the Ca2+ responses before, during KCl stimulation and during recovery in absence and presence of forodesine (Foro) and G6PDi. Box extends from 25th to 75th percentile, middle line represents the median, whiskers indicate min to max; n = 10–11 mice. (c) The levels of pentoseP,(d) phosphocreatine (PCreatine), (e) ATP and (f) AMP in relation to the AUC of Ca2+ imaging in the same slice in presence of forodesine (Foro; 20 µM) or G6PDi (50 µM) (n = 10–11 mice). Whiskers indicate min to max. Note that here the control was also KCl stimulated.
Supplementary information
Supplementary Table 1
Data supporting the assignment of m/z peaks for individual compounds.
Source data
Source Data Fig. 2
Source data for Fig. 2.
Source Data Fig. 3
Source data for Fig. 3.
Source Data Fig. 4
Source data for Fig. 4.
Source Data Fig. 5
Source data for Fig. 5.
Source Data Extended Data Fig. 1
Source data for Extended Data Fig. 1.
Source Data Extended Data Fig. 2
Source data for Extended Data Fig. 2.
Source Data Extended Data Fig. 3
Source data for Extended Data Fig. 3.
Source Data Extended Data Fig. 4
Source data for Extended Data Fig. 4.
Source Data Extended Data Fig. 5
Source data for Extended Data Fig. 5.
Rights and permissions
About this article
Cite this article
Miller, A., York, E.M., Stopka, S.A. et al. Spatially resolved metabolomics and isotope tracing reveal dynamic metabolic responses of dentate granule neurons with acute stimulation. Nat Metab 5, 1820–1835 (2023). https://doi.org/10.1038/s42255-023-00890-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s42255-023-00890-z