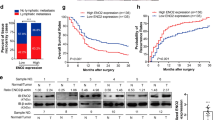
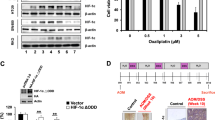
Abstract
Metabolic reprogramming is associated with resistance to antiangiogenic therapy in cancer. However, its molecular mechanisms have not been clearly elucidated. Here, we identify the glycolytic enzyme enolase 2 (ENO2) as a driver of resistance to antiangiogenic therapy in colorectal cancer (CRC) mouse models and human participants. ENO2 overexpression induces neuroendocrine differentiation, promotes malignant behaviour in CRC and desensitizes CRC to antiangiogenic drugs. Mechanistically, the ENO2-derived metabolite phosphoenolpyruvate (PEP) selectively inhibits histone deacetylase 1 (HDAC1) activity, which increases the acetylation of β-catenin and activates the β-catenin pathway in CRC. Inhibition of ENO2 with enolase inhibitors AP-III-a4 or POMHEX synergizes the efficacy of antiangiogenic drugs in vitro and in mice bearing drug-resistant CRC xenograft tumours. Together, our findings reveal that ENO2 constitutes a useful predictive biomarker and therapeutic target for resistance to antiangiogenic therapy in CRC, and uncover a previously undefined and metabolism-independent role of PEP in regulating resistance to antiangiogenic therapy by functioning as an endogenous HDAC1 inhibitor.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
All the data and materials relevant to the study are included in the manuscript, extended data and Supplementary Information. All other data files supporting the findings of this study are available from the corresponding authors upon reasonable request. The mass spectrometry proteomics data were deposited in the ProteomeXchange Consortium via the PRIDE partner repository with the dataset identifier PXD030202. Source data are provided with this paper.
References
Ebos, J. M. & Kerbel, R. S. Antiangiogenic therapy: impact on invasion, disease progression, and metastasis. Nat. Rev. Clin. Oncol. 8, 210–221 (2011).
Ebos, J. M., Lee, C. R. & Kerbel, R. S. Tumor and host-mediated pathways of resistance and disease progression in response to antiangiogenic therapy. Clin. Cancer Res. 15, 5020–5025 (2009).
Huang, M. et al. New insights into antiangiogenic therapy resistance in cancer: mechanisms and therapeutic aspects. Drug Resist. Updat. 64, 100849 (2022).
Davies, A. H., Beltran, H. & Zoubeidi, A. Cellular plasticity and the neuroendocrine phenotype in prostate cancer. Nat. Rev. Urol. 15, 271–286 (2018).
Wang, Z., Zhao, Y., An, Z. & Li, W. Molecular links between angiogenesis and neuroendocrine phenotypes in prostate cancer progression. Front. Oncol. 9, 1491 (2019).
Rubin, M. A., Bristow, R. G., Thienger, P. D., Dive, C. & Imielinski, M. Impact of lineage plasticity to and from a neuroendocrine phenotype on progression and response in prostate and lung cancers. Mol. Cell 80, 562–577 (2020).
Zhang, Y. et al. Androgen deprivation promotes neuroendocrine differentiation and angiogenesis through CREB-EZH2-TSP1 pathway in prostate cancers. Nat. Commun. 9, 4080 (2018).
van Veenendaal, L. M. et al. The clinical utility of neuron-specific enolase (NSE) serum levels as a biomarker for Merkel cell carcinoma (MCC). Ann. Surg. Oncol. 28, 1019–1028 (2021).
Yukimoto, R. et al. Specific activation of glycolytic enzyme enolase 2 in BRAF V600E-mutated colorectal cancer. Cancer Sci. 112, 2884–2894 (2021).
Yan, T. et al. Neuronal markers are expressed in human gliomas and NSE knockdown sensitizes glioblastoma cells to radiotherapy and temozolomide. BMC Cancer 11, 524 (2011).
Jiménez-Valerio, G. & Casanovas, O. Angiogenesis and metabolism: entwined for therapy resistance. Trends Cancer 3, 10–18 (2017).
Pisarsky, L. et al. Targeting metabolic symbiosis to overcome resistance to anti-angiogenic therapy. Cell Rep. 15, 1161–1174 (2016).
Ma, M. K. F. et al. Stearoyl-CoA desaturase regulates sorafenib resistance via modulation of ER stress-induced differentiation. J. Hepatol. 67, 979–990 (2017).
Wei, L. et al. Genome-wide CRISPR/Cas9 library screening identified PHGDH as a critical driver for Sorafenib resistance in HCC. Nat. Commun. 10, 4681 (2019).
Dando, I. et al. Oncometabolites in cancer aggressiveness and tumour repopulation. Biol. Rev. Camb. Philos. Soc. 94, 1530–1546 (2019).
Sun, L., Zhang, H. & Gao, P. Metabolic reprogramming and epigenetic modifications on the path to cancer. Protein Cell 13, 877–919 (2022).
Ho, P. C. et al. Phosphoenolpyruvate is a metabolic checkpoint of anti-tumor T cell responses. Cell 162, 1217–1228 (2015).
Gao, X., Wang, H., Yang, J. J., Liu, X. & Liu, Z. R. Pyruvate kinase M2 regulates gene transcription by acting as a protein kinase. Mol. Cell 45, 598–609 (2012).
Kim, J. et al. FOXA1 inhibits prostate cancer neuroendocrine differentiation. Oncogene 36, 4072–4080 (2017).
Qi, M. et al. Targeting FAPα-expressing hepatic stellate cells overcomes resistance to antiangiogenics in colorectal cancer liver metastasis models. J. Clin. Invest. 132, e157399 (2022).
Huang, M. et al. Tumor perivascular cell-derived extracellular vesicles promote angiogenesis via the Gas6/Axl pathway. Cancer Lett. 524, 131–143 (2022).
Liu, E. et al. Tau acetylates and stabilizes β-catenin thereby promoting cell survival. EMBO Rep. 21, e48328 (2020).
Hoffmeyer, K., Junghans, D., Kanzler, B. & Kemler, R. Trimethylation and acetylation of beta-catenin at lysine 49 represent key elements in ESC pluripotency. Cell Rep. 18, 2815–2824 (2017).
Zheng, Y. et al. Insulin-like growth factor 1-induced enolase 2 deacetylation by HDAC3 promotes metastasis of pancreatic cancer. Signal Transduct. Target. Ther. 5, 53 (2020).
Wolf, D., Rodova, M., Miska, E. A., Calvet, J. P. & Kouzarides, T. Acetylation of beta-catenin by CREB-binding protein (CBP). J. Biol. Chem. 277, 25562–25567 (2002).
Levy, L. et al. Acetylation of beta-catenin by p300 regulates beta-catenin–Tcf4 interaction. Mol. Cell. Biol. 24, 3404–3414 (2004).
Ge, X., Jin, Q., Zhang, F., Yan, T. & Zhai, Q. PCAF acetylates {beta}-catenin and improves its stability. Mol. Biol. Cell 20, 419–427 (2009).
Lechner, S. et al. Target deconvolution of HDAC pharmacopoeia reveals MBLAC2 as common off-target. Nat. Chem. Biol. 18, 812–820 (2022).
Lin, Y. H. et al. An enolase inhibitor for the targeted treatment of ENO1-deleted cancers. Nat. Metab. 2, 1413–1426 (2020).
Garcia, J. et al. Bevacizumab (Avastin) in cancer treatment: a review of 15 years of clinical experience and future outlook. Cancer Treat. Rev. 86, 102017 (2020).
Grothey, A., Blay, J. Y., Pavlakis, N., Yoshino, T. & Bruix, J. Evolving role of regorafenib for the treatment of advanced cancers. Cancer Treat. Rev. 86, 101993 (2020).
Gacche, R. N. & Assaraf, Y. G. Redundant angiogenic signaling and tumor drug resistance. Drug Resist. Update 36, 47–76 (2018).
Kuang, R. et al. GLUT3 upregulation promotes metabolic reprogramming associated with antiangiogenic therapy resistance. JCI Insight 2, e88815 (2017).
Liu, Y. et al. Neuroendocrine differentiation is a prognostic factor for stage II poorly differentiated colorectal cancer. BioMed. Res. Int. 2014, 789575 (2014).
Grabowski, P. et al. Neuroendocrine differentiation is a relevant prognostic factor in stage III-IV colorectal cancer. Eur. J. Gastroenterol. Hepatol. 13, 405–411 (2001).
Dost Gunay, F. S., Kırmızı, B. A., Ensari, A., İcli, F. & Akbulut, H. Tumor-associated macrophages and neuroendocrine differentiation decrease the efficacy of bevacizumab plus chemotherapy in patients with advanced colorectal cancer. Clin. Colorectal Cancer 18, e244–e250 (2019).
Kim, J. & DeBerardinis, R. J. Mechanisms and implications of metabolic heterogeneity in cancer. Cell Metab. 30, 434–446 (2019).
Li, X., Egervari, G., Wang, Y., Berger, S. L. & Lu, Z. Regulation of chromatin and gene expression by metabolic enzymes and metabolites. Nat. Rev. Mol. Cell Biol. 19, 563–578 (2018).
Li, W. et al. NADPH levels affect cellular epigenetic state by inhibiting HDAC3-Ncor complex. Nat. Metab. 3, 75–89 (2021).
Pan, A. et al. The mitochondrial β-oxidation enzyme HADHA restrains hepatic glucagon response by promoting β-hydroxybutyrate production. Nat. Commun. 13, 386 (2022).
Burgermeister, E. et al. Aryl hydrocarbon receptor nuclear translocator-like (ARNTL/BMAL1) is associated with bevacizumab resistance in colorectal cancer via regulation of vascular endothelial growth factor A. EBioMedicine 45, 139–154 (2019).
Depner, C. et al. EphrinB2 repression through ZEB2 mediates tumour invasion and anti-angiogenic resistance. Nat. Commun. 7, 12329 (2016).
Wang, S. et al. FOXF1 promotes angiogenesis and accelerates bevacizumab resistance in colorectal cancer by transcriptionally activating VEGFA. Cancer Lett. 439, 78–90 (2018).
Muller, F. L. et al. Passenger deletions generate therapeutic vulnerabilities in cancer. Nature 488, 337–342 (2012).
Leonard, P. G. et al. SF2312 is a natural phosphonate inhibitor of enolase. Nat. Chem. Biol. 12, 1053–1058 (2016).
Iaconelli, J. et al. HDAC6 inhibitors modulate Lys49 acetylation and membrane localization of beta-catenin in human iPSC-derived neuronal cells. ACS Chem. Biol. 10, 883–890 (2015).
Chen, X. et al. Bcl-3 promotes Wnt signaling by maintaining the acetylation of β-catenin at lysine 49 in colorectal cancer. Signal Transduct. Target. Ther. 5, 52 (2020).
Seto, E. & Yoshida, M. Erasers of histone acetylation: the histone deacetylase enzymes. Cold Spring Harb. Perspect. Biol. 6, a018713 (2014).
Shvedunova, M. & Akhtar, A. Modulation of cellular processes by histone and non-histone protein acetylation. Nat. Rev. Mol. Cell Biol. 23, 329–349 (2022).
Huang, M. et al. Perivascular cell-derived extracellular vesicles stimulate colorectal cancer revascularization after withdrawal of antiangiogenic drugs. J. Extracell. Vesicles 10, e12096 (2021).
Cascone, T. et al. Upregulated stromal EGFR and vascular remodeling in mouse xenograft models of angiogenesis inhibitor-resistant human lung adenocarcinoma. J. Clin. Invest. 121, 1313–1328 (2011).
van Malenstein, H. et al. Long-term exposure to sorafenib of liver cancer cells induces resistance with epithelial-to-mesenchymal transition, increased invasion and risk of rebound growth. Cancer Lett. 329, 74–83 (2013).
Tong, J., Tan, S., Zou, F., Yu, J. & Zhang, L. FBW7 mutations mediate resistance of colorectal cancer to targeted therapies by blocking Mcl-1 degradation. Oncogene 36, 787–796 (2017).
Amodio, V. et al. EGFR blockade reverts resistance to KRASG12C inhibition in colorectal cancer. Cancer Discov. 10, 1129–1139 (2020).
Michels, B. E. et al. Pooled in vitro and in vivo CRISPR–Cas9 screening identifies tumor suppressors in human colon organoids. Cell Stem Cell 26, 782–792 (2020).
Chen, M. et al. Pericyte-targeting prodrug overcomes tumor resistance to vascular disrupting agents. J. Clin. Invest. 127, 3689–3701 (2017).
Ye, G. et al. The FAP α-activated prodrug Z-GP-DAVLBH inhibits the growth and pulmonary metastasis of osteosarcoma cells by suppressing the AXL pathway. Acta Pharm. Sin. B 12, 1288–1304 (2022).
Wang, S. et al. Zoledronic acid and thymosin α1 elicit antitumor immunity against prostate cancer by enhancing tumor inflammation and cytotoxic T cells. J. Immunother. Cancer 11, e006381 (2023).
Yang, L. et al. Finding missing proteins from the epigenetically manipulated human cell with stringent quality criteria. J. Proteome Res. 14, 3645–3657 (2015).
Fekete, J. T. & Gyorffy, B. ROCplot.org: validating predictive biomarkers of chemotherapy/hormonal therapy/anti-HER2 therapy using transcriptomic data of 3,104 breast cancer patients. Int. J. Cancer 145, 3140–3151 (2019).
Zhu, P. et al. lnc-β-Catm elicits EZH2-dependent β-catenin stabilization and sustains liver CSC self-renewal. Nat. Struct. Mol. Biol. 23, 631–639 (2016).
Sun, H. et al. The cycloartane triterpenoid ADCX impairs autophagic degradation through Akt overactivation and promotes apoptotic cell death in multidrug-resistant HepG2/ADM cells. Biochem. Pharmacol. 146, 87–100 (2017).
Wen, Q. et al. lncRNA SYTL5-OT4 promotes vessel co-option by inhibiting the autophagic degradation of ASCT2. Drug Resist. Updat. 69, 100975 (2023).
Gao, J. et al. Discovery and biological evaluation of a small-molecule inhibitor of CRM1 that suppresses the growth of triple-negative breast cancer cells. Traffic 22, 221–229 (2021).
Acknowledgements
This study was supported by grants from National Natural Science Foundation of China (grant numbers: 81973340, 82204427, 82204428, 82273941, 82293681 (82293680) and 82102781); National High-level Personnel of Special Support Program (D.Z.); Local Innovative and Research Teams Project of the Guangdong Pearl River Talents Program (grant number: 2017BT01Y036); Natural Science Foundation of Guangdong Province (grant numbers: 2023A1515010361, 2022A1515011813, 2021A1515010882 and 2021A1515012080); Guangdong Basic and Applied Basic Research Foundation (grant number: 2021A1515110242); Key-Area Research and Development Program of Guangdong Province (grant number: 2020B1111110004); Science and Technology Projects in Guangzhou (grant numbers: 202201010173, 202102070001 and 2023A03J1030); and China Postdoctoral Science Foundation (grant number: 2022M721356). We are grateful to K. Li, B. Qin (Sun Yat-sen University), C. Shi, L. Tan (Jinan University) and M. Xie (Guangzhou Medical University) for assistance with PDO experiments. We thank Y. Yang (Fudan University) for advice on animal experiments. We also acknowledge T. Liu and G. Chen (Jinan University) for their valuable discussion and revision on this paper. We thank the College of Pharmacy Public Research Platform (Jinan University) for technical support.
Author information
Authors and Affiliations
Contributions
Conception and design of the study: C.W., M.H., Y.L., W.Y. and D.Z. Acquisition of data: C.W. and Y.L. (all experiments), L.X., J.P., C.J. and W.H. (clinical analysis), Y.Z. (western blotting), Z.L., S.L., Z.Z. and Y.W. (HDAC1-PEP binding), Z.T. and J.F. (HDAC1 and HDAC2 activity), H.Z. and J.L. (LC–MS/MS), Y.Z. and M. Chen (animal study) and M. Cheng (molecular docking study). Analysis and interpretation of data: C.W., M.H., Y.L., T.L. and M. Chen. Writing of the manuscript: C.W., M.H., Y.L., D.Z. and W.Y. Revision of the manuscript: M. Chen, N.Y., X.L., Q.Q., T.L., D.Z. and W.Y. All authors have seen and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Metabolism thanks Heather Christofk and the other, anonymous, reviewers for their contribution to the peer review of this work. Primary Handling Editor: Alfredo Giménez-Cassina, in collaboration with the Nature Metabolism team
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Establishment of bevacizumab-resistant and regorafenib-resistant CRC xenograft tumors.
a, Diagram depicts the establishment of Bev-resistant CRC xenograft tumors. BALB/c nude mice bearing HCT116 or SW620 xenograft tumors were treated with IgG or bevacizumab (10 mg/kg, intraperitoneal injection) twice a week until resistance to bevacizumab, and the first, second, and third generations (G1, G2, and G3) of bevacizumab-resistant CRC tumors were established successively (created with BioRender.com). Bev, bevacizumab. b,c, Mice bearing Bev-resistant or -sensitive CRC xenograft tumors were treated with IgG or bevacizumab (10 mg/kg, intraperitoneal injection) twice a week. (b) The growth curves and (c) tumor weight of xenograft tumors are shown. n = 5 or 6 mice per group. d, Diagram depicts the establishment of Rego-resistant CRC cells in vitro (created with BioRender.com). Rego, regorafenib. e, Rego-resistant and -sensitive HCT116 and SW620 cells were treated with the indicated concentrations of regorafenib for 24 h, and cell viability was determined by MTT assay. n = 3 biologically independent experiments. f, BALB/c nude mice bearing Rego-resistant or -sensitive CRC xenograft tumors were treated with vehicle or regorafenib (20 mg/kg, intraperitoneal injection, once a day), and tumor volumes were calculated every other day. n = 5 mice per group. g, Mice bearing Rego-resistant SW620 xenograft tumors were treated with vehicle, bevacizumab (10 mg/kg), or regorafenib (20 mg/kg) for 17 days. The growth curves and weight of CRC xenograft tumors are shown. Scale bar, 1 cm. n = 5 mice per group. h, Diagram depicts the TMT analysis of Bev-sensitive and -resistant HCT116 xenograft tumors (created with BioRender.com). i, GO enrichment analysis of significantly changed proteins (Fold change > 1.1 or < 0.85, and P < 0.05. n = 3 mice per group) identified in (h). j, The protein level of ENO2 in regorafenib-resistant CRC cells was evaluated by Western blotting. Representative of n = 3 independent replicates. Data are presented as mean ± SEM. ns, not significant. P values were determined by unpaired two-tailed Student’s t-test (c; g right) or two-way ANOVA with Tukey’s multiple comparison test (b, e, f; g left).
Extended Data Fig. 2 ENO2 and neuroendocrine differentiation are associated with resistance to antiangiogenic drugs in CRC.
a–d, Western blotting and qPCR assay were conducted to evaluate the protein and mRNA levels of ENO2 and neuroendocrine differentiation markers SYP and CHGA in (a, b) Bev-sensitive, Bev-resistant, (c, d) Rego-sensitive, and Rego-resistant CRC xenograft tumors. Bev, bevacizumab. Rego, regorafenib. (b, d) Relative mRNA expression in each group is shown. n = 3 mice per group. Representative blots are shown. e, The serum levels of ENO2 in mice bearing Rego-sensitive or -resistant CRC tumors was determined by ELISA. n = 5 mice per group. f, IHC staining and quantification of CHGA in tumors from the indicated groups. Scale bar, 50 μm. g, IHC staining and quantification of ENO2, SYP, and CHGA in tumors from the indicated groups. Scale bar, 50 μm. (f, g, n = 5 mice per group. Representative IHC images are shown). h, Diagram depicts the establishment of Rego-resistant HCT116 xenograft tumors in vivo. i, IF staining and quantification of CD31+ microvessel density in HCT116 xenograft tumors in each group. Scale bar, 20 μm. n = 6 mice per group. Representative IF images are shown. j, Western blot analysis of ENO2 in Rego-sensitive and -resistant HCT116 cells. Representative of n = 3 independent replicates. k, The viability of CRC cells treated with regorafenib for 24 h was evaluated by MTT assay. n = 3 biologically independent experiments. l, Disease free survival analysis of COAD and READ patients with high or low levels of ENO2 from TCGA (defined by RNA sequencing with group cutoff values of 50% and 50%). Data are presented as the mean ± s.e.m. ns, not significant. P values were determined by unpaired two-tailed Student’s t-test (b, d, e, f, g and k) or one-way ANOVA with Tukey’s multiple comparison test (i).
Extended Data Fig. 3 ENO2 regulates neuroendocrine differentiation, proliferation, and regorafenib sensitivity in CRC cells.
a, Western blot analysis of ENO2, SYP, and TUBB3 in DLD1 and LoVo cells transfected with the ENO2 overexpression plasmid or ENO2 shRNA. Representative of n = 3 independent replicates. b, The mRNA levels of ENO2 and CHGA in CRC cells transfected as indicated were determined by qPCR assay. n = 3 biologically independent experiments. c, Morphological change of CRC cells transfected with the ENO2 overexpression plasmid. White arrows indicated neurite extensions. Scale bar, 100 μm. Representative of n = 3 independent replicates. d, The effect of ENO2 on CRC cell viability was evaluated by CCK8 assay. n = 3 biologically independent experiments. e, The effect of ENO2 on CRC cell proliferation was determined by EdU cell proliferation assay. Scar bar, 100 μm. EdU incorporation was quantified. f. HUVECs were treated with conditioned medium from ENO2-overexpressing or ENO2-silenced DLD1 and LoVo cells, and tube formation assay was performed. Quantification of the number of HUVEC tubular structures is shown. Scale bar, 100 μm. (e, f, representative of n = 3 biological replicates). g, The effect of ENO2 on the viability of DLD1 and LoVo cells treated with regorafenib for 24 h was evaluated by MTT assay. n = 3 biologically independent experiments. h, ENO2 was knocked out by Cas9 system in CRC cells, and Western blotting was conducted to evaluate the expression of ENO2, CHGA, SYP, and TUBB3 in Control and ENO2−/− CRC cells. ENO2−/−, ENO2 knockout. Representative of n = 3 independent replicates. i, CCK8 assay was performed to evaluate the effect of ENO2 on CRC cell viability. j, The viability of Control and ENO2−/− CRC cells treated with regorafenib for 24 h was evaluated by MTT assay. k. Quantification of the number of HUVEC tubular structures after treatment with conditioned medium from Control and ENO2−/− CRC cells is shown. (i–k, n = 3 biologically independent experiments). Data are presented as the mean ± s.e.m. P values were determined by unpaired two-tailed Student’s t-test (b, e-g, ENO2-transfected group; j, k), one-way ANOVA with Tukey’s multiple comparison test (b, e-g, siENO2-transfected group), or one-way ANOVA with Tukey’s multiple comparison test (d and i).
Extended Data Fig. 4 ENO2 regulates malignant behavior and phenotype in CRC cells.
a, b, The effect of ENO2 on the migration and invasion of CRC cells was determined by (a) Transwell migration and (b) invasion assays. Quantification of the numbers of (a) migrated and (b) invaded cells is shown. Scale bar, 100 μm. c, HUVECs were treated with recombinant human ENO2 protein (20 ng/mL), and tube formation assay was performed. Quantification of the number of endothelial tubular structures. Scale bar, 200 μm. rhENO2, recombinant human ENO2 protein. (a-c, representative of n = 3 biological replicates). d, The effect of ENO2 on the expression of proangiogenic factors in HCT116 cells were assessed by qPCR assay. n = 3 biologically independent experiments. e, f, The effect of ENO2 on the expression of EMT- and stemness-related markers in CRC cells was evaluated by (e) Western blotting and (f) qPCR assay. Fold change of mRNA level in each group is shown. (e, representative of n = 3 independent replicates. f, n = 3 biologically independent experiments). Data are presented as the mean ± s.e.m. P values were determined by unpaired two-tailed Student’s t-test (a, b, ENO2-transfected group; c; d; f left) or one-way ANOVA with Tukey’s multiple comparison test (a, b, siENO2-transfected group; f right).
Extended Data Fig. 5 ENO2 affects the therapeutic efficacy of bevacizumab and regorafenib in CRC xenograft and PDX models.
a, Representative images of IHC staining of Ki67, cleaved caspase-3, and CD31 in HCT116 xenograft tumors in mice treated with bevacizumab or regorafenib. Scale bar, 50 μm. Representative IHC images are shown. b, c, BALB/c nude mice bearing HCT116shNC or HCT116shENO2 tumors were treated with vehicle, bevacizumab, or regorafenib for 17 days. (b) Tumor volume was calculated every three days, and (c) the tumors were removed and weighed. Scale bar, 1 cm. (a–c, n = 6 mice per group). d, Western blot analysis of ENO2 in CRC PDOs infected with the indicated AAVs. Representative of n = 3 independent samples. e, IHC staining and quantification of ENO2 and SYP in CRC PDX models in each group. Scale bar, 50 μm. n = 5 mice per group. Representative IHC images are shown. Data are presented as the mean ± s.e.m. P values were determined by two-way ANOVA with Tukey’s multiple comparison test (b) or unpaired two-tailed Student’s t-test (c and e).
Extended Data Fig. 6 ENO2 activates the β-catenin pathway by enhancing the K49 acetylation of β-catenin in CRC.
a, TOP/FOP Flash reporter assay was performed to evaluate β-catenin transcription activity in ENO2 knockout HCT116 and SW620 cells. b–d, The expression of β-catenin target genes (CD44, CCND1, MYC, ASCL2, and LGR5) in ENO2-ovexrepssing, ENO2-silenced, and ENO2 knockout HCT116 and SW620 cells were evaluated by (b, c) qPCR assay and (d) Western blotting. e, Western blot analysis of β-catenin (Ser552) and non-phospho (Active) β-catenin (Ser33/37/Thr41) in CRC cells transfected with the ENO2 overexpression plasmid. f, Quantitative analysis of IF staining of β-catenin and Ac-K49-β-catenin in Fig. 4d is shown. g, Western blot analysis of ENO2 and Ac-K49-β-catenin in ENO2 knockout CRC cells. h, ENO2-silenced CRC cells were treated with MG132 (5 μM) for 24 h, and the levels of Ac-K49-β-catenin and β-catenin were determined by Western blotting. siNC, negative control siRNA. i, The knockdown efficiency of β-catenin siRNAs in HCT116 cells was assessed by Western blotting. j, k, Mice bearing HCT116 xenograft tumors were infected with rAAV-Tie2-shNC or rAAV-Tie2-shKdr. j, IHC staining and quantification of VEGFR2, CD31, ENO2, and Ac-K49-β-catenin in CRC xenograft tumors infected with the indicated rAAVs. Scare bar, 50 μm. Representative IHC images are shown. k, Western blot analysis of KDR/VEGFR2 and ENO2 in CRC xenograft tumors in each group. Representative blots are shown. (a–c, f, n = 3 biologically independent experiments. d, e, g–i, representative of n = 3 independent replicates. j, n = 5 mice per group. k, n = 3 mice per group). Data are presented as the mean ± s.e.m. P values were determined by unpaired two-tailed Student’s t-test (a; b left; c, f, j) or one-way ANOVA with Tukey’s multiple comparison test (b right).
Extended Data Fig. 7 ENO2-derived PEP increases β-catenin acetylation and transcriptional activity in CRC cells.
a, b, LC-MS/MS analysis was conducted to determine the concentrations of intracellular PEP in (a) antiangiogenic drug-sensitive and -resistant HCT116 cells, and (b) CRC cells treated without or with PEP (n = 3). Bev, bevacizumab. Rego, regorafenib. (a, b, n = 3 biologically independent experiments). c, The expression of Ac-K49-β-catenin in CRC cells treated with a gradient concentration of PEP was determined by Western blotting. Representative of n = 3 independent replicates. d, TOP/FOP Flash reporter assay was performed to assess the effect of PEP on β-catenin transcriptional activity in HCT116 cells. n = 3 biologically independent experiments. e–g, Western blotting was performed to evaluate the effect of PEP on (e) β-catenin phosphorylation and (f, g) the expression of β-catenin target genes (c-Myc and Cyclin D1) in CRC cells. Representative of n = 3 independent replicates. Data are presented as the mean ± s.e.m. P values were determined by one-way ANOVA with Tukey’s multiple comparison test (a, b and d).
Extended Data Fig. 8 ENO2-derived PEP induces β-catenin acetylation by inhibiting HDAC1 activity in CRC.
a, Co-IP assay was performed to evaluate the effect of ENO2 on the binding of β-catenin to CBP, PCAF, and P300 in HCT116 cells. b, c, ENO2-silenced HCT116 cells were treated with different HDAC inhibitors (ENT, Entinostat, 5 μM; Vorinostat, 10 μM; BEL, Belinostat, 1 μM; TSA, Trichostatin A, 1 μM; SAN, Santacruzamate A, 1 μM; TUB, Tubacin, 5 μM; TMP, TMP269, 10 μM) for 24 h, and (b) Ac-K49-β-catenin expression and (c) β-catenin transcriptional activity were determined. d, e, Western blot analysis of the expression of HDACs in (d) ENO2-transfected HCT116 cells and (e) in HCT116 cells transfected as indicated. f, g, ENO2-silenced HCT116 cells were transfected with the indicated siRNAs, and (f) Ac-K49-β-catenin expression and (g) β-catenin transcriptional activity were evaluated. h, The effects of PEP (1 mM) and HDAC1 on CRC cell viability were assessed by MTT assay. i, The levels of β-catenin target genes in CRC cells transfected and treated as indicated were evaluated by qPCR assay. j, The interaction between β-catenin and HDAC1 in ENO2-silenced HCT116 cells was assessed by Co-IP assay. k, The inhibitory effect of ENO2 on HDAC1 activity was evaluated in HCT116 cells. l, The effect of PEP on the interaction of β-catenin with HDAC1 in HCT116 cells was determined by Co-IP assay. m, The interaction of PEP with wild-type or the mutant HDAC1 (K58R, H141D, and Y303D) in SW620 cells was determined by DARTS approach. n, The effects of PEP (1 mM) and HDAC1 on CRC cell viability were assessed by MTT assay. o, qPCR assay was conducted to assess the effect of HDAC1 on the expression of β-catenin target genes in PEP-treated CRC cells. (a, b, d–f, j, l, m, representative of n = 3 independent replicates. c, g–i, k, n, o, n = 3 biologically independent experiments). Data are presented as the mean ± s.e.m. ns, not significant. P values were determined by unpaired two-tailed Student’s t-test (c, g, i, k and o) or two-way ANOVA with Tukey’s multiple comparison test (h and n).
Extended Data Fig. 9 Inhibition of ENO2 enhances the efficacy of antiangiogenic therapy in drug-resistant CRC.
a, Rego-resistant CRC cells were treated with AP-III-a4 (10 μM) and/or various concentrations of regorafenib, and cell viability was evaluated by MTT assay (n = 3). Rego, regorafenib. b, MTT assay was performed to evaluate the viability of Rego-resistant SW620 cells treated with regorafenib + AP-III-a4 or regorafenib + POMHEX for 24 h. (a, b, n = 3 biologically independent experiments). c, d, IHC staining and quantification of ENO2, SYP, and cleaved caspase-3 in (c) Rego-resistant SW620 and (d) Bev-resistant HCT116 xenograft tumors in each group. Bev, bevacizumab. Scale bar, 50 μm. n = 5 mice per group. Representative IHC images are shown. Data are presented as the mean ± s.e.m. ns, not significant. P values were determined by two-way ANOVA with Tukey’s multiple comparison test (a), unpaired two-tailed Student’s t-test (b) or one-way ANOVA with Tukey’s multiple comparison test (c and d).
Extended Data Fig. 10 The ENO2/PEP/HDAC1 signaling contributes to ENO2-mediated β-catenin acetylation in CRC cells.
a, Western blot analysis of ENO1, ENO3, and PKM2 in drug-sensitive, Bev-resistant, and Rego-resistant CRC xenograft tumors and the corresponding drug-sensitive cells and tumors. Bev, bevacizumab. Rego, regorafenib. b, IHC staining and quantification of ENO1, ENO3, and PKM2 in antiangiogenic drug-sensitive and -resistant CRC xenograft tumors. Scale bar, 50 µm. n = 5 mice per group. Representative IHC images are shown. c, Western blot analysis of ENO1, ENO2, ENO3, and PKM2 in antiangiogenic drug-sensitive and -resistant HCT116 cells. d, Western blot analysis of ENO1 and ENO2 in parental and drug-resistant HCT116 cells. e, Western blot analysis of ENO1, ENO2, and ENO3 in HCT116 cells transfected or treated as indicated. f, Western blot analysis of Ac-K49-β-catenin, β-catenin, CHGA, SYP, and TUBB3 in CRC cells transfected or treated as indicated. g, Identification of direct binding of PEP to rhHDAC1 and rhHDAC2 was determined by DARTS approach. rhHDAC1, recombinant human HDAC1. rhHDAC2, recombinant human HDAC2. h, i, The binding sites and hydrogen-binding numbers of the interaction of PEP with HDAC1 (PDB: 4BKX) and HDAC2 (PDB: 6XEB) were predicted using AutoDock Vina software. j. The binding modes of the interaction of PEP with HDAC1 and HDAC2 were predicted by molecular dynamics (MD) simulation using GROMACS 2020.3 software. k, An in vitro enzyme activity assay was conducted to evaluate the effect of PEP on the activity of HDAC1 and HDAC2. l, Western blot analysis of Ac-K9-Histone H3 and Ac-K27-Histone H3 in PEP-treated CRC cells transfected as indicated. (a, c, d-f, g, l, representative of n = 3 independent replicates. b, n = 5 mice per group. k, n = 3 biologically independent experiments). Data are presented as the mean ± s.e.m. ns, not significant. P values were determined by unpaired two-tailed Student’s t-test (b and k).
Supplementary information
Supplementary Table 1
List of differentially expressed proteins in Bev-resistant versus Bev-sensitive HCT116 xenograft tumours.
Supplementary Tables 2–9
Supplementary Table 2. Characteristics of 52 CRC participants (n = 52 lesions) treated with chemotherapy plus bevacizumab postoperatively at Sun Yat-sen University Cancer Center. Supplementary Table 3. Multivariate Cox model analysis of factors affecting the DFS of CRC participants (n = 52) treated with chemotherapy plus bevacizumab postoperatively at Sun Yat-sen University Cancer Center. Supplementary Table 4. Cox regression analyses of prognostic factors for PFS in CRC participants (n = 52) treated with chemotherapy plus bevacizumab postoperatively at Sun Yat-sen University Cancer Center. Supplementary Table 5. siRNA sequences used for cell transfection. Supplementary Table 6. shRNA sequences used for cell transfection. Supplementary Table 7. Clinical characteristics of CRC participants for PDX and PDO models. Supplementary Table 8. Antibodies specific for the following proteins/peptides were used in this study. Supplementary Table 9. Primer sequences used for qPCR.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 2
Unprocessed western blots.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 4
Unprocessed western blots.
Source Data Fig. 5
Statistical source data.
Source Data Fig. 5
Unprocessed western blots.
Source Data Fig. 6
Statistical source data.
Source Data Fig. 6
Unprocessed western blots.
Source Data Fig. 7
Statistical source data.
Source Data Fig. 7
Unprocessed western blots.
Source Data Extended Data Fig./Table 1
Statistical source data.
Source Data Extended Data Figs. 1 and 2
Unprocessed western blots.
Source Data Extended Data Fig./Table 2
Statistical source data.
Source Data Extended Data Fig./Table 3
Statistical source data.
Source Data Extended Data Fig./Table 3
Unprocessed western blots.
Source Data Extended Data Fig./Table 4
Statistical source data.
Source Data Extended Data Fig./Table 4
Unprocessed western blots.
Source Data Extended Data Fig./Table 5
Statistical source data.
Source Data Extended Data Fig./Table 5
Unprocessed western blots.
Source Data Extended Data Fig./Table 6
Statistical source data.
Source Data Extended Data Fig./Table 6
Unprocessed western blots.
Source Data Extended Data Fig./Table 7
Statistical source data.
Source Data Extended Data Fig./Table 7
Unprocessed western blots.
Source Data Extended Data Fig./Table 8
Statistical source data.
Source Data Extended Data Fig./Table 8
Unprocessed western blots.
Source Data Extended Data Fig./Table 9
Statistical source data.
Source Data Extended Data Fig./Table 10
Statistical source data.
Source Data Extended Data Fig./Table 10
Unprocessed western blots.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, C., Huang, M., Lin, Y. et al. ENO2-derived phosphoenolpyruvate functions as an endogenous inhibitor of HDAC1 and confers resistance to antiangiogenic therapy. Nat Metab 5, 1765–1786 (2023). https://doi.org/10.1038/s42255-023-00883-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s42255-023-00883-y
This article is cited by
-
Research progress of exosomes in the angiogenesis of digestive system tumour
Discover Oncology (2024)