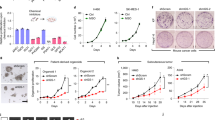
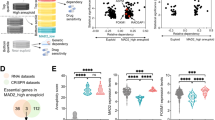
Abstract
Redox metabolites have been observed to fluctuate through the cell cycle in cancer cells, but the functional impacts of such metabolic oscillations remain unknown. Here, we uncover a mitosis-specific nicotinamide adenine dinucleotide phosphate (NADPH) upsurge that is essential for tumour progression. Specifically, NADPH is produced by glucose 6-phosphate dehydrogenase (G6PD) upon mitotic entry, which neutralizes elevated reactive oxygen species (ROS) and prevents ROS-mediated inactivation of mitotic kinases and chromosome missegregation. Mitotic activation of G6PD depends on the phosphorylation of its co-chaperone protein BAG3 at threonine 285, which results in dissociation of inhibitory BAG3. Blocking BAG3T285 phosphorylation induces tumour suppression. A mitotic NADPH upsurge is present in aneuploid cancer cells with high levels of ROS, while nearly unobservable in near-diploid cancer cells. High BAG3T285 phosphorylation is associated with worse prognosis in a cohort of patients with microsatellite-stable colorectal cancer. Our study reveals that aneuploid cancer cells with high levels of ROS depend on a G6PD-mediated NADPH upsurge in mitosis to protect them from ROS-induced chromosome missegregation.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Code availability
No custom codes were used in this study.
References
Pavlova, N. N. & Thompson, C. B. The emerging hallmarks of cancer metabolism. Cell Metab. 23, 27–47 (2016).
Harris, I. S. & DeNicola, G. M. The complex interplay between antioxidants and ROS in cancer. Trends Cell Biol. 30, 440–451 (2020).
Vander Heiden, M. G. & DeBerardinis, R. J. Understanding the intersections between metabolism and cancer biology. Cell 168, 657–669 (2017).
Hosios, A. M. & Vander Heiden, M. G. The redox requirements of proliferating mammalian cells. J. Biol. Chem. 293, 7490–7498 (2018).
Salazar-Roa, M. & Malumbres, M. Fueling the cell division cycle. Trends Cell Biol. 27, 69–81 (2017).
Icard, P., Fournel, L., Wu, Z., Alifano, M. & Lincet, H. Interconnection between metabolism and cell cycle in cancer. Trends Biochem. Sci. 44, 490–501 (2019).
Burhans, W. C. & Heintz, N. H. The cell cycle is a redox cycle: linking phase-specific targets to cell fate. Free Radic. Biol. Med. 47, 1282–1293 (2009).
Chiu, J. & Dawes, I. W. Redox control of cell proliferation. Trends Cell Biol. 22, 592–601 (2012).
da Veiga Moreira, J. et al. Cell cycle progression is regulated by intertwined redox oscillators. Theor. Biol. Med. Model. 12, 10 (2015).
Menon, S. G. & Goswami, P. C. A redox cycle within the cell cycle: ring in the old with the new. Oncogene 26, 1101–1109 (2007).
Zou, Y. et al. Analysis of redox landscapes and dynamics in living cells and in vivo using genetically encoded fluorescent sensors. Nat. Protoc. 13, 2362–2386 (2018).
Patterson, J. C. et al. ROS and oxidative stress are elevated in mitosis during asynchronous cell cycle progression and are exacerbated by mitotic arrest. Cell Syst. 8, 163–167 (2019).
Havens, C. G., Ho, A., Yoshioka, N. & Dowdy, S. F. Regulation of late G1/S phase transition and APC Cdh1 by reactive oxygen species. Mol. Cell. Biol. 26, 4701–4711 (2006).
Conour, J. E., Graham, W. V. & Gaskins, H. R. A combined in vitro/bioinformatic investigation of redox regulatory mechanisms governing cell cycle progression. Physiol. Genomics 18, 196–205 (2004).
Hayes, J. D., Dinkova-Kostova, A. T. & Tew, K. D. Oxidative stress in cancer. Cancer Cell 38, 167–197 (2020).
Ju, H. Q., Lin, J. F., Tian, T., Xie, D. & Xu, R. H. NADPH homeostasis in cancer: functions, mechanisms and therapeutic implications. Signal Transduct. Target. Ther. 5, 231 (2020).
Vasudevan, A. et al. Aneuploidy as a promoter and suppressor of malignant growth. Nat. Rev. Cancer 21, 89–103 (2021).
Danielsen, H. E., Pradhan, M. & Novelli, M. Revisiting tumour aneuploidy—the place of ploidy assessment in the molecular era. Nat. Rev. Clin. Oncol. 13, 291–304 (2016).
Newman, D. L., Thurgood, L. A. & Gregory, S. L. The impact of aneuploidy on cellular homeostasis. Free Radic. Res. 53, 705–713 (2019).
Dephoure, N. et al. Quantitative proteomic analysis reveals posttranslational responses to aneuploidy in yeast. eLife 3, e03023 (2014).
Zhu, J., Tsai, H. J., Gordon, M. R. & Li, R. Cellular stress associated with aneuploidy. Dev. Cell 44, 420–431 (2018).
Santaguida, S. & Amon, A. Short- and long-term effects of chromosome mis-segregation and aneuploidy. Nat. Rev. Mol. Cell Biol. 16, 473–485 (2015).
Li, R. & Zhu, J. Effects of aneuploidy on cell behaviour and function. Nat. Rev. Mol. Cell Biol. 23, 250–265 (2022).
Tao, R. et al. Genetically encoded fluorescent sensors reveal dynamic regulation of NADPH metabolism. Nat. Methods 14, 720–728 (2017).
Jiang, P. et al. p53 regulates biosynthesis through direct inactivation of glucose-6-phosphate dehydrogenase. Nat. Cell Biol. 13, 310–316 (2011).
Murphy, M. P. et al. Guidelines for measuring reactive oxygen species and oxidative damage in cells and in vivo. Nat. Metab. 4, 651–662 (2022).
Albrecht, S. C., Barata, A. G., Grosshans, J., Teleman, A. A. & Dick, T. P. In vivo mapping of hydrogen peroxide and oxidized glutathione reveals chemical and regional specificity of redox homeostasis. Cell Metab. 14, 819–829 (2011).
Yang, H. C., Stern, A. & Chiu, D. T. G6PD: a hub for metabolic reprogramming and redox signaling in cancer. Biomed. J. 44, 285–292 (2021).
Behl, C. Breaking BAG: the co-chaperone BAG3 in health and disease. Trends Pharm. Sci. 37, 672–688 (2016).
Kong, D. H. et al. BAG3 elevation inhibits cell proliferation via direct interaction with G6PD in hepatocellular carcinomas. Oncotarget 7, 700–711 (2016).
Cunningham, A. D., Colavin, A., Huang, K. C. & Mochly-Rosen, D. Coupling between protein stability and catalytic activity determines pathogenicity of G6PD variants. Cell Rep. 18, 2592–2599 (2017).
Soto, M., Raaijmakers, J. A. & Medema, R. H. Consequences of genomic diversification induced by segregation errors. Trends Genet. 35, 279–291 (2019).
Matthews, H. K., Bertoli, C. & de Bruin, R. A. M. Cell cycle control in cancer. Nat. Rev. Mol. Cell Biol. 23, 74–88 (2022).
Lens, S. M., Voest, E. E. & Medema, R. H. Shared and separate functions of Polo-like kinases and Aurora kinases in cancer. Nat. Rev. Cancer 10, 825–841 (2010).
Su, Z. et al. Global redox proteome and phosphoproteome analysis reveals redox switch in Akt. Nat. Commun. 10, 5486 (2019).
Kemper, E. K., Zhang, Y., Dix, M. M. & Cravatt, B. F. Global profiling of phosphorylation-dependent changes in cysteine reactivity. Nat. Methods 19, 341–352 (2022).
Kettenbach, A. N. et al. Quantitative phosphoproteomics identifies substrates and functional modules of Aurora and Polo-like kinase activities in mitotic cells. Sci. Signal. 4, rs5 (2011).
Carmena, M., Ruchaud, S. & Earnshaw, W. C. Making the Auroras glow: regulation of Aurora A and B kinase function by interacting proteins. Curr. Opin. Cell Biol. 21, 796–805 (2009).
Wang, G. F. et al. Oxidative stress induces mitotic arrest by inhibiting Aurora A-involved mitotic spindle formation. Free Radic. Biol. Med. 103, 177–187 (2017).
Byrne, D. P. et al. Aurora A regulation by reversible cysteine oxidation reveals evolutionarily conserved redox control of Ser/Thr protein kinase activity. Sci. Signal. 13, eaax2713 (2020).
Tsuchiya, Y. et al. Covalent Aurora A regulation by the metabolic integrator coenzyme A. Redox Biol. 28, 101318 (2020).
Lim, D. C. et al. Redox priming promotes Aurora A activation during mitosis. Sci. Signal. 13, eabb6707 (2020).
Cohen-Sharir, Y. et al. Aneuploidy renders cancer cells vulnerable to mitotic checkpoint inhibition. Nature 590, 486–491 (2021).
Ippolito, M. R. et al. Gene copy-number changes and chromosomal instability induced by aneuploidy confer resistance to chemotherapy. Dev. Cell 56, 2440–2454 (2021).
Lukow, D. A. et al. Chromosomal instability accelerates the evolution of resistance to anti-cancer therapies. Dev. Cell 56, 2427–2439 (2021).
Santaguida, S. et al. Chromosome mis-segregation generates cell-cycle-arrested cells with complex karyotypes that are eliminated by the immune system. Dev. Cell 41, 638–651 (2017).
Vilar, E. & Gruber, S. B. Microsatellite instability in colorectal cancer—the stable evidence. Nat. Rev. Clin. Oncol. 7, 153–162 (2010).
Sinicrope, F. A. et al. Prognostic impact of microsatellite instability and DNA ploidy in human colon carcinoma patients. Gastroenterology 131, 729–737 (2006).
Lengauer, C., Kinzler, K. W. & Vogelstein, B. Genetic instability in colorectal cancers. Nature 386, 623–627 (1997).
Stingele, S. et al. Global analysis of genome, transcriptome and proteome reveals the response to aneuploidy in human cells. Mol. Syst. Biol. 8, 608 (2012).
Sarmiento-Salinas, F. L. et al. Breast cancer subtypes present a differential production of reactive oxygen species (ROS) and susceptibility to antioxidant treatment. Front. Oncol. 9, 480 (2019).
Isnaini, I., Permatasari, N., Mintaroem, K., Prihardina, B. & Widodo, M. A. Oxidants–antioxidants profile in the breast cancer cell line MCF-7. Asian Pac. J. Cancer Prev. 19, 3175–3178 (2018).
Cassidy-Stone, A. et al. Chemical inhibition of the mitochondrial division dynamin reveals its role in Bax/Bak-dependent mitochondrial outer membrane permeabilization. Dev. Cell 14, 193–204 (2008).
Lucantoni, F., Dussmann, H. & Prehn, J. H. M. Metabolic targeting of breast cancer cells with the 2-deoxy-d-glucose and the mitochondrial bioenergetics inhibitor MDIVI-1. Front. Cell Dev. Biol. 6, 113 (2018).
Yu, F. et al. Aurora-A promotes the establishment of spindle assembly checkpoint by priming the Haspin–Aurora-B feedback loop in late G2 phase. Cell Discov. 3, 16049 (2017).
Gao, Y. F. et al. Cdk1-phosphorylated CUEDC2 promotes spindle checkpoint inactivation and chromosomal instability. Nat. Cell Biol. 13, 924–933 (2011).
Zhao, H. et al. AMPK-mediated activation of MCU stimulates mitochondrial Ca2+ entry to promote mitotic progression. Nat. Cell Biol. 21, 476–486 (2019).
Seo, Y. H. & Carroll, K. S. Profiling protein thiol oxidation in tumor cells using sulfenic acid-specific antibodies. Proc. Natl Acad. Sci. USA 106, 16163–16168 (2009).
Zhong, B. et al. Glucose-6-phosphate dehydrogenase neutralizes stresses by supporting reductive glutamine metabolism and AMPK activation. Signal Transduct. Target. Ther. 6, 46 (2021).
Vaughn, A. E. & Deshmukh, M. Glucose metabolism inhibits apoptosis in neurons and cancer cells by redox inactivation of cytochrome c. Nat. Cell Biol. 10, 1477–1483 (2008).
Jiang, Y. et al. Aurora A-mediated pyruvate kinase M2 phosphorylation promotes biosynthesis with glycolytic metabolites and tumor cell cycle progression. J. Biol. Chem. 298, 102561 (2022).
Li, M. et al. Aldolase B suppresses hepatocellular carcinogenesis by inhibiting G6PD and pentose phosphate pathways. Nat. Cancer 1, 735–747 (2020).
Cheng, A. et al. Aurora-A mediated phosphorylation of LDHB promotes glycolysis and tumor progression by relieving the substrate-inhibition effect. Nat. Commun. 10, 5566 (2019).
Ruan, C. et al. An integrative multi-omics approach uncovers the regulatory role of CDK7 and CDK4 in autophagy activation induced by silica nanoparticles. Autophagy 17, 1426–1447 (2021).
Wiredja, D. D., Koyuturk, M. & Chance, M. R. The KSEA App: a web-based tool for kinase activity inference from quantitative phosphoproteomics. Bioinformatics 33, 3489–3491 (2017).
Sacco, F. et al. Phosphoproteomics reveals the GSK3–PDX1 axis as a key pathogenic signaling node in diabetic islets. Cell Metab. 29, 1422–1432 (2019).
Tsutsumi, R. et al. Assay to visualize specific protein oxidation reveals spatio-temporal regulation of SHP2. Nat. Commun. 8, 466 (2017).
Alam, M. S. Proximity ligation assay (PLA). Curr. Protoc. Immunol. 123, e58 (2018).
Cheng, A. et al. Energy restriction causes metaphase delay and chromosome mis-segregation in cancer cells. Cell Cycle 20, 1195–1208 (2021).
Xing, F. et al. Accelerating precision anti-cancer therapy by time-lapse and label-free 3D tumor slice culture platform. Theranostics 11, 9415–9430 (2021).
Perez-Riverol, Y. et al. The PRIDE database resources in 2022: a hub for mass spectrometry-based proteomics evidences. Nucleic Acids Res. 50, D543–D552 (2022).
Acknowledgements
We thank Y. Yang and Y. Zhao at the East China University of Science and Technology for providing the iNap1 sensor plasmids; and B. Li at Capital Medical University for providing materials and reagents. This work was supported by the National Key R&D Program of China (2022YFA1303100 to X.Y. and Z.Y.), the National Science Foundation of China (92057104 to Z.Y., 31970670 and 32170736 to J.G. and 32000528 to A.C.) and the Fundamental Research Funds for the Central Universities (WK2070000189 to A.C., YD9100002011 and YD9100002028 to Z.Y.). This work was also supported in part by the Open Project of the CAS Key Laboratory of Innate Immunity and Chronic Disease and Research Funds of the Center for Advanced Interdisciplinary Science and Biomedicine of IHM of the USTC (QYPY20220017 to Z.Y.), the ‘Laboratory for Synthetic Chemistry and Chemical Biology’ under the Health@InnoHK programme by the Innovation and Technology Commission of Hong Kong and the Hong Kong Research Grant Council (T12-710/16-R to J.S.). All correspondence and requests for materials should be addressed to Z.Y. (zhenye@ustc.edu.cn).
Author information
Authors and Affiliations
Contributions
A.C., X.Y., J.G. and Z.Y., conceptualisation; A.C., T.X., W.Y. and T.W., investigation; D.Z., H.G., H.Z., Y.W., X.P., Y.W., L.L., K.Z. and A.C., data curation; A.C., J.S. and Z.Y., writing and editing; Z.Y. and J.G., supervision; Z.Y., A.C., J.G., X.Y. and J.S., funding acquisition.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Metabolism thanks Stephen Gregory and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Yanina-Yasmin Pesch, in collaboration with the Nature Metabolism team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Cancer cells exhibit an NADPH upsurge during mitosis.
a, Time-lapse microscopy of NADPH dynamics with genetically encoded sensor iNap1. Scale bar, 10 μm. Images were pseudo-colored by F425/F485. b, Images for ex vivo imaging in Fig. 1b. c, Quantified data of Fig. 1b at interphase and mitosis (n = 15 biologically independent cells, ***P < 0.0001). d, Images of iNap1 in interphase and mitotic HeLa cells in one view. I and M indicated interphase and mitosis, respectively. Scale bar, 10 μm.
Extended Data Fig. 2 G6PD-mediated mitotic NADPH upsurge is essential for accurate chromosome segregation.
a, The mRNA expression was analyzed by qPCR upon the treatment of two independent shRNA for 72 h (n = 3 biologically independent samples). b, Relative changes of G6PD activity upon the inhibitor treatments for 30 min were measured by enzymatic activity kit (n = 3 biologically independent samples, ***P < 0.0001). c, Relative changes of iNap1 signal in mitotic cells upon G6PD knockdown ex vivo (n = 124 biologically independent cells for NT and n = 141 for G6PD KD group, ***P < 0.0001) (top). Extracts of NT (non-targeting) or G6PD KD HeLa xenograft were immunoblotted with the indicated antibodies (bottom). d, Schematic diagram of the fate of 13C-label from [1,2-13C2] glucose in glycolysis and PPP (left). Isotopomer distributions of pyruvate and lactate from [1,2-13C2] glucose were determined in interphase, mitotic and G6PDi-treated mitotic HeLa cells (n = 3 biologically independent samples for each group, ***P = 0.0010, *P = 0.0118, ***P < 0.0001, ***P < 0.0001, from left to right) (right). e, Representative images of interphase and mitotic ROS in HeLa cells, Chromosomes were labeled with mCherry-H2B (red), ROS were staining with CM-H2DCFDA (green), Scale bar, 10 μm (left). The relative changes of CM-H2DCFDA signal were shown (n = 26 biologically independent cells for each group, ***P < 0.0001) (right). f, Relative changes of ROS in mitotic HeLa cells upon the treatment of NT or G6PD shRNA for 72 h were measured by CM-H2DCFDA staining (n = 4 biologically independent experiments, ***P = 0.0002, ***P < 0.0001, from left to right). g, Time-lapse microscopy of H2O2 dynamics with Orp1-roGFP2 during mitosis after the treatment of DMSO or G6PD inhibitor in HeLa. Images were pseudo-colored by F425/F485. Scale bar, 10 μm (left). The relative changes of Orp1- roGFP2 signal at time point 36 mins (metaphase) to 0 mins (NEB) were shown (n = 10 biologically independent cells for each group, ***P < 0.0001) (right). h, Representative mitotic phenotypes of HeLa cells upon the treatment of NT or G6PD shRNAs were analyzed by time-lapse microscopy that imaged tith H2B-GFP reporter. Scale bar, 10 μm. i, Extracts of NT or G6PD KD HeLa cells were immunoblotted with the indicated antibodies. j, Quantified data of misalignment, lagging chromosomes and micronucleus were shown (n = 3 biologically independent experiments, *P = 0.0135, *P = 0.0109, ***P = 0.0008, ***P < 0.0001, ***P = 0.0007, ***P = 0.0002, from left to right).
Extended Data Fig. 3 G6PD activity in mitosis is modulated by dynamic interaction with BAG3.
a, Overview of the procedure for identifying the potential regulators of G6PD that is cell cycle dependent. DTB, double thymidine block (image created with BioRender.com). b, Co-localization of BAG3 and G6PD in HeLa cells, Scale bar, 10 μm. c, Schematic diagrams of phosphorylation sites of BAG3 in mitosis identified by MS. d, Schematic diagrams of the functional domains and different deletion mutants of BAG3. e, Purified recombinant GST-G6PD proteins were mixed with purified recombinant FLAG-BAG3 WT or different mutants. GST pull-down assays were performed and the eluted samples were immunoblotted with the indicated antibodies. f, The cell cycle-dependent interaction between G6PD and BAG3WT or BAG3T285A in HeLa was confirmed by PLA. Quantified PLA signals from single interphase and mitotic cells were shown. (n = 22, 28, 21, 19 biologically independent cells, ***P < 0.0001, (NS) P = 0.7511, from left to right). g, Purified recombinant GST-G6PD proteins were mixed with/without purified recombinant His-BAG3 and His-p53. Then the relative changes of G6PD activity were measured by enzymatic activity kit (n = 3 biologically independent samples for each group, ***P < 0.0001, **P = 0.0012). h, Interphase and mitotic HeLa cells with BAG3 KO and reconstituted expression of BAG3WT or BAG3T285A were treated with and without 2 mM DSS and immunoblotted with the indicated antibodies. The positions of various forms of G6PD were indicated. i, Purified recombinant GST-BAG3 proteins were mixed with purified recombinant His-G6PD WT or different mutants. GST pull-down assays were performed and the eluted samples were immunoblotted with the indicated antibodies. j, Relative changes of iNap1 signal in mitotic cells after BAG3 or G6PD knockout (n = 73, 84, 41 biologically independent cells, (NS) P = 0.9013, ***P < 0.0001, from left to right) (top). Extracts of HeLaWT, HeLaBAG3 KO or HeLaG6PD KO cells were immunoblotted with the indicated antibodies (bottom). k, Quantified data of lagging chromosomes and micronucleus in HeLaWT, HeLaBAG3 KO or HeLaG6PD KO cells were shown (n = 3 biologically independent samples, (NS) P = 0.9841, ***P < 0.0001, (NS) P = 0.5076, **P = 0.0086, from left to right).
Extended Data Fig. 4 CDK1 and AMPK mediate the phosphorylation of BAG3 T285 in mitosis.
a, Representative images of BAG3 pT285 signal in mitotic HeLaWT and HeLaAMPKα1/2 DKO cells upon the treatment of RO3306 for 1 h. Scale bar, 10 μm (left). Relative changes of BAG3 pT285 signal were shown (n = 67, 49, 79 biologically independent cells, from left to right, ***P < 0.0001) (right). b, Relative changes of BAG3 pT285 signal in mitotic HeLa cells upon the treatment of JNJ7706621 (CDK inhibitor) and BAY3827 (AMPK inhibitor), RO3306 or BAY3827 were shown (n = 27, 27, 31, 34 biologically independent cells, from left to right, ***P < 0.0001). c, Extracts of HeLaWT and HeLaAMPKα1/2 DKO cells treated with/without RO3306 were immunoblotted with the indicated antibodies. d, The interaction between G6PD and BAG3 in HeLaWT and HeLaAMPKα1/2 DKO cells upon treatment of Compound C and/or RO3306 were confirmed by Co-IP and subsequently immunoblotting with the indicated antibodies. e, HeLaWT or HeLaAMPKα1/2 DKO mitotic cells were treated with/without RO3306. Then, the interphase and mitotic cells were collected and treated with/without 2 mM DSS and were immunoblotted with the indicated antibodies. The positions of various forms of G6PD were indicated. f, Relative changes of G6PD activity in mitotic HeLa cells upon the treatment of JNJ7706621 and/or BAY3827 were measured by enzymatic activity kit (n = 3 biologically independent samples for each group, ***P < 0.0001). g, Time-lapse microscopy of NADPH dynamics with iNap1 during HeLa cell division after the treatment of RO3306 and Compound C. Images were pseudo-colored by F425/F485. Scale bar, 10 μm (left). Relative ratios of iNap1 signal at time point 40 mins (metaphase) to the signal at 0 mins (NEB) were shown (n = 12 biologically independent cells for left and n = 15 for right, ***P < 0.0001) (right). h, Relative changes of ROS in mitotic HeLa cells upon the treatment of JNJ7706621 and/or BAY3827 were measured by CM-H2DCFDA staining (n = 3 biologically independent samples, ***P < 0.0001, ***P = 0.0004, from left to right).
Extended Data Fig. 5 Blocking BAG3 T285 phosphorylation suppresses tumor growth.
a, The endogenous BAG3 was replaced by BAG3WT or BAG3T285A mutant in HeLa cells. The proliferation of cultured cells was evaluated with/without 5 mM NAC (n = 3 biologically independent samples for each group, ***P < 0.0001).
Extended Data Fig. 6 The NADPH upsurge prevents ROS-induced inactivation of mitotic kinases.
a, Immunofluorescence imaging of mCherry-H2B showing the enrichment of mitotic cells after double thymidine block and shake-off, Scale bar, 20 μm. b, Quantification of phosphoproteome data with Kinase–Substrate Enrichment Analysis (KSEA) scores and FDR (Q value) from interphase/mitosis comparisons. c, Extracts of mitotic HeLa cell with the treatment of G6PD shRNA and/or NAC were immunoblotted with the indicated antibodies. d, Immunofluorescence images of Aurora-A and Aurora-B in mitotic HeLa cells with/without the treatment of G6PD inhibitor. Scale bar, 10 μm. e, Extracts of mitotic HeLa cells treated with G6PD inhibitor at different time points were immunoblotted with the indicated antibodies. f, Quantified PLA signals from single mitotic cells at each condition were shown (n = 25, 24, 27, 28, 32 biologically independent cells, from left to right, ***P < 0.0001) (top). Representative images of the oxidized Aurora-A by dimedone-PLA (red) were shown for each condition. The white line in the picture was based on WGA staining. Scale bar, 10 μm (bottom). g, Schematic diagrams of FLAG-tag and Aurora kinase domain (129-403aa), containing WT and several cysteine point mutants. The primary antibodies for PLA were labeled. h, Overview of the procedure for cell treatment, collection and protein purification. Briefly, FLAG-Aurora A kinase domain (129-403aa) with/without C290 mutant was expressed in HeLa cells. Then mitotic HeLa cells were treated with/without G6PD inhibitor, collected by double thymidine block and shake-off, extracted with cell lysis buffer containing 5 mM IAA and 5 mM dimedone, purified with FLAG beads and immunoblotted with the indicated antibodies in Fig. 6h (image created with BioRender.com). i, Alignment of amino acid sequences containing active site of Aurora kinase from several model species: Dr (Danio rerio); Xl (Xenopus laevis); Rn (Rattus norvegicus); Mm (Mus musculus); Hs (Homo Sapiens). j, Extracts of mitotic HeLa cells with BAG3 KO and reconstituted expression of BAG3WT or BAG3T285A upon the treatment of 5 mM NAC were immunoblotted with the indicated antibodies.
Extended Data Fig. 7 CDK1/AMPK-BAG3-G6PD axis safeguards genome stability of ROS-high aneuploid cancer cell.
a, Time-lapse microscopy of NADPH dynamics with iNap1 from late G2 to next G1 in near-diploid and aneuploid cells. Images were pseudo-colored by F425/F485. Scale bar, 10 μm. b, Detailed information for different cell lines used in Fig. 7a. c, Representative images of ROS in near-diploid and aneuploid cells, chromosomes were labeled with mCherry-H2B (red), ROS were staining with CM-H2DCFDA (green). d, The relative changes of interphase vs mitotic ROS in near-diploid and aneuploid cells were measured by CM-H2DCFDA staining (n per group was shown, (NS) P = 0.7414, (NS) P = 0.8818, (NS) P = 0.5976, ***P < 0.0001, ***P < 0.0001, ***P < 0.0001, ***P < 0.0001, from left to right). e, Extracts of near-diploid and aneuploid cancer cells were immunoblotted with the indicated antibodies. f, Extracts of near-diploid and induced aneuploid cancer cells were immunoblotted with the indicated antibodies. g, The expression of G6PD in near-diploid and aneuploid cell lines were analyzed (n = 220 biologically independent cell lines for near-diploid and n = 667 for aneuploid, ***P < 0.0001), data from cBioportal. h, The activity of NRF2 pathway in near-diploid and aneuploid cell lines were analyzed (n = 259 biologically independent cell lines for near-diploid and n = 680 for aneuploid, ***P < 0.0001), data from CCLE. i, The interaction between G6PD and BAG3 in near-diploid (LOVO and HCT116) and aneuploid (HeLa and HT29) cells was tested by Co-IP with G6PD or BAG3 antibody and the eluted samples were subsequently immunoblotted with the indicated antibodies. j, Relative changes of BAG3 pT285 signal in mitotic HT29, U2OS, SW480 and U251 cells upon the treatment of JNJ7706621 and BAY3827 or RO3306 and BAY3827 (n = 34, 39, 83, 54, 25, 27, 72, 60, 30, 28, 56, 60, 27, 26, 74, 76 biologically independent cells, from left to right, ***P < 0.0001). k, Near-diploid and aneuploid cancer cells were treated with/without G6PD inhibitor and collected by nocodazole synchronization and shake-off. Aurora-A was purified by IP and subsequently, the phosphorylation of Aurora-A T288 and oxidization of Aurora-A were immunoblotted with the indicated antibodies. l, Relative changes of ROS in near-diploid and induced aneuploid cancer cells with/without the treatment of G6PD inhibitor were measured by CM-H2DCFDA staining (n = 3 biologically independent samples for each group, (NS) P = 0.1318, ***P < 0.0001, ***P = 0.0003, (NS) P = 0.0756, ***P < 0.0001, ***P = 0.0004, from left to right). m, Relative proliferation rates were analyzed in near-diploid and aneuploid cells with/without the treatment of G6PD inhibitor (n = 3 biologically independent samples for each group, ***P < 0.0001, **P = 0.0038, (NS) P = 0.2105, (NS) P = 0.2102, (NS) P = 0.0857, *P = 0.0133, ***P < 0.0001, ***P < 0.0001, ***P < 0.0001, ***P < 0.0001, ***P < 0.0001, ***P < 0.0001, ***P < 0.0001, ***P < 0.0001, from left to right). n, The proliferative capability of RKO and HCT116 cells used in Fig. 7j was evaluated (n = 3 biologically independent samples for each group, (NS) P = 0.9138, (NS) P = 0.7697).
Extended Data Fig. 8 Mdivi-1 treatment induces mitotic NADPH upsurge in MCF7 cells.
a, Quantified data of mitochondrial network/branch junctions with/without the treatment of Mdivi-1 (Mitochondrial fission inhibitor, 50 μM) were shown (n = 40 biologically independent cells for left and n = 34 for right, ***P < 0.0001). b, Relative changes of ROS in MCF7 upon the treatment of Mdivi-1 were measured by CM-H2DCFDA staining (n = 3 biologically independent samples, ***P = 0.0002). c, Extracts of MCF7 and Mdivi-1-treated MCF7 cells were immunoblotted with the indicated antibodies. d, Quantifications of iNap1 signal from MCF7 and Mdivi-1-treated MCF7 in different cell cycle (n = 49, 95, 69, 107 biologically independent cells, (NS) P = 0.6032, ***P < 0.0001, from left to right). e, Mitotic cell extracts of MCF7 with/without the treatment of Mdivi-1 and NAC (1 mM) were immunoblotted with the indicated antibodies.
Supplementary information
Supplementary Information
Supplementary Fig. 1 and Tables 1–4.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 2
Unprocessed western blots and/or gels.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 3
Unprocessed western blots and/or gels.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 4
Unprocessed western blots and/or gels.
Source Data Fig. 5
Statistical source data.
Source Data Fig. 6
Statistical source data.
Source Data Fig. 6
Unprocessed western blots and/or gels.
Source Data Fig. 7
Statistical source data.
Source Data Fig. 7
Unprocessed western blots and/or gels.
Source Data Fig. 8
Statistical source data.
Source Data Extended Data Fig. 1
Statistical source data.
Source Data Extended Data Fig. 2
Statistical source data.
Source Data Extended Data Fig. 2
Unprocessed western blots and/or gels.
Source Data Extended Data Fig. 3
Statistical source data.
Source Data Extended Data Fig. 3
Unprocessed western blots and/or gels.
Source Data Extended Data Fig. 4
Statistical source data.
Source Data Extended Data Fig. 4
Unprocessed western blots and/or gels.
Source Data Extended Data Fig. 5
Statistical source data.
Source Data Extended Data Fig. 6
Statistical source data.
Source Data Extended Data Fig. 6
Unprocessed western blots and/or gels.
Source Data Extended Data Fig. 7
Statistical source data.
Source Data Extended Data Fig. 7
Unprocessed western blots and/or gels.
Source Data Extended Data Fig. 8
Statistical source data.
Source Data Extended Data Fig. 8
Unprocessed western blots and/or gels.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Cheng, A., Xu, T., You, W. et al. A mitotic NADPH upsurge promotes chromosome segregation and tumour progression in aneuploid cancer cells. Nat Metab 5, 1141–1158 (2023). https://doi.org/10.1038/s42255-023-00832-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s42255-023-00832-9
This article is cited by
-
The two sides of chromosomal instability: drivers and brakes in cancer
Signal Transduction and Targeted Therapy (2024)
-
Construction and validation of an efferocytosis-related prognostic signature in lung adenocarcinoma
Journal of Cancer Research and Clinical Oncology (2023)