Abstract
The additional therapeutic effects of regular exercise during a dietary weight loss program in people with obesity and prediabetes are unclear. Here, we show that whole-body (primarily muscle) insulin sensitivity (primary outcome) was 2-fold greater (P = 0.006) after 10% weight loss induced by calorie restriction plus exercise training (Diet+EX; n = 8, 6 women) than 10% weight loss induced by calorie restriction alone (Diet-ONLY; n = 8, 4 women) in participants in two concurrent studies. The greater improvement in insulin sensitivity was accompanied by increased muscle expression of genes involved in mitochondrial biogenesis, energy metabolism and angiogenesis (secondary outcomes) in the Diet+EX group. There were no differences between groups in plasma branched-chain amino acids or markers of inflammation, and both interventions caused similar changes in the gut microbiome. Few adverse events were reported. These results demonstrate that regular exercise during a diet-induced weight loss program has profound additional metabolic benefits in people with obesity and prediabetes.
Trial Registration: ClinicalTrials.gov (NCT02706262 and NCT02706288)
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
Source data for Figs. 1, 2, 3, 4, 5c and 6 and Extended Data Tables 1, 2, 3 and 4 are provided with this paper. The RNA-seq data generated during this study are available at the National Center for Biotechnology Information (NCBI) Gene Expression Omnibus (GEO) database under accession number GSE230002. We used DAVID Bioinformatics Resources v6.8 (http://david.ncifcrf.gov) to analyze the muscle RNA-seq data. The 16S data have been uploaded to the European Nucleotide Archive (ENA; https://www.ebi.ac.uk/ena) under the study identifier PRJEB61649. The Greengenes 13_8 database was used for taxonomic assignment of the ASVs detected in this study (https://data.qiime2.org/2022.11/common/gg-13-8-99-515-806-nb-classifier.qza). Source data are provided with this paper.
References
Klein, S., Wadden, T. & Sugerman, H. J. AGA technical review on obesity. Gastroenterology 123, 882–932 (2002).
Magkos, F. et al. Effects of moderate and subsequent progressive weight loss on metabolic function and adipose tissue biology in humans with obesity. Cell Metab. 23, 591–601 (2016).
Perseghin, G. et al. Increased glucose transport–phosphorylation and muscle glycogen synthesis after exercise training in insulin-resistant subjects. N. Engl. J. Med. 335, 1357–1362 (1996).
Devlin, J. T. & Horton, E. S. Effects of prior high-intensity exercise on glucose metabolism in normal and insulin-resistant men. Diabetes 34, 973–979 (1985).
Ding, C. et al. Dose-dependent effects of exercise and diet on insulin sensitivity and secretion. Med. Sci. Sports Exerc. 51, 2109–2116 (2019).
Ryan, B. J. et al. Moderate-intensity exercise and high-intensity interval training affect insulin sensitivity similarly in obese adults. J. Clin. Endocrinol. Metab. 105, e2941–e2959 (2020).
Jensen, M. D. et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. J. Am. Coll. Cardiol. 63, 2985–3023 (2014).
Garvey, W. T. et al. American Association of Clinical Endocrinologists and American College of Endocrinology Comprehensive Clinical Practice Guidelines for Medical Care of Patients with Obesity. Endocr. Pract. 22, 1–203 (2016).
US Preventative Services Task Force. Behavioral weight loss interventions to prevent obesity-related morbidity and mortality in adults: a US Preventative Services Task Force Recommendation Statement. JAMA 320, 1163–1171 (2018).
Weiss, E. P. et al. Calorie restriction and matched weight loss from exercise: independent and additive effects on glucoregulation and the incretin system in overweight women and men. Diabetes Care 38, 1253–1262 (2015).
Bouchonville, M. et al. Weight loss, exercise or both and cardiometabolic risk factors in obese older adults: results of a randomized controlled trial. Int. J. Obes. 38, 423–431 (2014).
Larson-Meyer, D. E. et al. Effect of calorie restriction with or without exercise on insulin sensitivity, beta-cell function, fat cell size, and ectopic lipid in overweight subjects. Diabetes Care 29, 1337–1344 (2006).
Tamura, Y. et al. Effects of diet and exercise on muscle and liver intracellular lipid contents and insulin sensitivity in type 2 diabetic patients. J. Clin. Endocrinol. Metab. 90, 3191–3196 (2005).
Dengel, D. R., Pratley, R. E., Hagberg, J. M., Rogus, E. M. & Goldberg, A. P. Distinct effects of aerobic exercise training and weight loss on glucose homeostasis in obese sedentary men. J. Appl. Physiol. 81, 318–325 (1996).
Snel, M. et al. Effects of adding exercise to a 16-week very low-calorie diet in obese, insulin-dependent type 2 diabetes mellitus patients. J. Clin. Endocrinol. Metab. 97, 2512–2520 (2012).
Bogardus, C. et al. Effects of physical training and diet therapy on carbohydrate metabolism in patients with glucose intolerance and non-insulin-dependent diabetes mellitus. Diabetes 33, 311–318 (1984).
Toledo, F. G. S., Watkins, S. & Kelley, D. E. Changes induced by physical activity and weight loss in the morphology of intermyofibrillar mitochondria in obese men and women. J. Clin. Endocrinol. Metab. 91, 3224–3227 (2006).
Coker, R. H. et al. The impact of exercise training compared to caloric restriction on hepatic and peripheral insulin resistance in obesity. J. Clin. Endocrinol. Metab. 94, 4258–4266 (2009).
Toledo, F. G. S. et al. Mitochondrial capacity in skeletal muscle is not stimulated by weight loss despite increases in insulin action and decreases in intramyocellular lipid content. Diabetes 57, 987–994 (2008).
Joseph, P. L. et al. Benefits and barriers to exercise among individuals with class III obesity. Am. J. Health Behav. 43, 1136–1147 (2019).
Koh, H.-C. E. et al. Heterogeneity in insulin-stimulated glucose uptake among different muscle groups in healthy lean people and people with obesity. Diabetologia 64, 1158–1168 (2021).
Barnard, R. J., Massey, M. R., Cherny, S., O’Brien, L. T. & Pritikin, N. Long-term use of a high-complex-carbohydrate, high-fiber, low-fat diet and exercise in the treatment of NIDDM patients. Diabetes Care 6, 268–273 (1983).
Gardner, C. D. et al. Comparison of the Atkins, Zone, Ornish, and LEARN diets for change in weight and related risk factors among overweight premenopausal women: the A TO Z Weight Loss Study: a randomized trial. JAMA 297, 969–977 (2007).
Sullivan, S. & Klein, S. Effect of short-term Pritikin diet therapy on the metabolic syndrome. J. Cardiometab. Syndr. 1, 308–312 (2006).
Mifflin, M. D. et al. A new predictive equation for resting energy expenditure in healthy individuals. Am. J. Clin. Nutr. 51, 241–247 (1990).
Korenblat, K. M., Fabbrini, E., Mohammed, B. S. & Klein, S. Liver, muscle, and adipose tissue insulin action is directly related to intrahepatic triglyceride content in obese subjects. Gastroenterology 134, 1369–1375 (2008).
Matsuda, M. & DeFronzo, R. A. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care 22, 1462–1470 (1999).
Morton, J. T. et al. Establishing microbial composition measurement standards with reference frames. Nat. Commun. 10, 2719 (2019).
Way, K. L., Hackett, D. A., Baker, M. K. & Johnson, N. A. The effect of regular exercise on insulin sensitivity in type 2 diabetes mellitus: a systematic review and meta-analysis. Diabetes Metab. J. 40, 253–271 (2016).
DiMenna, F. J. & Arad, A. D. The acute vs. chronic effect of exercise on insulin sensitivity: nothing lasts forever. Cardiovasc. Endocrinol. Metab. 10, 149–161 (2021).
Holloszy, J. O. Adaptations of muscular tissue to training. Prog. Cardiovasc. Dis. 18, 445–458 (1976).
Hargreaves, M. & Cameron-Smith, D. Exercise, diet, and skeletal muscle gene expression. Med. Sci. Sports Exerc. 34, 1505–1508 (2002).
Morino, K., Petersen, K. F. & Shulman, G. I. Molecular mechanisms of insulin resistance in humans and their potential links with mitochondrial dysfunction. Diabetes 55, S9–S15 (2006).
Koliaki, C. & Roden, M. Alterations of mitochondrial function and insulin sensitivity in human obesity and diabetes mellitus. Annu. Rev. Nutr. 36, 337–367 (2016).
Liu, Y., Christensen, P. M., Hellsten, Y. & Gliemann, L. Effects of exercise training intensity and duration on skeletal muscle capillarization in healthy subjects: a meta-analysis. Med. Sci. Sports Exerc. 54, 1714–1728 (2022).
Hamrin, K. et al. Prolonged exercise-induced stimulation of skeletal muscle glucose uptake is due to sustained increases in tissue perfusion and fractional glucose extraction. J. Clin. Endocrinol. Metab. 96, 1085–1092 (2011).
Goodyear, L. J., Hirshman, M. F., Valyou, P. M. & Horton, E. S. Glucose transporter number, function, and subcellular distribution in rat skeletal muscle after exercise training. Diabetes 41, 1091–1099 (1992).
Petersen, M. C. & Shulman, G. I. Mechanisms of insulin action and insulin resistance. Physiol. Rev. 98, 2133–2223 (2018).
Fuchs, A. et al. Associations among adipose tissue immunology, inflammation, exosomes and insulin sensitivity in people with obesity and nonalcoholic fatty liver disease. Gastroenterology 161, 968–981.e12 (2021).
Horowitz, J. F. & Klein, S. Oxidation of nonplasma fatty acids during exercise is increased in women with abdominal obesity. J. Appl. Physiol. 89, 2276–2282 (2000).
Hari, A. et al. Exercise training rapidly increases hepatic insulin extraction in NAFLD. Med. Sci. Sports Exerc. 52, 1449–1455 (2020).
Wirth, A., Holm, G. & Björntorp, P. Effect of physical training on insulin uptake by the perfused rat liver. Metabolism 31, 457–462 (1982).
Frøsig, C. et al. Effects of endurance exercise training on insulin signaling in human skeletal muscle: interactions at the level of phosphatidylinositol 3-kinase, Akt, and AS160. Diabetes 56, 2093–2102 (2007).
Dabke, K., Hendrick, G. & Devkota, S. The gut microbiome and metabolic syndrome. J. Clin. Invest. 129, 4050–4057 (2019).
Vrieze, A. et al. Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology 143, 913–916.e7 (2012).
Kootte, R. S. et al. Improvement of insulin sensitivity after lean donor feces in metabolic syndrome is driven by baseline intestinal microbiota composition. Cell Metab. 26, 611–619.e6 (2017).
Smith, G. I., Mittendorfer, B. & Klein, S. Metabolically healthy obesity: facts and fantasies. J. Clin. Invest. 129, 3978–3989 (2019).
Le, T.-A. et al. Effect of colesevelam on liver fat quantified by magnetic resonance in nonalcoholic steatohepatitis: a randomized controlled trial. Hepatology 56, 922–932 (2012).
Smith, G. I., Commean, P. K., Reeds, D. N., Klein, S. & Mittendorfer, B. Effect of protein supplementation during diet-induced weight loss on muscle mass and strength: a randomized controlled study. Obesity 26, 854–861 (2018).
Donnelly, J. E. et al. American College of Sports Medicine Position Stand. Appropriate physical activity intervention strategies for weight loss and prevention of weight regain for adults. Med. Sci. Sports Exerc. 41, 459–471 (2009).
Mittendorfer, B., Horowitz, J. F. & Klein, S. Gender differences in lipid and glucose kinetics during short-term fasting. Am. J. Physiol. Endocrinol. Metab. 281, E1333–E1339 (2001).
Yamaguchi, S. et al. Adipose tissue NAD+ biosynthesis is required for regulating adaptive thermogenesis and whole-body energy homeostasis in mice. Proc. Natl Acad. Sci. USA 116, 23822–23828 (2019).
Gold, L. et al. Aptamer-based multiplexed proteomic technology for biomarker discovery. PLoS ONE 5, e15004 (2010).
McDonald, D. et al. American Gut: an open platform for citizen science microbiome research. mSystems 3, e00031-18 (2018).
Bolyen, E. et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 37, 852–857 (2019).
Janssen, S. et al. Phylogenetic placement of exact amplicon sequences improves associations with clinical information. mSystems 3, e00021-18 (2018).
Allison, D. B., Paultre, F., Maggio, C., Mezzitis, N. & Pi-Sunyer, F. X. The use of areas under curves in diabetes research. Diabetes Care 18, 245–250 (1995).
Van Cauter, E., Mestrez, F., Sturis, J. & Polonsky, K. S. Estimation of insulin secretion rates from C-peptide levels: comparison of individual and standard kinetic parameters for C-peptide clearance. Diabetes 41, 368–377 (1992).
Smith, G. I. et al. Influence of adiposity, insulin resistance, and intrahepatic triglyceride content on insulin kinetics. J. Clin. Invest. 130, 3305–3314 (2020).
Matthews, D. R. et al. Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28, 412–419 (1985).
Mittendorfer, B., Patterson, B. W., Smith, G. I., Yoshino, M. & Klein, S. β cell function and plasma insulin clearance in people with obesity and different glycemic status. J. Clin. Invest. 132, e154068 (2022).
Ritchie, M. E. et al. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 43, e47 (2015).
Kars, M. et al. Tauroursodeoxycholic acid may improve liver and muscle but not adipose tissue insulin sensitivity in obese men and women. Diabetes 59, 1899–1905 (2010).
Acknowledgements
We thank the staff of the Center for Human Nutrition and the CTRU at Washington University School of Medicine for assistance in conducting the metabolic studies and their technical assistance in processing and analyzing the study samples. We also thank the study participants for their participation. This study was supported by the National Institutes of Health grants P30 DK056341 (Washington University Nutrition and Obesity Research Center to S.K.), P30 DK020579 (Washington University Diabetes Research Center) and R01 DK104995 (NAD+ and metabolic flexibility to S.K.); postdoctoral training grants T32 HL130357 to J.W.B. and B.D.K., and T32 DK007120 to M.L.K.; and UL1 TR000448 (Washington University Institute of Clinical and Translational Sciences), as well as support from the Sam and Marilyn Fox Foundation to S.K.
Author information
Authors and Affiliations
Contributions
J.W.B., B.D.K., G.I.S., G.R., J.Y., R.K. and B.W.P. performed sample and data analyses. J.W.B., B.D.K., G.I.S., G.G.S., K.K. and M.L.K. conducted the clinical studies. G.I.S. and S.K. designed the study. J.W.B., B.D.K., G.I.S., B.W.P., R.K. and S.K. interpreted the data and wrote the manuscript. All authors critically reviewed and edited the paper.
Corresponding author
Ethics declarations
Competing interests
S.K. serves on scientific advisory boards for Altimmune and Merck. All other authors declare they have no competing interests.
Peer review
Peer review information
Nature Metabolism thanks Seth Creasy, Jason Gill and Emily Manoogian for their contribution to the peer review of this work. Primary Handling Editor: Ashley Castellanos-Jankiewicz, in collaboration with the Nature Metabolism team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1
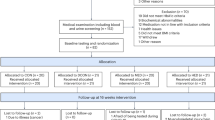
Schematic diagrams of overall study protocol (a), schedule of individual assessments before (b) and after (c) the intervention and the protocol for inpatient 24-h study blood sampling schedule and hyperinsulinemic-euglycemic clamp procedure (HECP) plus muscle biopsy (Bx) (d). Blood sample timepoints are indicated by arrows and running figure indicates time of a 60-min bout of exercise performed in the Diet+EX group after the intervention. M=meal.
Extended Data Fig. 2 Effect of each intervention on the gut microbiome.
Measures of alpha diversity using both Chao richness (top) and Shannon entropy (bottom), before (T0) and at ~1 month (T1, 5.2 ± 0.7% weight loss), ~2 months (T2, 8.6 ± 0.8% weight loss), and ~4-5 months (T3, 10.5 ± 2.7% weight loss) after starting the intervention in the Diet-ONLY (n = 7) and Diet+EX (n = 8) groups. Data are means ± SEM (a). Beta diversity using Distanced-based Redundancy Analysis of the weight UniFrac distance, conditional on the between-person variability (b). Log-ratio analysis of 10 ASVs that increased the most and the 10 ASVs that decreased the most from T0 to T1 with treatment groups combined shown over time when stratified by study group. Data are log-ratio means ± SEM (c).
Extended Data Fig. 3
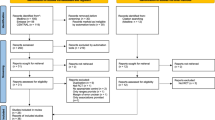
Flow of study participants.
Supplementary information
Supplementary Information
Study protocols.
Supplementary Tables 1–3
Contains supplementary tables 1, 2 and 3.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Statistical source data.
Source Data Fig. 6
Statistical source data.
Source Data Extended Data Fig./Table 1
Statistical source data.
Source Data Extended Data Fig./Table 2
Statistical source data.
Source Data Extended Data Fig./Table 3
Statistical source data.
Source Data Extended Data Fig./Table 4
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Beals, J.W., Kayser, B.D., Smith, G.I. et al. Dietary weight loss-induced improvements in metabolic function are enhanced by exercise in people with obesity and prediabetes. Nat Metab 5, 1221–1235 (2023). https://doi.org/10.1038/s42255-023-00829-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s42255-023-00829-4