Abstract
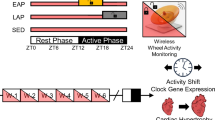
Physical endurance and energy conservation are essential for survival in the wild. However, it remains unknown whether and how meal timing regulates physical endurance and muscle diurnal rhythms. Here, we show that day/sleep time-restricted feeding (DRF) enhances running endurance by 100% throughout the circadian cycle in both male and female mice, compared to either ad libitum feeding or night/wake time-restricted feeding. Ablation of the circadian clock in the whole body or the muscle abolished the exercise regulatory effect of DRF. Multi-omics analysis revealed that DRF robustly entrains diurnal rhythms of a mitochondrial oxidative metabolism-centric network, compared to night/wake time-restricted feeding. Remarkably, muscle-specific knockdown of the myocyte lipid droplet protein perilipin-5 completely mimics DRF in enhancing endurance, enhancing oxidative bioenergetics and outputting rhythmicity to circulating energy substrates, including acylcarnitine. Together, our work identifies a potent dietary regimen to enhance running endurance without prior exercise, as well as providing a multi-omics atlas of muscle circadian biology regulated by meal timing.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
RNA-seq data in this paper have been deposited into the Gene Expression Omnibus under accession number GSE194106. The published human transcriptome data are available at GSE28422. The MS proteomics data have been deposited to the ProteomeXchange Consortium via the iProX59 partner repository under dataset identifier PXD039355. Graphs of these multi-omics data can be found at http://www.circametdb.org.cn/. Raw quantification data are deposited to the Science Data Bank (https://www.scidb.cn/en/). Source data are provided with this paper. Any other relevant data are available from the corresponding author upon request.
Code availability
Custom code is deposited to the Science Data Bank (https://doi.org/10.57760/sciencedb.08081).
References
Gabriel, B. M. & Zierath, J. R. Circadian rhythms and exercise—re-setting the clock in metabolic disease. Nat. Rev. Endocrinol. 15, 197–206 (2019).
Baskin, K. K., Winders, B. R. & Olson, E. N. Muscle as a ‘mediator’ of systemic metabolism. Cell Metab. 21, 237–248 (2015).
Fan, W. et al. PPARδ promotes running endurance by preserving glucose. Cell Metab. 25, 1186–1193 (2017).
Bass, J. & Lazar, M. A. Circadian time signatures of fitness and disease. Science 354, 994–999 (2016).
Dyar, K. A. et al. Muscle insulin sensitivity and glucose metabolism are controlled by the intrinsic muscle clock. Mol. Metab. 3, 29–41 (2014).
Schroder, E. A. et al. Intrinsic muscle clock is necessary for musculoskeletal health. J. Physiol. 593, 5387–5404 (2015).
Woldt, E. et al. Rev-erb-α modulates skeletal muscle oxidative capacity by regulating mitochondrial biogenesis and autophagy. Nat. Med. 19, 1039–1046 (2013).
Hong, S. et al. Dissociation of muscle insulin sensitivity from exercise endurance in mice by HDAC3 depletion. Nat. Med. 23, 223–234 (2017).
Takahashi, J. S. Transcriptional architecture of the mammalian circadian clock. Nat. Rev. Genet. 18, 164–179 (2017).
Jordan, S. D. et al. CRY1/2 selectively repress PPARδ and limit exercise capacity. Cell Metab. 26, 243–255 (2017).
Ezagouri, S. et al. Physiological and molecular dissection of daily variance in exercise capacity. Cell Metab. 30, 78–91 (2019).
Adamovich, Y. et al. Clock proteins and training modify exercise capacity in a daytime-dependent manner. Proc. Natl Acad. Sci. USA 118, e2101115118 (2021).
Sato, S. et al. Time of exercise specifies the impact on muscle metabolic pathways and systemic energy homeostasis. Cell Metab. 30, 92–110 (2019).
Sato, S. et al. Atlas of exercise metabolism reveals time-dependent signatures of metabolic homeostasis. Cell Metab. 34, 329–345 (2022).
Reinke, H. & Asher, G. Cross-talk between metabolism and circadian clocks. Nat. Rev. Mol. Cell Biol. 20, 227–241 (2019).
Guan, D. & Lazar, M. A. Interconnections between circadian clocks and metabolism. J. Clin. Invest. 131, e148278 (2021).
Allada, R. & Bass, J. Circadian mechanisms in medicine. N. Engl. J. Med. 384, 550–561 (2021).
Panda, S. Circadian physiology of metabolism. Science 354, 1008–1015 (2016).
Chaix, A., Manoogian, E. N. C., Melkani, G. C. & Panda, S. Time-restricted eating to prevent and manage chronic metabolic diseases. Annu. Rev. Nutr. 39, 291–315 (2019).
Li, M.-D. Clock-modulated checkpoints in time-restricted eating. Trends Mol. Med. 28, 25–35 (2022).
Manoogian, E. N., Chow, L. S., Taub, P. R., Laferrère, B. & Panda, S. Time-restricted eating for the prevention and management of metabolic diseases. Endocr. Rev. 49, 405–436 (2021).
Petersen, M. C. et al. Complex physiology and clinical implications of time-restricted eating. Physiol. Rev. 102, 1991–2034 (2022).
Chaix, A., Deota, S., Bhardwaj, R., Lin, T. & Panda, S. Sex- and age-dependent outcomes of 9-hour time-restricted feeding of a Western high-fat high-sucrose diet in C57BL/6J mice. Cell Rep. 36, 109543 (2021).
Damiola, F. et al. Restricted feeding uncouples circadian oscillators in peripheral tissues from the central pacemaker in the suprachiasmatic nucleus. Genes Dev. 14, 2950–2961 (2000).
Stokkan, K.-A., Yamazaki, S., Tei, H., Sakaki, Y. & Menaker, M. Entrainment of the circadian clock in the liver by feeding. Science 291, 490–493 (2001).
Xin, H. et al. A multi-tissue multi-omics analysis reveals distinct kinetics in entrainment of diurnal transcriptomes by inverted feeding. iScience 24, 102335 (2021).
Manella, G. et al. The liver-clock coordinates rhythmicity of peripheral tissues in response to feeding. Nat. Metab. 3, 829–842 (2021).
Zhang, Z., Shui, G. & Li, M. -D. Time to eat reveals the hierarchy of peripheral clocks. Trends Cell Biol. 31, 869–872 (2021).
Al-Nawaiseh, A. M., Bataineh, M. F., Kilani, H. A., Bellar, D. M. & Judge, L. W. Time-restricted feeding and aerobic performance in elite runners: Ramadan fasting as a model. Front. Nutr. 8, 718936 (2021).
Marcaletti, S., Thomas, C. & Feige, J. N. Exercise performance tests in mice. Curr. Protoc. Mouse Biol. 1, 141–154 (2011).
Schiaffino, S. & Reggiani, C. Fiber types in mammalian skeletal muscles. Physiol. Rev. 91, 1447–1531 (2011).
Tabebordbar, M. et al. Directed evolution of a family of AAV capsid variants enabling potent muscle-directed gene delivery across species. Cell 184, 4919–4938 (2021).
Opperhuizen, A. L. et al. Feeding during the resting phase causes profound changes in physiology and desynchronization between liver and muscle rhythms of rats. Eur. J. Neurosci. 44, 2795–2806 (2016).
Mukherji, A. et al. Shifting eating to the circadian rest phase misaligns the peripheral clocks with the master SCN clock and leads to a metabolic syndrome. Proc. Natl Acad. Sci. USA 112, E6691–E6698 (2015).
Schmitt, K. et al. Circadian control of DRP1 activity regulates mitochondrial dynamics and bioenergetics. Cell Metab. 27, 657–666 (2018).
Ohta, H., Yamazaki, S. & McMahon, D. G. Constant light desynchronizes mammalian clock neurons. Nat. Neurosci. 8, 267–269 (2005).
Mason, R. R. & Watt, M. J. Unraveling the roles of PLIN5: linking cell biology to physiology. Trends Endocrinol. Metab. 26, 144–152 (2015).
Wolins, N. E. et al. OXPAT/PAT-1 is a PPAR-induced lipid droplet protein that promotes fatty acid utilization. Diabetes 55, 3418–3428 (2006).
Simcox, J. et al. Global analysis of plasma lipids identifies liver-derived acylcarnitines as a fuel source for brown fat thermogenesis. Cell Metab. 26, 509–522 (2017).
Levine, D. C. et al. NADH inhibition of SIRT1 links energy state to transcription during time-restricted feeding. Nat. Metab. 3, 1621–1632 (2021).
Yasumoto, Y. et al. Short-term feeding at the wrong time is sufficient to desynchronize peripheral clocks and induce obesity with hyperphagia, physical inactivity and metabolic disorders in mice. Metabolism 65, 714–727 (2016).
Arble, D. M., Bass, J., Laposky, A. D., Vitaterna, M. H. & Turek, F. W. Circadian timing of food intake contributes to weight gain. Obesity 17, 2100–2102 (2009).
Acosta-Rodríguez, V. A., de Groot, M. H. M., Rijo-Ferreira, F., Green, C. B. & Takahashi, J. S. Mice under caloric restriction self-impose a temporal restriction of food intake as revealed by an automated feeder system. Cell Metab. 26, 267–277 (2017).
Acosta-Rodríguez, V. et al. Circadian alignment of early onset caloric restriction promotes longevity in male C57BL/6J mice. Science 376, 1192–1202 (2022).
Murphy, R. M., Watt, M. J. & Febbraio, M. A. Metabolic communication during exercise. Nat. Metab. 2, 805–816 (2020).
Hargreaves, M. & Spriet, L. L. Skeletal muscle energy metabolism during exercise. Nat. Metab. 2, 817–828 (2020).
Meier, F. et al. diaPASEF: parallel accumulation–serial fragmentation combined with data-independent acquisition. Nat. Methods 17, 1229–1236 (2020).
Song, J. W. et al. Omics-driven systems interrogation of metabolic dysregulation in COVID-19 pathogenesis. Cell Metab. 32, 188–202 (2020).
Chen, Y. et al. Proteomic analysis identifies prolonged disturbances in pathways related to cholesterol metabolism and myocardium function in the COVID-19 recovery stage. J. Proteome Res. 20, 3463–3474 (2021).
Lundell, L. S. et al. Time-restricted feeding alters lipid and amino acid metabolite rhythmicity without perturbing clock gene expression. Nat. Commun. 11, 4643 (2020).
Narkar, V. A. et al. AMPK and PPARδ agonists are exercise mimetics. Cell 134, 405–415 (2008).
Xin, H. et al. Protocol for setup and circadian analysis of inverted feeding in mice. STAR Protoc. 2, 100701 (2021).
Zhang, Z. et al. Impaired function of the suprachiasmatic nucleus rescues the loss of body temperature homeostasis caused by time-restricted feeding. Sci. Bull. 65, 1268–1280 (2020).
Storch, K. F. et al. Intrinsic circadian clock of the mammalian retina: importance for retinal processing of visual information. Cell 130, 730–741 (2007).
Meng, Z. X. et al. Baf60c drives glycolytic metabolism in the muscle and improves systemic glucose homeostasis through Deptor-mediated Akt activation. Nat. Med. 19, 640–645 (2013).
Tian, H. et al. Precise metabolomics reveals a diversity of aging‐associated metabolic features. Small Methods 6, e2200130 (2022).
Lam, S. M. et al. A multi-omics investigation of the composition and function of extracellular vesicles along the temporal trajectory of COVID-19. Nat. Metab. 3, 909–922 (2021).
Xin, H. et al. Circadian signatures of adipose tissue in diet-induced obesity. Front. Physiol. 13, 953237 (2022).
Chen, T. et al. iProX in 2021: connecting proteomics data sharing with big data. Nucleic Acids Res. 50, D1522–D1527 (2022).
Acknowledgements
Per1/Per2-DKO mice were gifted from E. E. Zhang, and bred by J. R. He. Bmal1fl/fl mice were gifted from W. He. We thank D. Zhu, Z. Gan, Z. Meng and L. Xie for suggestions. This study was supported by National Natural Science Foundation of China Grants (32271208 to M.-D.L., 92057109 to M.-D.L., 81900776 to M.-D.L. and 32150005 to C.C.L.W.), Chongqing Science Fund for Distinguished Young Scholars (CSTB2022NSCQ-JQX0009 to M.-D.L.), National Key Research and Development Programme of China (2022YFA0806001 to C.C.L.W.), CAMS Innovation Fund for Medical Sciences (2022-I2M-1-004 to C.C.L.W.).
Author information
Authors and Affiliations
Contributions
Conceptualization: M.-D.L.; Methodology: H.X., R.H., M.Z., J.C., G.S., C.C.L.W. and M.-D.L.; Investigation: M.-D.L., H.X., R.H., M.Z., J.C., T.Z., S.J., X.L., H.T., S.M.L., X.B., J.Z. and L.L.; Funding acquisition: M.-D.L. and C.C.L.W.; Project administration: M.-D.L.; Resources: M.-D.L., C.C.L.W., G.S., S.T., F.D. and Z.Z.; Supervision: M.-D.L., C.C.L.W. and G.S.; Writing—original draft: M.-D.L., H.X., R.H., M.Z. and J.C.; Writing—review and editing: M.-D.L., C.C.L.W. and G.S.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Metabolism thanks Chih-Hao Lee, Kristin Stanford and the other, anonymous, reviewer for their contribution to the peer review of this work. Primary Handling Editor: Ashley Castellanos-Jankiewicz, in collaboration with the Nature Metabolism team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Exercise phenotyping in time-restricted fed mice.
a, Running power in 3-week TRF female mice, as measured by time to exhaustion, distance, cumulative stimulations, and maximum speed (n = 14 NRF or 16 DRF mice). b, Running endurance in the early night (ZT14, n = 15 NRF or 16 DRF mice), as measured by time to exhaustion and distance. c, Running endurance in 3-week DRF mice (Male: n = 15 mice; Female: n = 16 mice), as measured by time to exhaustion, distance, and blood glucose. d, Running power in 3-week DRF mice (Male: n = 15 AL or 16 DRF mice; Female: n = 16 mice), as measured by time to exhaustion, distance, and maximum speed. e, Immunofluorescence (IF) of slow- (type 1)/fast- (type 2B, 2 A, 2X) myofibers and histochemical staining of bioenergetic enzyme activity in soleus muscle after 3 weeks of TRF (n = 8 mice, one image per mouse). MyHC, myosin heavy chain. SDH, succinate dehydrogenase. GPDH, alpha-glycerophosphate dehydrogenase. Arrows indicate positively stained fibers. Scale bars, 200 µm. f, Total locomotor activity in 3-week TRF female mice (n = 9 AL or 8 DRF/NRF mice). g, Respiratory exchange ratio (RER) in 1-week TRF female mice (n = 4 mice). h, The scheme for testing endurance in trained female mice (n = 12 mice, slope: 15o). i, Myofiber typing in GA muscle from ad libitum (AL)-fed exercised (Exe) mice or TRF-fed sedentary (Sed) mice, as measured by IF and bioenergetic enzyme activity (n = 11 Sed-NRF, 12 Sed-DRF (IF/GPDH), 11 Sed-DRF (SDH) or 16 Exe-AL mice). TRF, time-restricted feeding; NRF, night/wake time-restricted feeding; DRF, day/sleep time-restricted feeding (food available from ZT0 to ZT12); AL, ad libitum feeding. Data were represented as the mean ± s.e.m. Statistical tests: ANOVA, post-hoc Sidak/Tukey test, Kruskal-Wallis test, or two-sided unpaired Student’s/Wilcoxon test; *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
Extended Data Fig. 2 Striated muscle-specific knockdown of Per2 gene by systemic administration of MyoAAV does not alter running endurance under DRF in female mice.
a, Daytime locomotor activity in Per1−/− Per2−/− (Per1/2 DKO) male mice before and after 3 weeks of DRF beginning at the age of 34 weeks (3 weeks AL feeding > 3 weeks DRF) (n = 8 mice), as measured by percentage of daytime activity and 24-h beam breaks. b, Daytime locomotor activity in Per2−/− female mice before and after 3 weeks of DRF starting at the age of 7 weeks (n = 10 mice). c, RT-qPCR analysis of muscle and liver tissues in MyoAAV-transduced (MKD) female mice under DRF (N = 5 Ctrl-MKD mice, N = 6 Per2-MKD mice). d, Representative immunofluorescence image of PER2 protein in TA muscle tissue. This experiment was repeated independently twice with similar results (n = 200 cells examined over two independent experiments). GA muscle from Per1/2 DKO mice, as well as mock primary antibody-treated Per2-MKD, serves as the negative control. e, Daytime locomotor activity in MKD female mice before and after 3 weeks of DRF starting at the age of 8 weeks (n = 5 Ctrl-MKD or 6 Per2-MKD mice). f, Running endurance of MKD female mice in the early day (ZT2, n = 16 mice), as measured by time to exhaustion, distance and cumulative stimulations. Mice received MyoAAV by tain vein injection at the age of 8 weeks, tested 3 weeks later (AL), and fed DRF for 3 weeks before the next running test. g, Tissue mass in Bmal1 or Per2 MKO mice under DRF (n = 8 control or 7 MKO mice). Per2-MKD, striated muscle-specific knockdown of Per2; Ctrl-MKD, control group. Data are presented as the mean ± s.e.m. or minima, maxima, median, 25th and 75th percentiles. Statistical tests: two-sided paired Student’s t-test, ANOVA, post-hoc Sidak’s test, or two-sided Wilcoxon test, *P < 0.05, **P < 0.01, ***P < 0.001.
Extended Data Fig. 3 General features and circadian clock oscillation of the multi-omics dataset.
a, Exercise performance in 1-week TRF mice as measured by time to exhaustion (n = 16 mice). b, Workflow of the multi-omics study. c-d, Diurnal expression of the muscle clock genes in TRF mice (c, female: n = 4 mice; d, male: n = 4 mice). e, Phase distribution of oscillating muscle genes and phase-shift between dual-oscillating genes in TRF mice. f-h, Diurnal expression of dual-oscillating (f), DRF-only (g), NRF-only (g), and mitochondrial (h) rhythmic genes in skeletal muscle (n = 4 mice per time-point). Data were represented as the mean ± s.e.m. or minima, maxima, median, 25th and 75th percentiles. Statistical test: ANOVA, two-sided unpaired Student’s t-test with Bonferroni correction, MetaCycle: P-values from JTK and LS methods were adjusted by Fisher’s method.
Extended Data Fig. 4 Global profiling of muscle metabolites.
Heatmap showing cycling metabolites in GA muscle during TRF. Inset shows phase distribution of muscle cycling metabolites.
Extended Data Fig. 5 Global profiling of muscle lipids and serum metabolites.
a, Heatmap showing cycling lipid species in GA muscle. Inset shows phase distribution of muscle cycling lipids. Abbreviations see Data S4. b, Diurnal expression of cycling serum metabolites (n = 7 mice for 2 time points). c, Principal component analysis of the four groups. D00, DRF ZT0; D12, DRF ZT12; N00, NRF ZT0; N12, NRF ZT12.
Extended Data Fig. 6 Global profiling of the muscle proteome.
a, 48-h profile of cycling muscle proteins from 3-week DRF mice. Samples were collected every 2 h for 48 h containing 25 time points (n = 2 mice per time point). b, Representative cycling muscle proteins (n = 2 mice per time point). Data were represented as mean and points. c, Representative immunoblots of pSer637-DRP1 in anterior hypothalamus (aHyp) of the brain in ad libitum fed mice (n = 2 mice, repeated three times with similar results). ALP, alkaline phosphatase treatment. d, Immunoblot analysis of key regulators involved in mitochondrial dynamics by DRF in GA muscle. Data were represented as the mean ± s.e.m. MetaCycle: P-values from JTK and LS methods were adjusted by Fisher’s method.
Extended Data Fig. 7 Daily rhythms of acylcarnitine and amino acids persist in the muscle of 3-week DRF female mice.
a, Heatmap showing cycling metabolites in GA muscle from 3-week DRF female mice. b, Class of cycling metabolites in muscle. c, Heatmap showing cycling acylcarnitines, amino acids and fatty acids. d, Daily abundance levels of selected cycling muscle metabolites representing acylcarnitines and amino acids. Stearoylcarnitine, 18:0-carnitine; L-Palmitoylcarnitine, 16:0-carnitine. Samples were collected every 2 h for 48 h containing 25 time points (n = 2 mice per time point). Data were shown as the mean ± s.e.m. Statistical tests: RAIN and MetaCycle.
Extended Data Fig. 8 Diurnal expression of rhythmic transcripts in DRF muscle.
a, Diurnal expression of Pdp2 and Hmgcs2 in GA muscle from TRF female mice, as measured by RNA sequencing (n = 4 mice). b, Diurnal gene expression in GA muscle from TRF female mice (n = 4 mice). c, Diurnal gene expression in GA muscle from TRF male mice (n = 4 mice). d, Diurnal gene expression in GA muscle from female mice subjected to DRF and constant lightness (n = 4 mice). e, Diurnal gene expression in GA muscle from Per1−/−Per2−/− mutant mice subjected to constant darkness and DRF (N = 14 mice, n = 2 mice per time point for 7 time points). CT, circadian time, or hours under constant darkness; τ, period length. f, Gene expression in vastus lateralis muscle biopsies before (T1, T3) and after (T2, T4) resistance exercise. Y (young, n = 8 males/8 females), O (old, n = 6 males/6 females); T1/T2 is from the sedentary state, T3/T4 is from the trained state (Source: GSE28422). Data are presented as mean ± s.e.m. or minima, maxima, median, 25th and 75th percentiles. Statistical tests: multiple t-tests with Bonferroni correction, MetaCycle (rhythmicity), Circacompare (phase shift), or ANOVA, two-sided Wilcoxon test; ns, not significant, AR, arrhythmia.
Extended Data Fig. 9 The role of myocyte Plin5 in the regulation of exercise capacity under DRF or ad libitum.
a, Treadmill running scheme for studying the role of myocyte Plin5 in exercise capacity. KD, TA muscle-specific knockdown of Plin5 gene; WT, control group dosed with negative control miRNA. b, Representative immunoblots of PLIN5 protein in different muscle depots from the ipsilateral and contralateral sides of the AAV injection site (one technical replicate of two biological replicates). c, Running power in female mice transduced with AAV-pre-miR-Plin5 (KD) or AAV-pre-miR-Ctrl (WT) and fed ad libitum (AL) or DRF for 3 weeks (n = 16 mice), as measured by time to exhaustion, distance, maximum speed, and cumulative stimulations. d, Cumulative stimulations and body weight in the endurance test for KD and WT mice fed AL or DRF for 3 weeks (n = 16 mice). Data were represented as the mean ± s.e.m. 2-way ANOVA or two-sided unpaired Student’s t test, *P < 0.05.
Extended Data Fig. 10 Muscle phenotyping in Plin5 KD mice.
a, Running endurance in DRF Plin5 KD mice resuming AL feeding for 1 to 3 weeks (n = 16 mice), as measured by time to exhaustion, distance, and cumulative number of stimulations. b, Running endurance and histochemical staining of bioenergetic enzyme activity in GA muscle from diet reversed (DRF > AL, return to AL for 3 weeks after 3 weeks of DRF) and DRF mice (n = 16 mice, one image per mouse). Scale bars, 100 μm. c, Representative immunoblots for PLIN5 in GA muscle and heart (HT) of MKD mice (n = 5 mice). d, Distribution of phase-shift among dual-oscillating muscle genes in KD and WT mice. KD, TA-specific Plin5 knockdown. e, Diurnal expression of circadian clock genes in the muscle (n = 2 mice for 8 time points). f, Pathway analysis of differentially expressed muscle genes from ZT21 to ZT3 between KD and WT mice (n = 6 mice). Data were represented as the mean ± s.e.m. 2-way ANOVA or two-sided unpaired Student’s t test, *P < 0.05, ****P < 0.0001.
Supplementary information
Supplementary Information
Supplementary Methods and Supplementary Table 4
Supplementary Tables 1–3
Table 1: Rhythmicity results of genes, proteins, metabolites and lipids. Table 2: PSEA results of oscillating genes in skeletal muscle. Table 3: Rhythmicity results of metabolites in the serum.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Statistical source data.
Source Data Fig. 6
Statistical source data.
Source Data Fig. 7
Statistical source data.
Source Data Fig. 8
Statistical source data.
Source Data Extended Data Fig. 1
Statistical source data.
Source Data Extended Data Fig. 2
Statistical source data.
Source Data Extended Data Fig. 3
Statistical source data.
Source Data Extended Data Fig. 4
Statistical source data.
Source Data Extended Data Fig. 5
Statistical source data.
Source Data Extended Data Fig. 6
Statistical source data.
Source Data Extended Data Fig. 7
Statistical source data.
Source Data Extended Data Fig. 8
Unprocessed western blots and statistical source data.
Source Data Extended Data Fig. 9
Unprocessed western blots and statistical source data.
Source Data Extended Data Fig. 10
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xin, H., Huang, R., Zhou, M. et al. Daytime-restricted feeding enhances running endurance without prior exercise in mice. Nat Metab 5, 1236–1251 (2023). https://doi.org/10.1038/s42255-023-00826-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s42255-023-00826-7
This article is cited by
-
Lipid droplets in pathogen infection and host immunity
Acta Pharmacologica Sinica (2024)
-
Multi-omics profiling reveals rhythmic liver function shaped by meal timing
Nature Communications (2023)
-
Time-restricted feeding makes the mouse run like a pro
Nature Metabolism (2023)