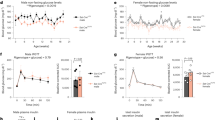
Abstract
Little is known about regulated glucagon secretion by human islet α-cells compared to insulin secretion from β-cells, despite conclusive evidence of dysfunction in both cell types in diabetes mellitus. Distinct insulins in humans and mice permit in vivo studies of human β-cell regulation after human islet transplantation in immunocompromised mice, whereas identical glucagon sequences prevent analogous in vivo measures of glucagon output from human α-cells. Here, we use CRISPR–Cas9 editing to remove glucagon codons 2–29 in immunocompromised NSG mice, preserving the production of other proglucagon-derived hormones. Glucagon knockout NSG (GKO-NSG) mice have metabolic, liver and pancreatic phenotypes associated with glucagon-signalling deficits that revert after transplantation of human islets from non-diabetic donors. Glucagon hypersecretion by transplanted islets from donors with type 2 diabetes revealed islet-intrinsic defects. We suggest that GKO-NSG mice provide an unprecedented resource to investigate human α-cell regulation in vivo.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request. Source data for Fig.1 are presented with the paper.
References
Gromada, J., Franklin, I. & Wollheim, C. B. α-cells of the endocrine pancreas: 35 years of research but the enigma remains. Endocr. Rev. 28, 84–116 (2007).
McKnight, K. D., Wang, P. & Kim, S. K. Deconstructing pancreas development to reconstruct human islets from pluripotent stem cells. Cell Stem Cell 6, 300–308 (2010).
Xin, Y. et al. RNA sequencing of single human islet cells reveals type 2 diabetes genes. Cell Metab. 24, 608–615 (2016).
Arda, H. E. et al. Age-dependent pancreatic gene regulation reveals mechanisms governing human β cell function. Cell Metab. 23, 909–920 (2016).
Rodriguez-Diaz, R. et al. Alpha cells secrete acetylcholine as a non-neuronal paracrine signal priming beta cell function in humans. Nat. Med 17, 888–892 (2011).
Ishikawa, F. et al. Development of functional human blood and immune systems in NOD/SCID/IL2 receptor ɣ chainnull mice. Blood 106, 1565–1573 (2005).
Shultz, L. D. et al. Human lymphoid and myeloid cell development in NOD/LtSz-scid IL2Rɣ null mice engrafted with mobilized human hemopoietic stem cells. J. Immunol. 174, 6477–6489 (2005).
Dai, C. et al. Age-dependent human β cell proliferation induced by glucagon-like peptide 1 and calcineurin signaling. J. Clin. Invest. 127, 3835–3844 (2017).
Dai, C. et al. Stress-impaired transcription factor expression and insulin secretion in transplanted human islets. J. Clin. Invest. 126, 1857–1870 (2016).
Peiris, H. et al. Discovering human diabetes-risk gene function with genetics and physiological assays. Nat. Commun. 9, 3855 (2018).
Bell, G. I., Sanchez-Pescador, R., Laybourn, P. J. & Najarian, R. C. Exon duplication and divergence in the human preproglucagon gene. Nature 304, 368–371 (1983).
Heinrich, G., Gros, P. & Habener, J. F. Glucagon gene sequence. Four of six exons encode separate functional domains of rat pre-proglucagon. J. Biol. Chem. 259, 14082–14087 (1984).
Drucker, D. J., Philippe, J., Mojsov, S., Chick, W. L. & Habener, J. F. Glucagon-like peptide I stimulates insulin gene expression and increases cyclic AMP levels in a rat islet cell line. Proc. Natl Acad. Sci. USA 84, 3434–3438 (1987).
Holst, J. J. The physiology of glucagon-like peptide 1. Physiol. Rev. 87, 1409–1439 (2007).
Cho, Y. M., Fujita, Y. & Kieffer, T. J. Glucagon-like peptide-1: glucose homeostasis and beyond. Annu. Rev. Physiol. 76, 535–559 (2014).
Drucker, D. J., Habener, J. F. & Holst, J. J. Discovery, characterization, and clinical development of the glucagon-like peptides. J. Clin. Invest. 127, 4217–4227 (2017).
Knop, F. K. EJE PRIZE 2018: A gut feeling about glucagon. Eur. J. Endocrinol. 178, R267–R280 (2018).
Hayashi, Y. et al. Mice deficient for glucagon gene-derived peptides display normoglycemia and hyperplasia of islet α-cells but not of intestinal L-cells. Mol. Endocrinol. 23, 1990–1999 (2009).
Gelling, R. W. et al. Lower blood glucose, hyperglucagonemia, and pancreatic cell hyperplasia in glucagon receptor knockout mice. Proc. Natl Acad. Sci. USA 100, 1438–1443 (2003).
Solloway, M. J. et al. Glucagon couples hepatic amino acid catabolism to mTOR-dependent regulation of α-cell mass. Cell Rep. 12, 495–510 (2015).
Dean, E. D. et al. Interrupted glucagon signaling reveals hepatic α cell axis and role for l-glutamine in α cell proliferation.Cell Metab. 25, 1362–1373 (2017).
Kim, J. et al. Amino acid transporter Slc38a5 controls glucagon receptor inhibition-induced pancreatic α cell hyperplasia in mice. Cell Metab. 25, 1348–1361 (2017).
Furuta, M. et al. Defective prohormone processing and altered pancreatic islet morphology in mice lacking active SPC2. Proc. Natl Acad. Sci. USA 94, 6646–6651 (1997).
Webb, G. C., Akbar, M. S., Zhao, C., Swift, H. H. & Steiner, D. F. Glucagon replacement via micro-osmotic pump corrects hypoglycemia and alpha-cell hyperplasia in prohormone convertase 2 knockout mice. Diabetes 51, 398–405 (2002).
Fowden, A. L. The role of insulin in fetal growth. Early Hum. Dev. 29, 177–181 (1992).
Milner, R. D. G. & Hill, D. J. Fetal growth control: the role of insulin and related peptides. Clin. Endocrinol. 21, 415–433 (1984).
Vuguin, P. M. et al. Ablation of the glucagon receptor gene increases fetal lethality and produces alterations in islet development and maturation. Endocrinology 147, 3995–4006 (2006).
Ouhilal, S. et al. Hypoglycemia, hyperglucagonemia, and fetoplacental defects in glucagon receptor knockout mice: a role for glucagon action in pregnancy maintenance. Am. J. Physiol. Metab. 302, E522–E531 (2012).
Cheng, X. et al. Glucagon contributes to liver zonation. Proc. Natl Acad. Sci. USA 115, E4111–E4119 (2018).
Ohneda, A., Aguilar-Parada, E., Eisentraut, A. M. & Unger, R. H. Control of pancreatic glucagon secretion by glucose. Diabetes 18, 1–10 (1969).
Miller, R. A. et al. Targeting hepatic glutaminase activity to ameliorate hyperglycemia. Nat. Med. 24, 518–524 (2018).
Holst, J. J., Wewer Albrechtsen, N. J., Pedersen, J. & Knop, F. K. Glucagon and amino acids are linked in a mutual feedback cycle: the liver-α-cell axis. Diabetes 66, 235–240 (2017).
Hancock, A. S., Du, A., Liu, J., Miller, M. & May, C. L. Glucagon deficiency reduces hepatic glucose production and improves glucose tolerance in adult mice. Mol. Endocrinol. 24, 1605–1614 (2010).
Vincent, M. et al. Abrogation of protein convertase 2 activity results in delayed islet cell differentiation and maturation, increased α-cell proliferation, and islet neogenesis. Endocrinology 144, 4061–4069 (2003).
Artner, I. et al. MafB: an activator of the glucagon gene expressed in developing islet α- and β-cells. Diabetes 55, 297–304 (2006).
Schaum, N. et al. Single-cell transcriptomics of 20 mouse organs creates a Tabula Muris. Nature 562, 367–372 (2018).
Sørensen, H. et al. Immunoneutralization of endogenous glucagon reduces hepatic glucose output and improves long-term glycemic control in diabetic ob/ob mice. Diabetes 55, 2843–8 (2006).
Svendsen, B. et al. Insulin secretion depends on intra-islet glucagon signaling. Cell Rep. 25, 1127–1134.e2 (2018).
Capozzi, M. E. et al. Glucagon lowers glycemia when β-cells are active. JCI Insight 5, e129954 (2019).
Müller, W. A., Faloona, G. R., Aguilar-Parada, E. & Unger, R. H. Abnormal alpha-cell function in diabetes—response to carbohydrate and protein ingestion. N. Engl. J. Med 283, 109–115 (1970).
Reaven, G. M., Chen, Y.-D. I., Golay, A., Swislocki, A. L. M. & Jaspan, J. B. Documentation of hyperglucagonemia throughout the day in nonobese and obese patients with noninsulin-dependent diabetes mellitus. J. Clin. Endocrinol. Metab. 64, 106–110 (1987).
Bozadjieva, N. et al. Loss of mTORC1 signaling alters pancreatic α cell mass and impairs glucagon secretion. J. Clin. Invest. 127, 4379–4393 (2017).
Müller, T. D., Finan, B., Clemmensen, C., DiMarchi, R. D. & Tschöp, M. H. The new biology and pharmacology of glucagon. Physiol. Rev. 97, 721–766 (2017).
Rodriguez-Diaz, R. et al. Paracrine interactions within the pancreatic islet determine the glycemic set point. Cell Metab. 27, 549–558.e4 (2018).
Schwartz, N. S., Clutter, W. E., Shah, S. D. & Cryer, P. E. Glycemic thresholds for activation of glucose counterregulatory systems are higher than the threshold for symptoms. J. Clin. Invest. 79, 777–81 (1987).
Efsun Arda, H. et al. A chromatin basis for cell lineage and disease risk in the human pancreas.Cell Syst. 7, 310–322.e4 (2018).
Brissova, M. et al. α cell function and gene expression are compromised in type 1 diabetes. Cell Rep. 22, 2667–2676 (2018).
Camunas-Soler, J. et al. Patch-seq links single-cell transcriptomes to human islet dysfunction in diabetes phenotypes. Cell Metab. 31, 1017–1031 (2020).
van der Meulen, T. et al. Urocortin3 mediates somatostatin-dependent negative feedback control of insulin secretion. Nat. Med. 21, 769–776 (2015).
Arrojo E Drigo, R. et al. Structural basis for delta cell paracrine regulation in pancreatic islets. Nat. Commun. 10, 3700 (2019).
Vergari, E. et al. Insulin inhibits glucagon release by SGLT2-induced stimulation of somatostatin secretion. Nat. Commun. 10, 139 (2019).
Cejvan, K., Coy, D. H. & Efendic, S. Intra-islet somatostatin regulates glucagon release via type 2 somatostatin receptors in rats. Diabetes 52, 1176–1181 (2003).
Hauge-Evans, A. C. et al. Somatostatin secreted by islet δ-cells fulfills multiple roles as a paracrine regulator of islet function. Diabetes 58, 403–411 (2009).
Patton, G. S. et al. Pancreatic immunoreactive somatostatin release. Proc. Natl Acad. Sci. USA 74, 2140–2143 (1977).
Weir, G. C., Samols, E., Day, J. A. & Patel, Y. C. Glucose and glucagon stimulate the secretion of somatostatin from the perfused canine pancreas. Metabolism 27, 1223–1226 (1978).
Kroon, E. et al. Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo. Nat. Biotechnol. 26, 443–452 (2008).
Micallef, S. J. et al. INSGFP/w human embryonic stem cells facilitate isolation of in vitro derived insulin-producing cells. Diabetologia 55, 694–706 (2012).
Basford, C. L. et al. The functional and molecular characterisation of human embryonic stem cell-derived insulin-positive cells compared with adult pancreatic beta cells. Diabetologia 55, 358–371 (2012).
Pagliuca, F. W. et al. Generation of functional human pancreatic β cells in vitro. Cell 159, 428–439 (2014).
Nair, G. G. et al. Recapitulating endocrine cell clustering in culture promotes maturation of human stem-cell-derived β cells. Nat. Cell Biol. 21, 263–274 (2019).
Chakravarthy, H. et al. Converting adult pancreatic islet α cells into β cells by targeting both Dnmt1 and Arx. Cell Metab. 25, 622–634 (2017).
Pauerstein, P. T. et al. A radial axis defined by semaphorin-to-neuropilin signaling controls pancreatic islet morphogenesis. Development 144, 3744–3754 (2017).
Acknowledgements
We thank past and current members of the Kim group for advice and encouragement, S. Park for assistance with gene targeting, K. Abraham (NIDDK/NIH) for guidance in the initial stages of this work, O. McGuinness and the Vanderbilt University Medical Center Hormone Core (DK059637 and DK020593) for amino acid measurements and advice, E. Walker for advice on glycogen quantification, the Stanford University Veterinary Service Center for animal care and advice, C. Sabatti and the Stanford Department of Biomedical Data Science Data Studio for advice on statistical analyses, the Stanford Cell Sciences Imaging Facility for microscope usage, and D. Serreze (The Jackson Laboratory) for generation of mouse lines. We also thank the Integrated Islet Distribution Program (UC4 DK098085-02), Alberta Diabetes Institute IsletCore, and International Institute for the Advancement of Medicine for processing and coordinating human islet distribution. This work was supported by the Type 1 Diabetes Mouse Resource (1UC4DK097610 to D. Serreze), a graduate research fellowship award from the National Science Foundation (DGF-114747 to K. Tellez), RO1 awards (DK107507; DK108817; CA21192701 to S. K. Kim) and a U01 award (DK120447 to P. MacDonald, University of Alberta). Work in the Stein lab was supported by NIH grants (DK106755, DK050203, and DK090570 to R. W. Stein). Work in the Kim lab was also supported by NIH grant P30 DK116074, the HL Snyder Foundation, the Mulberry Foundation, a gift from S. and M. Kirsch, and by the Stanford Islet Research Core, and the Diabetes Genomics and Analysis Core of the Stanford Diabetes Research Center.
Author information
Authors and Affiliations
Contributions
S.K.K., K.T. and Y.H. conceptualized the study; K.T., Y.H. and S.K.K. designed the methodology; K.T., Y.H. and X.G validated the results; K.T. and X.G. performed the formal analysis; K.T., Y.H., X.G. and C.A.C. carried out the investigation; K.T. curated the data; K.T. and S.K.K. wrote the original draft; K.T., Y.H., R.W.S., and S.K.K. contributed to writing, reviewing and editing the manuscript; K.T. and Y.H. carried out visualization studies; R.W.S. and S.K.K supervised the study; S.K.K. administered the project and S.K.K acquired funding for the study.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Primary Handling Editor: Elena Bellafante.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Design and characterization of GKO-NSG mice.
Related to Fig. 1. (a) Sequence from GKO-NSG founder (18-1) showing an in-frame deletion of 93 base pairs within exon 3 of the Gcg gene compared to the wild type NSG sequence (WT). Pink bar on top depicts exon 3 of Gcg. Blue bar represents nucleotide sequences encoding mature glucagon peptide. Red-highlighted dashes indicate deleted nucleotides in founder 18-1. (b) Representative immunostaining of GKO-NSG pancreatic islets with antibodies raised against mature glucagon (GCG, green) and proglucagon (Pro-GCG, red) - peptide sequences of GLP-1 (7-17). Similar results were seen across n = 3 NSG littermate control, n = 3 GKO-NSG, and n = 2 GKO-NSG Tx mice. (c) Body weight of male and female GKO-NSG and NSG control littermates at 3 and 8-weeks of age (3-week old female mice P = 0.025667 and 3-week old male mice P = 0.000454 by Repeated Measures ANOVA, with Tukey’s multiple comparisons test) (NSG mice, n = 7 males and 5 females; GKO-NSG mice, n = 11 males and 6 females). (d) Blood glucose measures of 2-3 month old GKO-NSG and NSG control mice during ad libitum feeding or after a 3-hour fast (fed: P = 0.000223; fasted: P = 0.003003 by Repeated Measures ANOVA, with Bonferroni’s multiple comparisons test) (NSG mice, n = 6 males and 4 females; GKO-NSG mice, n = 4 males and 8 females). (e) GKO-NSG and NSG control blood glucose measures over 180 minutes post oral glucose gavage (60 min: P = 0.001383; 90 min: P = 0.002618; 120 min: P = 0.040657 by Repeated Measures ANOVA, with Bonferroni’s multiple comparisons test) (6g/kg body weight) (NSG mice, n = 5 males and 3 females; GKO-NSG mice, n = 8 males and 2 females) and (f) plasma total GLP-1 levels from 2.5-3 month old GKO-NSG and NSG controls following oral glucose challenge (15 min: P = 0.000194, and 30 min: P = 0.000034 by Repeated Measures ANOVA, with Bonferroni’s multiple comparisons test) (NSG mice, n = 4 males; GKO-NSG mice, n = 5 males). (g) Quantification of active GLP-1 present in islet lysates from 5-7 month old NSG (n = 3 males) and GKO-NSG (n = 3 males) mice (P = 0.035323 by two-tailed Student’s t-test). Dashed lines indicate limit of detection. Scale bars, 50 μm. Data are represented as mean of biological replicates with individual data points overlaid and error bars indicate ± SEM. * P ≤ 0.05, ** P ≤ 0.01, *** P ≤ 0.001.
Extended Data Fig. 2 Blood glucose reduction following insulin challenge.
Related to Fig. 2. Percent of basal blood glucose 30-minutes post insulin injection (1U/kg body weight) from 4.5-6.5 month old NSG, GKO-NSG, and GKO-NSG Tx mice (P = 0.017712 by one-way ANOVA, with Tukey’s multiple comparison test) (NSG mice, n = 5 males; GKO-NSG mice, n = 2 males and 1 female; GKO-NSG Tx mice, n = 5 males). Data are represented as mean of biological replicates with individual data points overlaid and error bars indicate ± SEM. * P ≤ 0.05.
Extended Data Fig. 3 Concentrations of individual plasma amino acids showing no change in GKO-NSG mice.
Related to Fig. 3. Concentration of individual plasma amino acids that showed no significant changes in 6-7 month old GKO-NSG mice (NSG mice, n = 10 males and 2 females; GKO-NSG mice, n = 5 males and 3 females; GKO-NSG Tx mice, n = 6 males). Data are represented as mean of biological replicates with individual data points overlaid and error bars indicate ± SEM.
Extended Data Fig. 4 Further assessment of blood glucose, plasma insulin, and glucagon phenotypes in GKO-NSG mice after human islet transplantation.
Related to Fig. 5. Data are from 4-6 month old NSG control, GKO-NSG, and GKO-NSG mice post-transplantation (GKO-NSG Tx). (a) Plasma glucagon levels in ad libitum-fed mice (NSG vs. GKO-NSG Tx: P = 0.005112 by two-tailed Student’s t-test). Due to the distribution of data from GKO-NSG mice, these data points were omitted from statistical analysis. (NSG mice, n = 10 males and 3 females; GKO-NSG mice, n = 12 males and 1 female; GKO-NSG Tx mice, n = 6 males). Blood glucose (b) (P = 0.013846 by one-way ANOVA, with Tukey’s multiple comparison test) (NSG mice, n = 10 males and 3 females; GKO-NSG mice, n = 8 males and 2 females; GKO-NSG Tx mice, n = 6 males) and plasma insulin levels (c) (NSG mice, n = 10 males and 3 females; GKO-NSG mice, n = 7 males and 2 females; GKO-NSG Tx mice, n = 6 males) in fasted mice. (d) Mouse and human plasma insulin levels in ad libitum-fed GKO-NSG Tx mice (n =6 males). Dashed lines indicate limit of detection (d: black dashed line indicates limit of detection of mouse insulin and red dashed line indicates limit of detection of human insulin). Data are represented as mean of biological replicates with individual data points overlaid and error bars indicate ± SEM. * P ≤ 0.05, ** P ≤ 0.01, *** P ≤ 0.001.
Extended Data Fig. 5 In vitro characterization of donor human islets and more physiological assessment of GKO-NSG mice transplanted with islets either non-diabetic or T2D diabetic donors.
Related to Fig. 6. (a) In vitro glucagon secretion assay on islets from non-diabetic (n = 4 donors) and type 2 diabetic donors (n = 3 donors), shown as technical replicates from individual donors. (b) Glucagon content of donor islets transplanted into GKO-NSG mice (P =0.558605 by two-tailed Student’s t-test; non-diabetic donor n = 5, type 2 diabetic donor n = 3). Data in (c-e) are from 4-6 month old GKO-NSG mice post-transplantation with islets from non-diabetic (GKO-NSG Tx) or type 2 diabetic donors (GKO-NSG Tx T2D). For data presented in (c-e): GKO-NSG Tx mice, n = 6 males; GKO-NSG Tx T2D mice n = 1 male and 2 females. (c) Plasma glucagon levels in ad libitum-fed mice (P = 0.034687 by two-tailed Student’s t-test). Blood glucose (d) (P = 0.042886 by two-tailed Student’s t-test) and plasma insulin levels (e) in 6-hour fasted mice. (f) Percent of basal blood glucose 30-minutes post insulin injection (1U/kg body weight) from 4.5-6.5 month old GKO-NSG Tx and GKO-NSG Tx T2D mice (GKO-NSG Tx mice, n = 5 males; GKO-NSG Tx T2D n = 1 male and 1 female). Dashed lines indicate limit of detection. Data are represented as mean of biological replicates with individual data points overlaid, except in a, where individual data points represent technical replicates from single donors. Error bars indicate ± SEM. * P ≤ 0.05, ** P ≤ 0.01, *** P ≤ 0.001.
Supplementary information
Supplementary Information
Supplementary Tables 1–3
Source data
Source Data Fig. 1
Unprocessed genotyping gel
Rights and permissions
About this article
Cite this article
Tellez, K., Hang, Y., Gu, X. et al. In vivo studies of glucagon secretion by human islets transplanted in mice. Nat Metab 2, 547–557 (2020). https://doi.org/10.1038/s42255-020-0213-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s42255-020-0213-x
This article is cited by
-
Paracrine signalling by pancreatic δ cells determines the glycaemic set point in mice
Nature Metabolism (2024)
-
Acetyl-CoA-carboxylase 1 (ACC1) plays a critical role in glucagon secretion
Communications Biology (2022)
-
CRISPR-based genome editing in primary human pancreatic islet cells
Nature Communications (2021)
-
Engineering islets from stem cells for advanced therapies of diabetes
Nature Reviews Drug Discovery (2021)