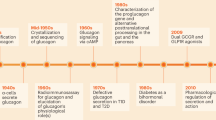
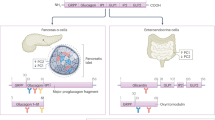
Abstract
Glucagon like peptide-1 (GLP-1), a peptide hormone from the intestinal tract, plays a central role in the coordination of postprandial glucose homeostasis through actions on insulin secretion, food intake and gut motility. GLP-1 forms the basis for a variety of current drugs for the treatment of type 2 diabetes and obesity, as well as new agents currently being developed. Here, we provide a concise overview of the core physiology of GLP-1 secretion and action, and the role of the peptide in human health, disease and therapeutics.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Moore, B. On the treatment of diabetus mellitus by acid extract of duodenal mucous membrane. Biochem. J. 1, 28–38 (1906).
Holst, J. J. The physiology of glucagon-like peptide 1. Physiol. Rev. 87, 1409–1439 (2007).
Thorens, B. Expression cloning of the pancreatic beta cell receptor for the gluco-incretin hormone glucagon-like peptide 1. Proc. Natl. Acad. Sci. USA 89, 8641–8645 (1992).
Nauck, M. A. et al. Normalization of fasting hyperglycaemia by exogenous glucagon-like peptide 1 (7-36 amide) in type 2 (non-insulin-dependent) diabetic patients. Diabetologia 36, 741–744 (1993).
Gribble, F. M. & Reimann, F. Function and mechanisms of enteroendocrine cells and gut hormones in metabolism. Nat. Rev. Endocrinol. 15, 226–237 (2019).
Sjölund, K., Sandén, G., Håkanson, R. & Sundler, F. Endocrine cells in human intestine: an immunocytochemical study. Gastroenterology 85, 1120–1130 (1983).
Lewis, J. E. et al. Selective stimulation of colonic L cells improves metabolic outcomes in mice. Diabetologia 63, 1396–1407 (2020).
Batterham, R. L. et al. Inhibition of food intake in obese subjects by peptide YY3-36. N. Engl. J. Med. 349, 941–948 (2003).
Gribble, F. M. & Reimann, F. Enteroendocrine cells: chemosensors in the intestinal epithelium. Annu. Rev. Physiol. 78, 277–299 (2016).
Brubaker, P. L., Schloos, J. & Drucker, D. J. Regulation of glucagon-like peptide-1 synthesis and secretion in the GLUTag enteroendocrine cell line. Endocrinology 139, 4108–4114 (1998).
Edfalk, S., Steneberg, P. & Edlund, H. Gpr40 is expressed in enteroendocrine cells and mediates free fatty acid stimulation of incretin secretion. Diabetes 57, 2280–2287 (2008).
Chu, Z. L. et al. A role for intestinal endocrine cell-expressed G protein-coupled receptor 119 in glycemic control by enhancing glucagon-like peptide-1 and glucose-dependent insulinotropic peptide release. Endocrinology 149, 2038–2047 (2008).
Hansen, K. B. et al. 2-Oleoyl glycerol is a GPR119 agonist and signals GLP-1 release in humans. J. Clin. Endocrinol. Metab. 96, E1409–E1417 (2011).
Mace, O. J., Schindler, M. & Patel, S. The regulation of K- and L-cell activity by GLUT2 and CasR in rat small intestine. J. Physiol. 590, 2917–2936 (2012).
Thomas, C. et al. TGR5-mediated bile acid sensing controls glucose homeostasis. Cell Metab 10, 167–177 (2009).
Bolognini, D., Tobin, A. B., Milligan, G. & Moss, C. E. The pharmacology and function of receptors for short-chain fatty acids. Mol. Pharmacol. 89, 388–398 (2016).
Pais, R., Rievaj, J., Larraufie, P., Gribble, F. & Reimann, F. Angiotensin II type 1 receptor-dependent GLP-1 and PYY secretion in mice and humans. Endocrinology 157, 3821–3831 (2016).
Pais, R. et al. Role of enteroendocrine L-cells in arginine vasopressin-mediated inhibition of colonic anion secretion. J. Physiol. (Lond.) 594, 4865–4878 (2016).
Psichas, A., Glass, L. L., Sharp, S. J., Reimann, F. & Gribble, F. M. Galanin inhibits GLP-1 and GIP secretion via the GAL1 receptor in enteroendocrine L and K cells. Br. J. Pharmacol. 173, 888–898 (2016).
Roberge, J. N., Gronau, K. A. & Brubaker, P. L. Gastrin-releasing peptide is a novel mediator of proximal nutrient-induced proglucagon-derived peptide secretion from the distal gut. Endocrinology 137, 2383–2388 (1996).
Jang, H. J. et al. Gut-expressed gustducin and taste receptors regulate secretion of glucagon-like peptide-1. Proc. Natl Acad. Sci. USA 104, 15069–15074 (2007).
Saltiel, M. Y. et al. Sweet taste receptor activation in the gut is of limited importance for glucose-stimulated GLP-1 and GIP secretion. Nutrients 9, E418 (2017).
Reimann, F. et al. Glucose sensing in L cells: a primary cell study. Cell Metab. 8, 532–539 (2008).
Gribble, F. M., Williams, L., Simpson, A. K. & Reimann, F. A novel glucose-sensing mechanism contributing to glucagon-like peptide-1 secretion from the GLUTag cell line. Diabetes 52, 1147–1154 (2003).
Gorboulev, V. et al. Na+-D-glucose cotransporter SGLT1 is pivotal for intestinal glucose absorption and glucose-dependent incretin secretion. Diabetes 61, 187–196 (2012).
Reimann, F., Williams, L., da Silva Xavier, G., Rutter, G. A. & Gribble, F. M. Glutamine potently stimulates glucagon-like peptide-1 secretion from GLUTag cells. Diabetologia 47, 1592–1601 (2004).
Matsumura, K., Miki, T., Jhomori, T., Gonoi, T. & Seino, S. Possible role of PEPT1 in gastrointestinal hormone secretion. Biochem. Biophys. Res. Commun. 336, 1028–1032 (2005).
Diakogiannaki, E. et al. Oligopeptides stimulate glucagon-like peptide-1 secretion in mice through proton-coupled uptake and the calcium-sensing receptor. Diabetologia 56, 2688–2696 (2013).
Parker, H. E. et al. Predominant role of active versus facilitative glucose transport for glucagon-like peptide-1 secretion. Diabetologia 55, 2445–2455 (2012).
Powell, D. R. et al. LX4211 increases serum glucagon-like peptide 1 and peptide YY levels by reducing sodium/glucose cotransporter 1 (SGLT1)-mediated absorption of intestinal glucose. J. Pharmacol. Exp. Ther. 345, 250–259 (2013).
Christiansen, C. B. et al. The impact of short-chain fatty acids on GLP-1 and PYY secretion from the isolated perfused rat colon. Am. J. Physiol. Gastrointest. Liver Physiol. 315, G53–G65 (2018).
Jørgensen, N. B. et al. Acute and long-term effects of Roux-en-Y gastric bypass on glucose metabolism in subjects with type 2 diabetes and normal glucose tolerance. Am. J. Physiol. Endocrinol. Metab. 303, E122–E131 (2012).
Martinussen, C. et al. The effect of acute dual SGLT1/SGLT2 inhibition on incretin release and glucose metabolism after gastric bypass surgery. Am. J. Physiol. Endocrinol. Metab. 318, E956–E964 (2020).
El-Ouaghlidi, A. et al. The dipeptidyl peptidase 4 inhibitor vildagliptin does not accentuate glibenclamide-induced hypoglycemia but reduces glucose-induced glucagon-like peptide 1 and gastric inhibitory polypeptide secretion. J. Clin. Endocrinol. Metab. 92, 4165–4171 (2007).
Reimann, F., Tolhurst, G. & Gribble, F. M. G-protein-coupled receptors in intestinal chemosensation. Cell Metab. 15, 421–431 (2012).
Goldspink, D. A. et al. Mechanistic insights into the detection of free fatty and bile acids by ileal glucagon-like peptide-1 secreting cells. Mol. Metab. 7, 90–101 (2018).
Goldspink, D. A. et al. Labeling and characterization of human GLP-1-secreting L-cells in primary ileal organoid culture. Cell Rep. 31, 107833 (2020).
Brighton, C. A. et al. Bile acids trigger GLP-1 release predominantly by accessing basolaterally located G protein-coupled bile acid receptors. Endocrinology 156, 3961–3970 (2015).
Christensen, L. W., Kuhre, R. E., Janus, C., Svendsen, B. & Holst, J. J. Vascular, but not luminal, activation of FFAR1 (GPR40) stimulates GLP-1 secretion from isolated perfused rat small intestine. Physiol. Rep. 3, e12551 (2015).
Modvig, I. M., Kuhre, R. E. & Holst, J. J. Peptone-mediated glucagon-like peptide-1 secretion depends on intestinal absorption and activation of basolaterally located calcium-sensing receptors. Physiol. Rep. 7, e14056 (2019).
Llewellyn-Smith, I. J., Reimann, F., Gribble, F. M. & Trapp, S. Preproglucagon neurons project widely to autonomic control areas in the mouse brain. Neuroscience 180, 111–121 (2011).
Orskov, C., Holst, J. J., Poulsen, S. S. & Kirkegaard, P. Pancreatic and intestinal processing of proglucagon in man. Diabetologia 30, 874–881 (1987).
Rouillé, Y. et al. Proteolytic processing mechanisms in the biosynthesis of neuroendocrine peptides: the subtilisin-like proprotein convertases. Front. Neuroendocrinol. 16, 322–361 (1995).
Larraufie, P. et al. Important role of the GLP-1 axis for glucose homeostasis after bariatric surgery. Cell Rep. 26, 1399–1408.e6 (2019).
Song, Y. et al. Gut-proglucagon-derived peptides are essential for regulating glucose homeostasis in mice. Cell Metab. 30, 976–986.e3 (2019).
Ban, K. et al. Cardioprotective and vasodilatory actions of glucagon-like peptide 1 receptor are mediated through both glucagon-like peptide 1 receptor-dependent and -independent pathways. Circulation 117, 2340–2350 (2008).
Richards, P. et al. Identification and characterization of GLP-1 receptor-expressing cells using a new transgenic mouse model. Diabetes 63, 1224–1233 (2014).
Pyke, C. et al. GLP-1 receptor localization in monkey and human tissue: novel distribution revealed with extensively validated monoclonal antibody. Endocrinology 155, 1280–1290 (2014).
He, S. et al. Gut intraepithelial T cells calibrate metabolism and accelerate cardiovascular disease. Nature 566, 115–119 (2019).
Baggio, L. L. et al. GLP-1 receptor expression within the human heart. Endocrinology 159, 1570–1584 (2018).
Dhir, G. & Cusi, K. Glucagon like peptide-1 receptor agonists for the management of obesity and non-alcoholic fatty liver disease: a novel therapeutic option. J. Investig. Med. 66, 7–10 (2018).
Gromada, J., Holst, J. J. & Rorsman, P. Cellular regulation of islet hormone secretion by the incretin hormone glucagon-like peptide 1. Pflugers Arch. 435, 583–594 (1998).
Creutzfeldt, W. The [pre-] history of the incretin concept. Regul. Pept. 128, 87–91 (2005).
Nauck, M. A., Bartels, E., Orskov, C., Ebert, R. & Creutzfeldt, W. Additive insulinotropic effects of exogenous synthetic human gastric inhibitory polypeptide and glucagon-like peptide-1-(7-36) amide infused at near-physiological insulinotropic hormone and glucose concentrations. J. Clin. Endocrinol. Metab. 76, 912–917 (1993).
Gasbjerg, L. S. et al. Separate and combined glucometabolic effects of endogenous glucose-dependent insulinotropic polypeptide and glucagon-like peptide 1 in healthy individuals. Diabetes 68, 906–917 (2019).
Jørgensen, N. B. et al. Exaggerated glucagon-like peptide 1 response is important for improved β-cell function and glucose tolerance after Roux-en-Y gastric bypass in patients with type 2 diabetes. Diabetes 62, 3044–3052 (2013).
Hansen, L., Deacon, C. F., Orskov, C. & Holst, J. J. Glucagon-like peptide-1-(7-36)amide is transformed to glucagon-like peptide-1-(9-36)amide by dipeptidyl peptidase IV in the capillaries supplying the L cells of the porcine intestine. Endocrinology 140, 5356–5363 (1999).
Vahl, T. P. et al. Glucagon-like peptide-1 (GLP-1) receptors expressed on nerve terminals in the portal vein mediate the effects of endogenous GLP-1 on glucose tolerance in rats. Endocrinology 148, 4965–4973 (2007).
Woerle, H. J., Carneiro, L., Derani, A., Göke, B. & Schirra, J. The role of endogenous incretin secretion as amplifier of glucose-stimulated insulin secretion in healthy subjects and patients with type 2 diabetes. Diabetes 61, 2349–2358 (2012).
Chambers, A. P. et al. The role of pancreatic preproglucagon in glucose homeostasis in mice. Cell Metab. 25, 927–934.e3 (2017).
Svendsen, B. et al. Insulin secretion depends on intra-islet glucagon signaling. Cell Rep. 25, 1127–1134.e2 (2018).
de Heer, J., Rasmussen, C., Coy, D. H. & Holst, J. J. Glucagon-like peptide-1, but not glucose-dependent insulinotropic peptide, inhibits glucagon secretion via somatostatin (receptor subtype 2) in the perfused rat pancreas. Diabetologia 51, 2263–2270 (2008).
Gasbjerg, L. S. et al. Evaluation of the incretin effect in humans using GIP and GLP-1 receptor antagonists. Peptides 125, 170183 (2020).
Wu, T., Rayner, C. K., Young, R. L. & Horowitz, M. Gut motility and enteroendocrine secretion. Curr. Opin. Pharmacol. 13, 928–934 (2013).
Meier, J. J. GLP-1 receptor agonists for individualized treatment of type 2 diabetes mellitus. Nat. Rev. Endocrinol. 8, 728–742 (2012).
Krieger, J. P. et al. Knockdown of GLP-1 receptors in vagal afferents affects normal food intake and glycemia. Diabetes 65, 34–43 (2016).
Nauck, M. A., Quast, D. R., Wefers, J. & Meier, J. J. GLP-1 receptor agonists in the treatment of type 2 diabetes: state-of-the-art. Mol. Metab. https://doi.org/10.1016/j.molmet.2020.101102 (2020).
Knudsen, L. B. & Lau, J. The discovery and development of liraglutide and semaglutide. Front. Endocrinol. (Lausanne) 10, 155 (2019).
Jepsen, S. L. et al. Paracrine crosstalk between intestinal L- and D-cells controls secretion of glucagon-like peptide-1 in mice. Am. J. Physiol. Endocrinol. Metab. 317, E1081–E1093 (2019).
Svane, M. S. et al. Peptide YY and glucagon-like peptide-1 contribute to decreased food intake after Roux-en-Y gastric bypass surgery. Int. J. Obes. (Lond) 40, 1699–1706 (2016).
Williams, E. K. et al. Sensory neurons that detect stretch and nutrients in the digestive system. Cell 166, 209–221 (2016).
Lu, V. B. et al. Adenosine triphosphate is co-secreted with glucagon-like peptide-1 to modulate intestinal enterocytes and afferent neurons. Nat. Commun. 10, 1029 (2019).
Trapp, S. & Cork, S. C. PPG neurons of the lower brain stem and their role in brain GLP-1 receptor activation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 309, R795–R804 (2015).
Kreisler, A. D. & Rinaman, L. Hindbrain glucagon-like peptide-1 neurons track intake volume and contribute to injection stress-induced hypophagia in meal-entrained rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 310, R906–R916 (2016).
Holt, M. K. et al. Preproglucagon neurons in the nucleus of the solitary tract are the main source of brain GLP-1, mediate stress-induced hypophagia, and limit unusually large intakes of food. Diabetes 68, 21–33 (2019).
Cheng, W. et al. Leptin receptor-expressing nucleus tractus solitarius neurons suppress food intake independently of GLP1 in mice. JCI Insight 5, 134359 (2020).
Sun, F. et al. Impact of GLP-1 receptor agonists on blood pressure, heart rate and hypertension among patients with type 2 diabetes: a systematic review and network meta-analysis. Diabetes Res. Clin. Pract. 110, 26–37 (2015).
Giblett, J. P., Clarke, S. J., Dutka, D. P. & Hoole, S. P. Glucagon-like peptide-1: a promising agent for cardioprotection during myocardial ischemia. JACC Basic Transl. Sci. 1, 267–276 (2016).
Holt, M. K. et al. PPG neurons in the nucleus of the solitary tract modulate heart rate but do not mediate GLP-1 receptor agonist-induced tachycardia in mice. Mol. Metab. 39, 101024 (2020).
Müller, T. D. et al. Glucagon-like peptide 1 (GLP-1). Mol. Metab. 30, 72–130 (2019).
Cheng, H. & Leblond, C. P. Origin, differentiation and renewal of the four main epithelial cell types in the mouse small intestine. III. Entero-endocrine cells. Am. J. Anat. 141, 503–519 (1974).
Mumphrey, M. B., Patterson, L. M., Zheng, H. & Berthoud, H. R. Roux-en-Y gastric bypass surgery increases number but not density of CCK-, GLP-1-, 5-HT-, and neurotensin-expressing enteroendocrine cells in rats. Neurogastroenterol. Motil. 25, e70–e79 (2013).
Nauck, M., Stöckmann, F., Ebert, R. & Creutzfeldt, W. Reduced incretin effect in type 2 (non-insulin-dependent) diabetes. Diabetologia 29, 46–52 (1986).
Vollmer, K. et al. Hyperglycemia acutely lowers the postprandial excursions of glucagon-like peptide-1 and gastric inhibitory polypeptide in humans. J. Clin. Endocrinol. Metab. 94, 1379–1385 (2009).
Wang, J. et al. Mutant neurogenin-3 in congenital malabsorptive diarrhea. N. Engl. J. Med. 355, 270–280 (2006).
Jackson, R. S. et al. Small-intestinal dysfunction accompanies the complex endocrinopathy of human proprotein convertase 1 deficiency. J. Clin. Invest. 112, 1550–1560 (2003).
Kay, R. G. et al. Peptidomic analysis of endogenous plasma peptides from patients with pancreatic neuroendocrine tumours. Rapid Commun. Mass Spectrom. 32, 1414–1424 (2018).
Tan, M., Lamendola, C., Luong, R., McLaughlin, T. & Craig, C. Safety, efficacy and pharmacokinetics of repeat subcutaneous dosing of avexitide (exendin 9-39) for treatment of post-bariatric hypoglycaemia. Diabetes Obes. Metab. 22, 1406–1416 (2020).
Deacon, C. F. Peptide degradation and the role of DPP-4 inhibitors in the treatment of type 2 diabetes. Peptides 100, 150–157 (2018).
Thethi, T. K., Pratley, R. & Meier, J. J. Efficacy, safety and cardiovascular outcomes of once-daily oral semaglutide in patients with type 2 diabetes: The PIONEER programme. Diabetes Obes. Metab. 22, 1263–1277 (2020).
Zhang, X. et al. Differential GLP-1R binding and activation by peptide and non-peptide agonists. Mol. Cell 80, 485–500.e7 (2020).
Lucey, M. et al. Disconnect between signalling potency and in vivo efficacy of pharmacokinetically optimised biased glucagon-like peptide-1 receptor agonists. Mol. Metab. 37, 100991 (2020).
Tschöp, M. H. et al. Unimolecular polypharmacy for treatment of diabetes and obesity. Cell Metab. 24, 51–62 (2016).
Frias, J. P. et al. Efficacy and safety of LY3298176, a novel dual GIP and GLP-1 receptor agonist, in patients with type 2 diabetes: a randomised, placebo-controlled and active comparator-controlled phase 2 trial. Lancet 392, 2180–2193 (2018).
Parker, V. E. R. et al. Efficacy, safety, and mechanistic insights of cotadutide, a dual receptor glucagon-like peptide-1 and glucagon agonist. J. Clin. Endocrinol. Metab. 105, dgz047 (2020).
Ämmälä, C. et al. Targeted delivery of antisense oligonucleotides to pancreatic β-cells. Sci. Adv. 4, eaat3386 (2018).
Reiner, T. et al. Accurate measurement of pancreatic islet beta-cell mass using a second-generation fluorescent exendin-4 analog. Proc. Natl. Acad. Sci. USA 108, 12815–12820 (2011).
Acknowledgements
Research in the laboratories of F.M.G. and F.R. is primarily supported by Wellcome (106262/Z/14/Z and 106263/Z/14/Z) and MRC-UK (MRC_MC_UU_12012/3) funding.
Author information
Authors and Affiliations
Contributions
F.M.G. and F.R. co-wrote the article.
Corresponding authors
Ethics declarations
Competing interests
F.M.G. is a consultant for Kallyope, and the laboratories of F.M.G. and F.R. receive additional research funding from AstraZeneca, Eli Lilly and LGC.
Additional information
Peer review information Primary Handling Editor: Christoph Schmitt. Nature Metabolism thanks Brian Finan and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Rights and permissions
About this article
Cite this article
Gribble, F.M., Reimann, F. Metabolic Messengers: glucagon-like peptide 1. Nat Metab 3, 142–148 (2021). https://doi.org/10.1038/s42255-020-00327-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s42255-020-00327-x