Abstract
Insulin and glucagon exert opposing effects on glucose metabolism and, consequently, pancreatic islet β-cells and α-cells are considered functional antagonists. The intra-islet hypothesis has previously dominated the understanding of glucagon secretion, stating that insulin acts to inhibit the release of glucagon. By contrast, glucagon is a potent stimulator of insulin secretion and has been used to test β-cell function. Over the past decade, α-cells have received increasing attention due to their ability to stimulate insulin secretion from neighbouring β-cells, and α-cell–β-cell crosstalk has proven central for glucose homeostasis in vivo. Glucagon is not only the counter-regulatory hormone to insulin in glucose metabolism but also glucagon secretion is more susceptible to changes in the plasma concentration of certain amino acids than to changes in plasma concentrations of glucose. Thus, the actions of glucagon also include a central role in amino acid turnover and hepatic fat oxidation. This Review provides insights into glucagon secretion, with a focus on the local paracrine actions on glucagon and the importance of α-cell–β-cell crosstalk. We focus on dysregulated glucagon secretion in obesity, non-alcoholic fatty liver disease and type 2 diabetes mellitus. Lastly, the future potential of targeting hyperglucagonaemia and applying dual and triple receptor agonists with glucagon receptor-activating properties in combination with incretin hormone receptor agonism is discussed.
Key points
-
Glucagon is a 29-amino acid peptide hormone mainly secreted from pancreatic α-cells and has primarily been recognized for its role in glucose homeostasis.
-
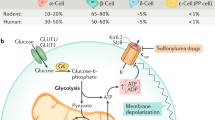
Glucagon secretion seems to be partly regulated by the direct effect of glucose on α-cells; however, paracrine regulation from neighbouring β-cells and δ-cells is also important.
-
Several amino acids are glucagonotropic, and glucagon increases hepatic uptake and turnover of amino acids and stimulates ureagenesis — a feedback cycle referred to as the liver–α-cell axis.
-
The importance of α-cell–β-cell crosstalk is increasingly recognized; studies suggest that α-cells are necessary for β-cell function (insulin secretion) and might preserve β-cell mass.
-
Fasting hyperglucagonaemia in diabetes mellitus might be both a pathophysiological trait in glucose metabolism and a helpful metabolic adaptation in hepatic lipid and amino acid metabolism.
-
Glucagon receptor antagonism improves glycaemic control in type 1 diabetes mellitus and type 2 diabetes mellitus but with adverse effects; future strategies targeting obesity and type 2 diabetes mellitus might involve glucagon co-agonism.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Banting, F. G., Best, C. H., Collip, J. B., Campbell, W. R. & Fletcher, A. A. Pancreatic extracts in the treatment of diabetes mellitus. Can. Med. Assoc. J. 12, 141–146 (1922).
Kimball, C. & Murlin, J. R. Aqueous extracts of pancreas III. Some precipitation reactions of insulin. J. Biol. Chem. 58, 337–346 (1923).
Sutherland, E. W. & Cori, C. F. Purification of the hyperglycemic-glycogenolytic factor from insulin and from gastric mucosa. J. Biol. Chem. 180, 825–837 (1949).
Unger, R. & Orci, L. The essential role of glucagon in the pathogenesis of diabetes mellitus. Lancet 305, 14–16 (1975).
Cherrington, A. D., Williams, P. E., Shulman, G. I. & Lacy, W. W. Differential time course of glucagon’s effect on glycogenolysis and gluconeogenesis in the conscious dog. Diabetes 30, 180–187 (1981).
Magnusson, I., Rothman, D. L., Gerard, D. P., Katz, L. D. & Shulman, G. I. Contribution of hepatic glycogenolysis to glucose production in humans in response to a physiological increase in plasma glucagon concentration. Diabetes 44, 185–189 (1995).
Bonner-Weir, S., Sullivan, B. A. & Weir, G. C. Human islet morphology revisited: human and rodent islets are not so different after all. J. Histochem. Cytochem. 63, 604–612 (2015).
Unger, R. H., Eisentraut, A. M., McCall, M. S. & Madison, L. L. Glucagon antibodies and an immunoassay for glucagon. J. Clin. Invest. 40, 1280–1289 (1961).
Holst, J. J. & Albrechtsen, N. J. W. Methods and guidelines for measurement of glucagon in plasma. Int. J. Mol. Sci. 20, 5416 (2019).
Muller, W., Faloona, G., Aguilar-Parada, E. & Unger, R. Abnormal alpha-cell function in diabetes — response to carbohydrate and protein ingestion. N. Engl. J. Med. 283, 109–115 (1970).
Sasaki, H. et al. Identification of glucagon in the gastrointestinal tract. J. Clin. Invest. 56, 135–145 (1975).
Wewer Albrechtsen, N. J. et al. Circulating glucagon 1-61 regulates blood glucose by increasing insulin secretion and hepatic glucose production. Cell Rep. 21, 1452–1460 (2017).
Holst, J. J., Albrechtsen, N. J. W., Gabe, M. B. N. & Rosenkilde, M. M. Oxyntomodulin: actions and role in diabetes. Peptides 100, 48–53 (2018).
Lund, A. et al. Evidence of extrapancreatic glucagon secretion in man. Diabetes 65, 585–597 (2016).
Jorsal, T. et al. Investigating intestinal glucagon after Roux-en-Y gastric bypass surgery. J. Clin. Endocrinol. Metab. 104, 6403–6416 (2019).
Lund, A. & Knop, F. K. Extrapancreatic glucagon: present status. Diabetes Res. Clin. Pract. 147, 19–28 (2019).
Kilimnik, G., Kim, A., Steiner, D. F., Friedman, T. C. & Hara, M. Intraislet production of GLP-1 by activation of prohormone convertase 1/3 in pancreatic α-cells in mouse models of β-cell regeneration. Islets 2, 149–155 (2010).
Marchetti, P. et al. A local glucagon-like peptide 1 (GLP-1) system in human pancreatic islets. Diabetologia 55, 3262–3272 (2012).
Knop, F. K. Resolution of type 2 diabetes following gastric bypass surgery: involvement of gut-derived glucagon and glucagonotropic signalling? Diabetologia 52, 2270–2276 (2009).
Jorsal, T. et al. Enteroendocrine K and L cells in healthy and type 2 diabetic individuals. Diabetologia 61, 284–294 (2018).
Thim, L. & Moody, A. J. The amino acid sequence of porcine glicentin. Peptides 2, 37–39 (1981).
Bataille, D. et al. Bioactive enteroglucagon (oxyntomodulin): present knowledge on its chemical structure and its biological activities. Peptides 2, 41–44 (1981).
Holst, J. J. Evidence that enteroglucagon (II) is identical with the C-terminal sequence (residues 33-69) of glicentin. Biochem. J. 207, 381–388 (1982).
Wewer Albrechtsen, N. J. et al. Hyperglucagonaemia analysed by glucagon sandwich ELISA: nonspecific interference or truly elevated levels? Diabetologia 57, 1919–1926 (2014).
Wewer Albrechtsen, N. J. et al. Inability of some commercial assays to measure suppression of glucagon secretion. J. Diabetes Res. 2016, 8352957 (2016).
Svoboda, M., Tastenoy, M., Vertongen, P. & Robberecht, P. Relative quantitative analysis of glucagon receptor mRNA in rat tissues. Mol. Cell. Endocrinol. 105, 131–137 (1994).
Hansen, L. H., Abrahamsen, N. & Nishimura, E. Glucagon receptor mRNA distribution in rat tissues. Peptides 16, 1163–1166 (1995).
Watanabe, M., Hayasaki, H., Tamayama, T. & Shimada, M. Insulin and glucagon receptor distribution. Braz. J. Med. Biol. Res. 31, 243–256 (1998).
van der Woning, B. et al. DNA immunization combined with scFv phage display identifies antagonistic GCGR specific antibodies and reveals new epitopes on the small extracellular loops. mAbs 8, 1126–1135 (2016).
Jiang, G. & Zhang, B. B. Glucagon and regulation of glucose metabolism. Am. J. Physiol. Endocrinol. Metab. 284, 671–678 (2003).
Müller, T. D., Finan, B., Clemmensen, C., DiMarchi, R. D. & Tschöp, M. H. The new biology and pharmacology of glucagon. Physiol. Rev. 97, 721–766 (2017).
Galsgaard, K. D., Pedersen, J., Knop, F. K., Holst, J. J. & Albrechtsen, N. J. W. Glucagon receptor signaling and lipid metabolism. Front. Physiol. 10, 413 (2019).
Richter, W. O., Robl, H. & Schwandt, P. Human glucagon and vasoactive intestinal polypeptide (VIP) stimulate free fatty acid release from human adipose tissue in vitro. Peptides 10, 333–335 (1989).
Wu, M. S. et al. Does glucagon increase plasma free fatty acid concentration in humans with normal glucose tolerance? J. Clin. Endocrinol. Metab. 70, 410–416 (1990).
Jensen, M. D., Heiling, V. J. & Miles, J. M. Effects of glucagon on free fatty acid metabolism in humans. J. Clin. Endocrinol. Metab. 72, 308–315 (1991).
Højbjerg Gravholt, C., Møller, N., Jensen, M. D., Christiansen, J. S. & Schmitz, O. Physiological levels of glucagon do not influence lipolysis in abdominal adipose tissue as assessed by microdialysis. J. Clin. Endocrinol. Metab. 86, 2085–2089 (2001).
Xiao, C., Pavlic, M., Szeto, L., Patterson, B. W. & Lewis, G. F. Effects of acute hyperglucagonemia on hepatic and intestinal lipoprotein production and clearance in healthy humans. Diabetes 60, 383–390 (2011).
Longuet, C. et al. The glucagon receptor is required for the adaptive metabolic response to fasting. Cell Metab. 8, 359–371 (2008).
Stephens, F. B., Constantin-Teodosiu, D. & Greenhaff, P. L. New insights concerning the role of carnitine in the regulation of fuel metabolism in skeletal muscle. J. Physiol. 581, 431–444 (2007).
Peng, I. C. et al. Glucagon regulates ACC activity in adipocytes through the CAMKK β/AMPK pathway. Am. J. Physiol. Endocrinol. Metab. 302, E1560 (2012).
Parilla, R., Goodman, M. N. & Toews, C. J. Effect of glucagon: insulin ratios on hepatic metabolism. Diabetes 23, 725–731 (1974).
Perry, R. J. et al. Hepatic acetyl CoA links adipose tissue inflammation to hepatic insulin resistance and type 2 diabetes. Cell 160, 745–758 (2015).
Petersen, K. F., Hansen, B. A. & Vilstrup, H. Time dependent stimulating effect of glucagon on the capacity of urea-N synthesis in rats. Horm. Metab. Res. 19, 53–56 (1987).
Boden, G., Rezvani, I. & Owen, O. E. Effects of glucagon on plasma amino acids. J. Clin. Invest. 73, 785–793 (1983).
Hamberg, O. & Vilstrup, H. Regulation of urea synthesis by glucose and glucagon in normal man. Clin. Nutr. 13, 183–191 (1994).
Pegorier, J. P., Salvado, J., Forestier, M. & Girard, J. Dominant role of glucagon in the initial induction of phosphoenolpyruvate carboxykinase mRNA in cultured hepatocytes from fetal rats. Eur. J. Biochem. 210, 1053–1059 (1992).
Watanabe, C. et al. Remodeling of hepatic metabolism and hyperaminoacidemia in mice deficient in proglucagon-derived peptides. Diabetes 61, 74–84 (2012).
Heibel, S. K. et al. Transcriptional regulation of N-acetylglutamate synthase. PLoS One 7, 29527 (2012).
Wewer Albrechtsen, N. J. et al. The liver-α-cell axis and type 2 diabetes. Endocr. Rev. 40, 1353–1366 (2019).
De Chiara, F. et al. Urea cycle dysregulation in non-alcoholic fatty liver disease. J. Hepatol. 69, 905–915 (2018).
Sands, J. M. Regulation of renal urea transporters. J. Am. Soc. Nephrol. 10, 635–646 (1999).
Le Cam, A. & Freychet, P. Glucagon stimulates the A system for neutral amino acid transport in isolated hepatocytes of adult rat. Biochem. Biophys. Res. Commun. 72, 893–901 (1976).
Richter, M. M. et al. The liver–α-cell axis in health and in disease. Diabetes 71, 1852–1861 (2022).
Davidson, I. W. F., Salter, J. M. & Best, C. H. Calorigenic action of glucagon. Nature 180, 1124 (1957).
Nair, K. S. Hyperglucagonemia increases resting metabolic rate in man during insulin deficiency. J. Clin. Endocrinol. Metab. 64, 896–901 (1987).
Calles-Escandón, J. Insulin dissociates hepatic glucose cycling and glucagon-induced thermogenesis in man. Metabolism 43, 1000–1005 (1994).
Al-Massadi, O., Fernø, J., Diéguez, C., Nogueiras, R. & Quiñones, M. Glucagon control on food intake and energy balance. Int. J. Mol. Sci. 20, 3905 (2019).
Heppner, K. M. et al. Glucagon regulation of energy metabolism. Physiol. Behav. 100, 545–548 (2010).
Habegger, K. M. et al. The metabolic actions of glucagon revisited. Nat. Rev. Endocrinol. 6, 689–697 (2010).
Le Sauter, J. & Geary, N. Hepatic portal glucagon infusion decreases spontaneous meal size in rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 261, R154–R161 (1991).
Langhans, W., Zieger, U., Scharrer, E. & Geary, N. Stimulation of feeding in rats by intraperitoneal injection of antibodies to glucagon. Science 218, 894–896 (1982).
Le Sauter, J., Noh, U. & Geary, N. Hepatic portal infusion of glucagon antibodies increases spontaneous meal size in rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 261, R162–R165 (1991).
Geary, N., Kissileff, H. R., Pi-Sunyer, F. X. & Hinton, V. Individual, but not simultaneous, glucagon and cholecystokinin infusions inhibit feeding in men. Am. J. Physiol. Regul. Integr. Comp. Physiol. 262, R975–R980 (1992).
Feczko, P. J., Simms, S. M., Iorio, J. & Halpert, R. Gastroduodenal response to low-dose glucagon. Am. J. Roentgenol. 140, 935–940 (1983).
Patel, G. K., Whalen, G. E., Soergel, K. H., Wu, W. C. & Meade, R. C. Glucagon effects on the human small intestine. Dig. Dis. Sci. 24, 501–508 (1979).
Unger, R. H. & Orci, L. Physiology and pathophysiology of glucagon. Physiol. Rev. 56, 778–826 (1976).
Mukharji, A., Drucker, D. J., Charron, M. J. & Swoap, S. J. Oxyntomodulin increases intrinsic heart rate through the glucagon receptor. Physiol. Rep. 1, 112 (2013).
Petersen, K. M., Bøgevig, S., Holst, J. J., Knop, F. K. & Christensen, M. B. Hemodynamic effects of glucagon: a literature review. J. Clin. Endocrinol. Metab. 103, 1804–1812 (2018).
Kazda, C. M. et al. Treatment with the glucagon receptor antagonist LY2409021 increases ambulatory blood pressure in patients with type 2 diabetes. Diabetes Obes. Metab. 19, 1071–1077 (2017).
Walker, J. N. et al. Regulation of glucagon secretion by glucose: paracrine, intrinsic or both? Diabetes Obes. Metab. 13, 95–105 (2011).
Braun, M. et al. Aminobutyric acid (GABA) is an autocrine excitatory transmitter in human pancreatic β-cells. Diabetes 59, 1694–1701 (2010).
Johansson, H., Gylfe, E. & Hellman, B. Cyclic AMP raises cytoplasmic calcium in pancreatic α2-cells by mobilizing calcium incorporated in response to glucose. Cell Calcium 10, 205–211 (1989).
Pipeleers, D. G., Schuit, F. C., Van Schravendijk, C. F. H. & Van De Winkel, M. Interplay of nutrients and hormones in the regulation of glucagon release. Endocrinology 117, 817–823 (1985).
Gromada, J., Franklin, I. & Wollheim, C. B. α-Cells of the endocrine pancreas: 35 years of research but the enigma remains. Endocr. Rev. 28, 84–116 (2007).
Gromada, J., Chabosseau, P. & Rutter, G. A. The α-cell in diabetes mellitus. Nat. Rev. Endocrinol. 14, 694–704 (2018).
Rorsman, P. & Hellman, B. Voltage-activated currents in guinea pig pancreatic α2 cells: evidence for Ca2+-dependent action potentials. J. Gen. Physiol. 91, 223–242 (1988).
Berts, A., Gylfe, E. & Hellman, B. Ca2+ oscillations in pancreatic islet cells secreting glucagon and somatostatin. Biochem. Biophys. Res. Commun. 208, 644–649 (1995).
Berts, A., Ball, A., Gylfe, E. & Hellman, B. Suppression of Ca2+ oscillations in glucagon-producing α2-cells by insulin/glucose and amino acids. Biochim. Biophys. Acta Mol. Cell Res. 1310, 212–216 (1996).
Heimberg, H., De Vos, A., Pipeleers, D., Thorens, B. & Schuit, F. Differences in glucose transporter gene expression between rat pancreatic α- and β-cells are correlated to differences in glucose transport but not in glucose utilization. J. Biol. Chem. 270, 8971–8975 (1995).
Gromada, J. et al. Adrenaline stimulates glucagon secretion in pancreatic A-cells by increasing the Ca2+ current and the number of granules close to the L-type Ca2+ channels. J. Gen. Physiol. 110, 217–228 (1997).
MacDonald, P. E. et al. A KATP channel-dependent pathway within α cells regulates glucagon release from both rodent and human islets of Langerhans. PLoS Biol. 5, 1236–1247 (2007).
Zhang, Q. et al. Role of K ATP channels in glucose-regulated glucagon secretion and impaired counterregulation in type 2 diabetes. Cell Metab. 18, 871–882 (2013).
Gilon, P. The role of α-cells in islet function and glucose homeostasis in health and type 2 diabetes. J. Mol. Biol. 432, 1367–1394 (2020).
Elliott, A. D., Ustione, A. & Piston, D. W. Somatostatin and insulin mediate glucose-inhibited glucagon secretion in the pancreatic α-cell by lowering cAMP. Am. J. Physiol. Endocrinol. Metab. 308, E130–E143 (2015).
Yu, Q., Shuai, H., Ahooghalandari, P., Gylfe, E. & Tengholm, A. Glucose controls glucagon secretion by directly modulating cAMP in alpha cells. Diabetologia 62, 1212–1224 (2019).
Gylfe, E. Glucose control of glucagon secretion — ‘there’s a brand-new gimmick every year’. Upsala J. Med. Sci. 121, 120–132 (2016).
Gromada, J. et al. ATP-sensitive K+ channel-dependent regulation of glucagon release and electrical activity by glucose in wild-type and SUR1−/− mouse α-cells. Diabetes 53, S181–S189 (2004).
Ravier, M. A. & Rutter, G. A. Glucose or insulin, but not zinc ions, inhibit glucagon secretion from mouse pancreatic α-cells. Diabetes 54, 1789–1797 (2005).
Bosco, D. et al. Unique arrangement of α- and β-cells in human islets of Langerhans. Diabetes 59, 1202–1210 (2010).
Franklin, I., Gromada, J., Gjinovci, A., Theander, S. & Wollheim, C. B. β-Cell secretory products activate α-cell ATP-dependent potassium channels to inhibit glucagon release. Diabetes 54, 1808–1815 (2005).
Kawamori, D. et al. Insulin signaling in α cells modulates glucagon secretion in vivo. Cell Metab. 9, 350–361 (2009).
Maruyama, H., Hisatomi, A., Orci, L., Grodsky, G. M. & Unger, R. H. Insulin within islets is a physiologic glucagon release inhibitor. J. Clin. Invest. 74, 2296–2299 (1984).
Gerich, J. E. et al. Comparison of the suppressive effects of elevated plasma glucose and free fatty acid levels on glucagon secretion in normal and insulin dependent diabetic subjects. Evidence for selective alpha cell insensitivity to glucose in diabetes mellitus. J. Clin. Invest. 58, 320–325 (1976).
Gerich, J. E., Charles, M. A. & Grodsky, G. M. Regulation of pancreatic insulin and glucagon secretion. Annu. Rev. Physiol. 38, 353–388 (1976).
Weir, G. C., Knowlton, S. D., Atkins, R. F., McKennan, K. X. & Martin, D. B. Glucagon secretion from the perfused pancreas of streptozotocin treated rats. Diabetes 25, 275–282 (1976).
Kawamori, D., Akiyama, M., Hu, J., Hambro, B. & Kulkarni, R. N. Growth factor signalling in the regulation of α-cell fate. Diabetes Obes. Metab. 13, 21–30 (2011).
Knop, F. K. et al. Impaired incretin effect and fasting hyperglucagonaemia characterizing type 2 diabetic subjects are early signs of dysmetabolism in obesity. Diabetes Obes. Metab. 14, 500–510 (2012).
Hare, K. J., Vilsbøll, T., Holst, J. J. & Knop, F. K. Inappropriate glucagon response after oral compared with isoglycemic intravenous glucose administration in patients with type 1 diabetes. Am. J. Physiol. Metab. 298, E832–E837 (2010).
Knop, F. K. EJE PRIZE 2018: a gut feeling about glucagon. Eur. J. Endocrinol. 178, R267–R280 (2018).
Knop, F. K., Vilsbøll, T., Madsbad, S., Holst, J. J. & Krarup, T. Inappropriate suppression of glucagon during OGTT but not during isoglycaemic i.v. glucose infusion contributes to the reduced incretin effect in type 2 diabetes mellitus. Diabetologia 50, 797–805 (2007).
Gylfe, E. & Gilon, P. Glucose regulation of glucagon secretion. Diabetes Res. Clin. Pract. 103, 1–10 (2014).
Blundell, T. L. et al. The crystal structure of rhombohedral 2 zinc insulin. Cold Spring Harb. Symp. Quant. Biol. 36, 233–241 (1972).
Ehrlich, J. C. & Ratner, I. M. Amyloidosis of the islets of Langerhans. A restudy of islet hyalin in diabetic and non-diabetic individuals. Am. J. Pathol. 38, 49–59 (1961).
Westermark, P. Amyloid of human islets of Langerhans — II. Electron microscopic analysis of isolated amyloid. Virchows Arch. A Pathol. Anat. Histol. 373, 161–166 (1977).
Cooper, G. J. S. et al. Purification and characterization of a peptide from amyloid-rich pancreases of type 2 diabetic patients. Proc. Natl Acad. Sci. USA 84, 8628–8632 (1987).
Silvestre, R. A., Peiró, E., Dégano, P., Miralles, P. & Marco, J. Inhibitory effect of rat amylin on the insulin responses to glucose and arginine in the perfused rat pancreas. Regul. Pept. 31, 23–31 (1990).
Gedulin, B. R., Rink, T. J. & Young, A. A. Dose-response for glucagonostatic effect of amylin in rats. Metabolism 46, 67–70 (1997).
Gedulin, B. R., Jodka, C. M., Herrmann, K. & Young, A. A. Role of endogenous amylin in glucagon secretion and gastric emptying in rats demonstrated with the selective antagonist, AC187. Regul. Pept. 137, 121–127 (2006).
Ryan, G. J., Jobe, L. J. & Martin, R. Pramlintide in the treatment of type 1 and type 2 diabetes mellitus. Clin. Ther. 27, 1500–1512 (2005).
Kong, M. F. et al. Infusion of pramlintide, a human amylin analogue, delays gastric emptying in men with IDDM. Diabetologia 40, 82–88 (1997).
Levetan, C. et al. Impact of pramlintide on glucose fluctuations and postprandial glucose, glucagon, and triglyceride excursions among patients with type 1 diabetes intensively treated with insulin pumps. Diabetes Care 26, 1–8 (2003).
Nyholm, B. et al. The amylin analog pramlintide improves glycemic control and reduces postprandial glucagon concentrations in patients with type 1 diabetes mellitus. Metabolism 48, 935–941 (1999).
Broderick, C. L., Brooke, G. S., DiMarchi, R. D. & Gold, G. Human and rat amylin have no effects on insulin secretion in isolated rat pancreatic islets. Biochem. Biophys. Res. Commun. 177, 932–938 (1991).
Inoue, K., Hiramatsu, S., Hisatomi, A., Umeda, F. & Nawata, H. Effects of amylin on the release of insulin and glucagon from the perfused rat pancreas. Horm. Metab. Res. 25, 135–137 (1993).
Olsen, H. L. et al. Glucose stimulates glucagon release in single rat α-cells by mechanisms that mirror the stimulus-secretion coupling in β-cells. Endocrinology 146, 4861–4870 (2005).
Gilon, P., Bertrand, G., Loubatières-Mariani, M. M., Remacle, C. & Henquin, J. C. The influence of 7-aminobutyric acid on hormone release by the mouse and rat endocrine pancreas. Endocrinology 129, 2521–2529 (1991).
Wendt, A. et al. Glucose inhibition of glucagon secretion from Rat α-cells is mediated by GABA released from neighboring β-cells. Diabetes 53, 1038–1045 (2004).
Rorsman, P. et al. Glucose-inhibition of glucagon secretion involves activation of GABAA-receptor chloride channels. Nature 341, 233–236 (1989).
Quoix, N. et al. Glucose and pharmacological modulators of ATP-sensitive K+ channels control [Ca2+]c by different mechanisms in isolated mouse α-cells. Diabetes 58, 412–421 (2009).
Vieira, E., Salehi, A. & Gylfe, E. Glucose inhibits glucagon secretion by a direct effect on mouse pancreatic alpha cells. Diabetologia 50, 370–379 (2007).
Hjortoe, G. M., Hagel, G. M., Terry, B. R., Thastrup, O. & Arkhammar, P. O. G. Functional identification and monitoring of individual α and β cells in cultured mouse islets of Langerhans. Acta Diabetol. 41, 185–193 (2004).
Patel, Y. C., Wheatley, T. & Ning, C. Multiple forms of immunoreactive somatostatin: comparison of distribution in neural and nonneural tissues and portal plasma of the rat. Endocrinology 109, 1943–1949 (1981).
Hauge-Evans, A. C. et al. Somatostatin secreted by islet δ-cells fulfills multiple roles as a paracrine regulator of islet function. Diabetes 58, 403–411 (2009).
Orci, L., Stefan, Y., Bonner-Weir, S., Perrelet, A. & Unger, R. ‘Obligatory’ association between A and D cells demonstrated by bipolar islets in neonatal pancreas. Diabetologia 21, 73–74 (1981).
Arrojo e Drigo, R. et al. Structural basis for delta cell paracrine regulation in pancreatic islets. Nat. Commun. 10, 3700 (2019).
Rorsman, P. & Huising, M. O. The somatostatin-secreting pancreatic δ-cell in health and disease. Nat. Rev. Endocrinol. 14, 404–414 (2018).
Gromada, J. et al. Gi2 proteins couple somatostatin receptors to low-conductance K+ channels in rat pancreatic α-cells. Pflug. Arch. Eur. J. Physiol. 442, 19–26 (2001).
Schuit, F. C., Derde, M. P. & Pipeleers, D. G. Sensitivity of rat pancreatic A and B cells to somatostatin. Diabetologia 32, 207–212 (1989).
Holst, J. J., Vilsbøll, T. & Deacon, C. F. The incretin system and its role in type 2 diabetes mellitus. Mol. Cell. Endocrinol. 297, 127–136 (2009).
Bagger, J. I. et al. Glucagonostatic potency of GLP-1 in patients with type 2 diabetes, patients with type 1 diabetes, and healthy control subjects. Diabetes 70, 1347–1356 (2021).
Zhang, Y. et al. GLP-1 receptor in pancreatic α-cells regulates glucagon secretion in a glucose-dependent bidirectional manner. Diabetes 68, 34–44 (2019).
Ørgaard, A. & Holst, J. J. The role of somatostatin in GLP-1-induced inhibition of glucagon secretion in mice. Diabetologia 60, 1731–1739 (2017).
Creutzfeldt, W. O. C. et al. Glucagonostatic actions reduction of fasting hyperglycemia by exogenous glucagon-like peptide I(7-36) amide in type I diabetic patients. Diabetes Care 19, 580–586 (1996).
Hare, K. J. et al. The glucagonostatic and insulinotropic effects of glucagon-like peptide 1 contribute equally to its glucose-lowering action. Diabetes 59, 1765–1770 (2010).
Junker, A. E. et al. Effects of glucagon-like peptide-1 on glucagon secretion in patients with non-alcoholic fatty liver disease. J. Hepatol. 64, 908–915 (2016).
Plamboeck, A. et al. The role of efferent cholinergic transmission for the insulinotropic and glucagonostatic effects of GLP-1. Am. J. Physiol. Regul. Integr. Comp. Physiol. 309, R544–R551 (2015).
Schirra, J. et al. Exendin(9-39)amide is an antagonist of glucagon-like peptide-1(7-36)amide in humans. J. Clin. Invest. 101, 1421–1430 (1998).
Gasbjerg, L. S., Bari, E. J., Christensen, M. & Knop, F. K. Exendin(9-39)NH2: recommendations for clinical use based on a systematic literature review. Diabetes Obes. Metab. 23, 2419–2436 (2021).
Christensen, M., Vedtofte, L., Holst, J. J., Vilsbøll, T. & Knop, F. K. Glucose-dependent insulinotropic polypeptide: a bifunctional glucose-dependent regulator of glucagon and insulin secretion in humans. Diabetes 60, 3103–3109 (2011).
Pederson, R. A. & Brown, J. C. Interaction of gastric inhibitory polypeptide, glucose, and arginine on insulin and glucagon secretion from the perfused rat pancreas. Endocrinology 103, 610–615 (1978).
Christensen, M. B., Calanna, S., Holst, J. J., Vilsbløll, T. & Knop, F. K. Glucose-dependent insulinotropic polypeptide: blood glucose stabilizing effects in patients with type 2 diabetes. J. Clin. Endocrinol. Metab. 99, 418–426 (2014).
Christensen, M. et al. Glucose-dependent insulinotropic polypeptide augments glucagon responses to hypoglycemia in type 1 diabetes. Diabetes 64, 72–78 (2015).
Lund, A., Vilsboll, T., Bagger, J. I., Holst, J. J. & Knop, F. K. The separate and combined impact of the intestinal hormones, GIP, GLP-1, and GLP-2, on glucagon secretion in type 2 diabetes. Am. J. Physiol. Endocrinol. Metab. 300, 1038–1046 (2011).
Mathiesen, D. S. et al. The effects of dual GLP-1/GIP receptor agonism on glucagon secretion — a review. Int. J. Mol. Sci. 20, 4092 (2019).
Chia, C. W. et al. Exogenous glucose-dependent insulinotropic polypeptide worsens postprandial hyperglycemia in type 2 diabetes. Diabetes 58, 1342–1349 (2009).
Bergmann, N. C. et al. No acute effects of exogenous glucose-dependent insulinotropic polypeptide on energy intake, appetite, or energy expenditure when added to treatment with a long-acting glucagon-like peptide 1 receptor agonist in men with type 2 diabetes. Diabetes Care 43, 588–596 (2020).
Gasbjerg, L. S. et al. GIP and GLP-1 receptor antagonism during a meal in healthy individuals. J. Clin. Endocrinol. Metab. 105, 725–738 (2020).
Stensen, S. et al. Effects of endogenous GIP in patients with type 2 diabetes. Eur. J. Endocrinol. 185, 33–45 (2021).
Assan, R., Attali, J. R., Ballerio, G., Boillot, J. & Girard, J. R. Glucagon secretion induced by natural and artificial amino acids in the perfused rat pancreas. Diabetes 26, 300–307 (1977).
Galsgaard, K. D. et al. Alanine, arginine, cysteine, and proline, but not glutamine, are substrates for, and acute mediators of, the liver-α-cell axis in female mice. Am. J. Physiol. Endocrinol. Metab. 318, E920–E929 (2020).
Rocha, D. M., Faloona, G. R. & Unger, R. H. Glucagon-stimulating activity of 20 amino acids in dogs. J. Clin. Invest. 51, 2346–2351 (1972).
Kuhara, T., Ikeda, S., Ohneda, A. & Sasaki, Y. Effects of intravenous infusion of 17 amino acids on the secretion of GH, glucagon, and insulin in sheep. Am. J. Physiol. Endocrinol. Metab. 260, E21–E26 (1991).
Ohneda, A., Parada, E., Eisentraut, A. M. & Unger, R. H. Characterization of response of circulating glucagon to intraduodenal and intravenous administration of amino acids. J. Clin. Invest. 47, 2305–2322 (1968).
Marliss, E. B., Aoki, T. T., Unger, R. H., Soeldner, J. S. & Cahill, G. F. Glucagon levels and metabolic effects in fasting man. J. Clin. Invest. 49, 2256–2270 (1970).
Dean, E. D. A primary role for α-cells as amino acid sensors. Diabetes 69, 542–549 (2020).
Finan, B., Capozzi, M. E. & Campbell, J. E. Repositioning glucagon action in the physiology and pharmacology of diabetes. Diabetes 69, 532–541 (2020).
Zmazek, J., Grubelnik, V., Markovič, R. & Marhl, M. Modeling the amino acid effect on glucagon secretion from pancreatic alpha cells. Metabolites 12, 348 (2022).
Müller, W. A., Faloona, G. R. & Unger, R. H. The effect of alanine on glucagon secretion. J. Clin. Invest. 50, 2215–2218 (1971).
Madison, L. L., Seyffert, W. A., Unger, R. H. & Barker, B. Effect of plasma free fatty acids on plasma glucagon and serum insulin concentrations. Metabolism 17, 301–304 (1968).
Luyckx, A. S. & Lefebvre, P. J. Arguments for a regulation of pancreatic glucagon secretion by circulating plasma free fatty acids (34511). Proc. Soc. Exp. Biol. Med. 133, 524–528 (1970).
Gerich, J. E., Langlois, M. & Schneider, V. Effects of alterations of plasma free fatty acid levels on pancreatic glucagon secretion in man. J. Clin. Invest. 53, 1284–1289 (1974).
Gross, R. & Mialhe, P. Free fatty acids and pancreatic function in the duck. Acta Endocrinol. 112, 100–104 (1986).
Collins, S. C., Salehi, A., Eliasson, L., Olofsson, C. S. & Rorsman, P. Long-term exposure of mouse pancreatic islets to oleate or palmitate results in reduced glucose-induced somatostatin and oversecretion of glucagon. Diabetologia 51, 1689–1693 (2008).
Radulescu, A., Gannon, M. C. & Nuttall, F. Q. The effect on glucagon, glucagon-like peptide-1, total and acyl-ghrelin of dietary fats ingested with and without potato. J. Clin. Endocrinol. Metab. 95, 3385–3391 (2010).
Raben, A., Holst, J. J., Madsen, J. & Astrup, A. Diurnal metabolic profiles after 14 d of an ad libitum high-starch, high-sucrose, or high-fat diet in normal-weight never-obese and postobese women. Am. J. Clin. Nutr. 73, 177–189 (2001).
Mandøe, M. J. et al. The 2-monoacylglycerol moiety of dietary fat appears to be responsible for the fat-induced release of GLP-1 in humans1. Am. J. Clin. Nutr. 102, 548–555 (2015).
Rodriguez-Diaz, R., Tamayo, A., Hara, M. & Caicedo, A. The local paracrine actions of the pancreatic α-cell. Diabetes 69, 550–558 (2020).
Cabrera, O. et al. The unique cytoarchitecture of human pancreatic islets has implications for islet cell function. Proc. Natl Acad. Sci. USA 103, 2334–2339 (2006).
Konstantinova, I. et al. EphA-Ephrin-A-mediated β cell communication regulates insulin secretion from pancreatic islets. Cell 129, 359–370 (2007).
Samols, E., Stagner, J. I., Ewart, R. B. L. & Marks, V. The order of islet microsvascular cellular perfusion is B → A → D in the perfused rat pancreas. J. Clin. Invest. 82, 350–353 (1988).
Almaça, J. & Caicedo, A. Blood flow in the pancreatic islet: not so isolated anymore. Diabetes 69, 1336–1338 (2020).
Kieffer, T. J., Heller, R. S., Unson, C. G., Weir, G. C. & Habener, J. F. Distribution of glucagon receptors on hormone-specific endocrine cells of rat pancreatic islets. Endocrinology 137, 5119–5125 (1996).
Kedees, M. H., Grigoryan, M., Guz, Y. & Teitelman, G. Differential expression of glucagon and glucagon-like peptide 1 receptors in mouse pancreatic alpha and beta cells in two models of alpha cell hyperplasia. Mol. Cell. Endocrinol. 311, 69–76 (2009).
Ishihara, H., Maechler, P., Gjinovci, A., Herrera, P. L. & Wollheim, C. B. Islet β-cell secretion determines glucagon release from neigbouring α-cells. Nat. Cell Biol. 5, 330–335 (2003).
Rodriguez-Diaz, R. et al. Paracrine interactions within the pancreatic islet determine the glycemic set point. Cell Metab. 27, 549–558.e4 (2018).
Wojtusciszyn, A., Armanet, M., Morel, P., Berney, T. & Bosco, D. Insulin secretion from human beta cells is heterogeneous and dependent on cell-to-cell contacts. Diabetologia 51, 1843–1852 (2008).
Huypens, P., Ling, Z., Pipeleers, D. & Schuit, F. Glucagon receptors on human islet cells contribute to glucose competence of insulin release. Diabetologia 43, 1012–1019 (2000).
Svendsen, B. et al. Insulin secretion depends on intra-islet glucagon signaling. Cell Rep. 25, 1127–1134.e2 (2018).
Zhu, L. et al. Intraislet glucagon signaling is critical for maintaining glucose homeostasis. JCI Insight 5, e127994 (2019).
Capozzi, M. E. et al. β Cell tone is defined by proglucagon peptides through cAMP signaling. JCI Insight 4, e126742 (2019).
Ahrén, B., Yamada, Y. & Seino, Y. The mediation by GLP-1 receptors of glucagon-induced insulin secretion revisited in GLP1- receptor knockout mice. Peptides 135, 170434 (2021).
Rodriguez-Diaz, R. et al. Alpha cells secrete acetylcholine as a non-neuronal paracrine signal priming beta cell function in humans. Nat. Med. 17, 888–892 (2011).
Fujita, Y. et al. Human pancreatic α- to β-cell area ratio increases after type 2 diabetes onset. J. Diabetes Investig. 9, 1270–1282 (2018).
Hædersdal, S., Lund, A., Knop, F. K. & Vilsbøll, T. The role of glucagon in the pathophysiology and treatment of type 2 diabetes. Mayo Clin. Proc. 93, 217–239 (2018).
Kelly, R. P. et al. Short-term administration of the glucagon receptor antagonist LY2409021 lowers blood glucose in healthy people and in those with type 2 diabetes. Diabetes Obes. Metab. 17, 414–422 (2015).
Kazda, C. M. et al. Evaluation of efficacy and safety of the glucagon receptor antagonist LY2409021 in patients with type 2 diabetes: 12- and 24-week phase 2 studies. Diabetes Care 39, 1241–1249 (2016).
Yabe, D. et al. Effects of DPP-4 inhibitor linagliptin and GLP-1 receptor agonist liraglutide on physiological response to hypoglycaemia in Japanese subjects with type 2 diabetes: a randomized, open-label, 2-arm parallel comparative, exploratory trial. Diabetes Obes. Metab. 19, 442–447 (2017).
Ahré́n, B. et al. Vildagliptin enhances islet responsiveness to both hyper- and hypoglycemia in patients with type 2 diabetes. J. Clin. Endocrinol. Metab. 94, 1236–1243 (2009).
Haedersdal, S. et al. Individual and combined glucose-lowering effects of glucagon receptor antagonism and dipeptidyl peptidase-4 inhibition. Diabetes 67, 274-LB (2018).
Kramer, C. K., Zinman, B., Choi, H., Connelly, P. W. & Retnakaran, R. The impact of chronic liraglutide therapy on glucagon secretion in type 2 diabetes: insight from the LIBRA trial. J. Clin. Endocrinol. Metab. 100, 3702–3709 (2015).
Hansen, L., Iqbal, N., Ekholm, E., Cook, W. & Hirshberg, B. Postprandial dynamics of plasma glucose, insulin, and glucagon in patients with type 2 diabetes treated with saxagliptin plus dapagliflozin add-on to metformin therapy. Endocr. Pract. 20, 1187–1197 (2014).
Okamoto, A., Yokokawa, H., Sanada, H. & Naito, T. Changes in levels of biomarkers associated with adipocyte function and insulin and glucagon kinetics during treatment with dapagliflozin among obese type 2 diabetes mellitus patients. Drugs R. D. 16, 255–261 (2016).
Daniele, G. et al. Dapagliflozin enhances fat oxidation and ketone production in patients with type 2 diabetes. Diabetes Care 39, 2036–2041 (2016).
Merovci, A. et al. Dapagliflozin improves muscle insulin sensitivity but enhances endogenous glucose production. J. Clin. Invest. 124, 509–514 (2014).
Ferrannini, E. et al. Metabolic response to sodium-glucose cotransporter 2 inhibition in type 2 diabetic patients. J. Clin. Invest. 124, 499–508 (2014).
Hædersdal, S. et al. The role of glucagon in the acute therapeutic effects of SGLT2 inhibition. Diabetes 69, 2619–2629 (2020).
Bagger, J. I., Knop, F. K., Lund, A., Holst, J. J. & Vilsbøll, T. Glucagon responses to increasing oral loads of glucose and corresponding isoglycaemic intravenous glucose infusions in patients with type 2 diabetes and healthy individuals. Diabetologia 57, 1720–1725 (2014).
Baron, A. D., Schaeffer, L., Shragg, P. & Kolterman, O. G. Role of hyperglucagonemia in maintenance of increased rates of hepatic glucose output in type II diabetics. Diabetes 36, 274–283 (1987).
Basu, R., Schwenk, W. F. & Rizza, R. A. Both fasting glucose production and disappearance are abnormal in people with ‘mild’ and ‘severe’ type 2 diabetes. Am. J. Physiol. Endocrinol. Metab. 287, 55–62 (2004).
Reaven, G. M., Chen, Y.-D. I., Golay, A., Swislocki, A. L. M. & Jaspan, J. B. Documentation of hyperglucagonemia throughout the day in nonobese and obese patients with noninsulin-dependent diabetes mellitus. J. Clin. Endocrinol. Metab. 64, 106–110 (1987).
Lund, A. et al. Higher endogenous glucose production during OGTT vs isoglycemic intravenous glucose infusion. J. Clin. Endocrinol. Metab. 101, 4377–4384 (2016).
Muscelli, E. et al. Separate impact of obesity and glucose tolerance on the patients. Diabetes 57, 1340–1348 (2008).
Shah, P., Basu, A, Basu, R. & Rizza, R. Impact of lack of suppression of glucagon on glucose tolerance in humans. Am. J. Physiol. 277, E283–E290 (1999).
Shah, P. et al. Lack of suppression of glucagon contributes to postprandial hyperglycemia in subjects with type 2 diabetees mellitus. J. Clin. Endocrinol. Metab. 85, 4053–4059 (2000).
Unger, R. H., Aguilar-Parada, E., Müller, W. A. & Eisentraut, A. M. Studies of pancreatic alpha cell function in normal and diabetic subjects. J. Clin. Invest. 49, 837–848 (1970).
Knop, F. K. et al. Reduced incretin effect in type 2 diabetes: cause or consequence of the diabetic state? Diabetes 56, 1951–1959 (2007).
Meier, J. J., Deacon, C. F., Schmidt, W. E., Holst, J. J. & Nauck, M. A. Suppression of glucagon secretion is lower after oral glucose administration than during intravenous glucose administration in human subjects. Diabetologia 50, 806–813 (2007).
Holst, J. et al. Regulation of glucagon secretion by incretins. Diabetes Obes. Metab. 13, 89–94 (2011).
Juel, C. T. B. et al. 53 rd EASD annual meeting of the European Association for the Study of Diabetes. Diabetologia 60, 1–608 (2017).
Wali, J. & Thomas, H. Pancreatic alpha cells hold the key to survival. eBioMedicine 2, 368–369 (2015).
Grøndahl, M. F. G. et al. Glucagon clearance is preserved in type 2 diabetes. Diabetes 71, 73–82 (2022).
Wewer Albrechtsen, N. J. et al. Hyperglucagonemia correlates with plasma levels of non-branched-chain amino acids in patients with liver disease independent of type 2 diabetes. Am. J. Physiol. Gastrointest. Liver Physiol. 314, G91–G96 (2018).
Suppli, M. P., Lund, A., Bagger, J. I., Vilsbøll, T. & Knop, F. K. Involvement of steatosis-induced glucagon resistance in hyperglucagonaemia. Med. Hypotheses 86, 100–103 (2016).
Suppli, M. P. et al. Glucagon resistance at the level of amino acid turnover in obese subjects with hepatic steatosis. Diabetes 69, 1090–1099 (2020).
Wewer Albrechtsen, N. J. et al. Evidence of a liver–alpha cell axis in humans: hepatic insulin resistance attenuates relationship between fasting plasma glucagon and glucagonotropic amino acids. Diabetologia 61, 671–680 (2018).
Holst, J. J., Albrechtsen, N. J. W., Pedersen, J. & Knop, F. K. G. Glucagon and amino acids are linked in a mutual feedback cycle: the liver-α-cell axis. Diabetes 66, 235–240 (2017).
Galsgaard, K. D. et al. Disruption of glucagon receptor signaling causes hyperaminoacidemia exposing a possible liver-alpha-cell axis. Am. J. Physiol. Endocrinol. Metab. 314, E93–E103 (2018).
Hædersdal, S. et al. 1952-P: Glucagon receptor antagonism increases plasma amino acids and glucagon. Diabetes 68, 1952-P (2019).
Solloway, M. J. et al. Glucagon couples hepatic amino acid catabolism to mTOR-dependent regulation of α-cell mass. Cell Rep. 12, 495–510 (2015).
Dean, E. D. et al. Interrupted glucagon signaling reveals hepatic α cell axis and role for L-glutamine in α cell proliferation. Cell Metab. 25, 1362–1373.e5 (2017).
Kim, J. et al. Amino acid transporter Slc38a5 controls glucagon receptor inhibition-induced pancreatic α cell hyperplasia in mice. Cell Metab. 25, 1348–1361.e8 (2017).
Lee, Y. H., Wang, M.-Y., Yu, X.-X. & Unger, R. H. Glucagon is the key factor in the development of diabetes. Diabetologia 59, 1372–1375 (2016).
Ellingsgaard, H. et al. Interleukin-6 regulates pancreatic α-cell mass expansion. Proc. Natl Acad. Sci. USA 105, 13163–13168 (2008).
Marroqui, L. et al. Pancreatic α cells are resistant to metabolic stress-induced apoptosis in type 2 diabetes. eBioMedicine 2, 378–385 (2015).
Pettus, J. et al. Glucagon receptor antagonist LGD-6972 significantly lowers HbA1c and is well tolerated after 12-week treatment in patients with type 2 diabetes mellitus (T2DM) on metformin. Diabetes 67, 73-OR (2018).
Kazierad, D. J., Chidsey, K., Somayaji, V. R., Bergman, A. J. & Calle, R. A. Efficacy and safety of the glucagon receptor antagonist PF-06291874: a 12-week, randomized, dose-response study in patients with type 2 diabetes mellitus on background metformin therapy. Diabetes Obes. Metab. 20, 2608–2616 (2018).
Pettus, J. et al. Effect of a glucagon receptor antibody (REMD-477) in type 1 diabetes: a randomized controlled trial. Diabetes Obes. Metab. 20, 1302–1305 (2018).
Pettus, J. et al. Glucagon receptor antagonist volagidemab in type 1 diabetes: a 12-week, randomized, double-blind, phase 2 trial. Nat. Med. 28, 2092–2099 (2022).
Pearson, M. J., Unger, R. H. & Holland, W. L. Clinical trials, triumphs, and tribulations of glucagon receptor antagonists. Diabetes Care 39, 1075–1077 (2016).
Ambery, P. et al. MEDI0382, a GLP-1 and glucagon receptor dual agonist, in obese or overweight patients with type 2 diabetes: a randomised, controlled, double-blind, ascending dose and phase 2a study. Lancet 391, 2607–2618 (2018).
Tillner, J. et al. A novel dual glucagon-like peptide and glucagon receptor agonist SAR425899: Results of randomized, placebo-controlled first-in-human and first-in-patient trials. Diabetes Obes. Metab. 21, 120–128 (2019).
Alba, M., Yee, J., Frustaci, M. E., Samtani, M. N. & Fleck, P. Efficacy and safety of glucagon‐like peptide‐1/glucagon receptor co‐agonist JNJ ‐64565111 in individuals with obesity without type 2 diabetes mellitus: a randomized dose‐ranging study. Clin. Obes. 11, e12432 (2021).
Linong, J. et al. Safety and efficacy of a GLP-1 and glucagon receptor dual agonist mazdutide (IBI362) 9 mg and 10 mg in Chinese adults with overweight or obesity: a randomised, placebo-controlled, multiple-ascending-dose phase 1b trial. Lancet 54, 101691 (2022).
Finan, B. et al. A rationally designed monomeric peptide triagonist corrects obesity and diabetes in rodents. Nat. Med. 21, 27–36 (2015).
Knerr, P. J. et al. Next generation GLP-1/GIP/glucagon triple agonists normalize body weight in obese mice. Mol. Metab. 63, 101533 (2022).
Coskun, T. et al. LY3298176, a novel dual GIP and GLP-1 receptor agonist for the treatment of type 2 diabetes mellitus: from discovery to clinical proof of concept. Mol. Metab. 18, 3–14 (2018).
Frias, J. P. et al. Efficacy and safety of LY3298176, a novel dual GIP and GLP-1 receptor agonist, in patients with type 2 diabetes: a randomised, placebo-controlled and active comparator-controlled phase 2 trial. Lancet 392, 2180–2193 (2018).
Cegla, J. et al. Coinfusion of low-dose GLP-1 and glucagon in man results in a reduction in food intake. Diabetes 63, 3711–3720 (2014).
Author information
Authors and Affiliations
Contributions
S.H. and A.A. researched data for the article. All authors contributed substantially to discussion of the content. S.H. wrote the article. All authors reviewed and/or edited the manuscript before submission.
Corresponding authors
Ethics declarations
Competing interests
S.H. has served as a consultant for Novo Nordisk. F.K.K. has served on scientific advisory panels, been part of speakers bureaus, served as a consultant to and/or received research support from Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Carmot Therapeutics, Eli Lilly, Gubra, MedImmune, MSD/Merck, Mundipharma, Norgine, Novo Nordisk, Sanofi, ShouTi, Zealand Pharma and Zucara, and is a minority shareholder in Antag Therapeutics. T.V. has served on scientific advisory panels, been part of speakers bureaus, and served as a consultant to and/or received research support from Amgen, AstraZeneca, Boehringer Ingelheim, BMS, Eli Lilly, Gilead, GSK, Mundipharma, MSD/Merck, Novo Nordisk, Sanofi and Sun Pharmaceuticals. A.A. has no competing interests.
Peer review
Peer review information
Nature Reviews Endocrinology thanks Nigel Irwin and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hædersdal, S., Andersen, A., Knop, F.K. et al. Revisiting the role of glucagon in health, diabetes mellitus and other metabolic diseases. Nat Rev Endocrinol 19, 321–335 (2023). https://doi.org/10.1038/s41574-023-00817-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41574-023-00817-4
This article is cited by
-
Connections between body composition and dysregulation of islet α- and β-cells in type 2 diabetes
Diabetology & Metabolic Syndrome (2024)
-
Poly-Agonist Pharmacotherapies for Metabolic Diseases: Hopes and New Challenges
Drugs (2024)
-
Advances in basic research on glucagon and alpha cells
Diabetology International (2024)
-
A century of glucagon
Nature Reviews Endocrinology (2023)