Abstract
Brain structure and function are intimately linked, however this association remains poorly understood and the complexity of this relationship has remained understudied. Healthy aging is characterised by heterogenous levels of structural integrity changes that influence functional network dynamics. Here, we use the multilayer brain network analysis on structural (diffusion weighted imaging) and functional (magnetoencephalography) data from the Cam-CAN database. We found that the level of similarity of connectivity patterns between brain structure and function in the parietal and temporal regions (alpha frequency band) is associated with cognitive performance in healthy older individuals. These results highlight the impact of structural connectivity changes on the reorganisation of functional connectivity associated with the preservation of cognitive function, and provide a mechanistic understanding of the concepts of brain maintenance and compensation with aging. Investigation of the link between structure and function could thus represent a new marker of individual variability, and of pathological changes.
Similar content being viewed by others
Introduction
The brain is one of the most complex biological systems. One of its fascinating aspects, which remains largely unknown, is how wide varieties of brain rhythms and temporally-specific activity patterns1 can emerge from a static network architecture2. Addressing this issue is a major fundamental endeavour for cognitive neuroscience, which can also improve our understanding of brain changes across the lifespan and our ability to detect pathological processes. Previous work has mostly focused on characterising brain structure (i.e., grey matter and white matter) or brain function (i.e., memory, motor function or cognitive control)3. These unimodal studies greatly advanced our understating of brain networks and of their associations with cognition4. However, brain network analysis methods, such as graph theory, have been applied across modalities to study the interaction between structure and function, showing strong associations between these dimensions5,6. Since these seminal studies, the relationship between brain structure and function has been the focus of intense reflection and methodological development since this relationship is central to many cognitive domains, evolves with age and is affected by pathologies5. Here, we investigate these issues in light of age-related brain changes associated with changes in brain structure that influence neural dynamics7, which could further our understanding of the large heterogeneity of individual cognitive trajectories observed during this life period. In particular, structure–function interactions could be central to further understanding the preservation (i.e., maintenance8 or compensation9) or the decline of cognitive performance during ageing.
Studying the relationships between white matter fibres (acquired by DWI—diffusion-weighted imaging) and blood-oxygen-level-dependent (BOLD) signal (acquired by fMRI—functional magnetic resonance imaging), previous studies have shown correlations between brain structure and function throughout the lifespan, and particularly across development10,11, and during the performance of cognitive tasks12. Also, in a healthy older population, Burzynska et al.13 showed that individuals with preserved white matter fibre integrity had a higher BOLD signal associated with better cognitive performance (see also14,15). Many studies have thus focused on this link between structure and function using high-spatial-resolution techniques such as fMRI. However, due to their constrained temporal resolution, age-related changes in the dynamics of the involved networks remain largely understudied.
Previous work has also demonstrated interactions between brain structure and function using high-temporal resolution techniques, such as magnetoencephalography (MEG) or electroencephalography (EEG). Indeed, fluctuations in the synchrony and transfer entropy (i.e., directionality) of brain activity have long been considered as noise to be controlled, whereas today, they have been reappraised as a fundamental aspect of brain communication16,17. These studies have notably highlighted that EEG connectivity is associated with structural connectivity measures in young adults18. With healthy ageing, Hinault et al.19,20 showed that a decrease in white matter fibre integrity is negatively associated with neural synchronisation between brain regions. However, for all these studies, the interpretation of these interactions is limited as it does not account for the full complexity of such a relationship.
A recent approach enables evaluating the relationships between different neuroimaging modalities by constructing a multiplex network model of the brain21. This approach allows the creation of a network in which each region is connected to itself across different layers22. This technique has already been used in pathology, such as schizophrenia23 and Alzheimer’s disease24,25, allowing to highlight brain changes that were not detected in unimodal analyses. Recently, the study by Battiston et al.26, investigating network connectivity by combining fMRI and DWI data in a two-layer multiplex network, revealed relevant relationships between structural and functional brain networks, showing that this technique is an appropriate choice for the study of brain network connectivity. Some studies have also investigated changes in functional connectivity in healthy27 or pathological28,29 participants using a multiplex approach applied on MEG data. Thus, multiplex brain networks can be used to study the structure-function relationship in healthy ageing. It seems, therefore, necessary to quantify the heterogeneity of this structure/function relationship in relation to cognitive heterogeneity. Moreover, previous work30 suggested that alterations in brain structure in the presence of delayed and/or noisier brain communications. Such a combination of DWI (structural) and MEG (functional) data in a multiplex connectome in healthy ageing is therefore important to identify markers of individual differences and early brain ageing effects, preceding major structural changes and loss of functional communications. These changes can lead to deleterious functional consequences20,31 or compensatory functional adjustments32. In particular, the functional role of the regions could be affected by changes in brain structure. Indeed, recent work using fMRI has shown in humans the presence of a functional asymmetry between brain regions in terms of afferent and efferent information transfer33. Other computer modelling work has also shown a relationship between network topology and information directionality, in particular, by identifying certain brain regions (or nodes) as targets and sources of information34. This method, therefore, appears ideal to clarify the association between brain structure and cortical dynamics, to identify the mechanisms underlying cognitive heterogeneity with ageing, and the functional adjustments allowing the maintenance of cognitive function.
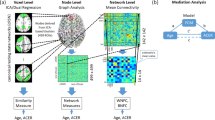
Here, we propose a multiplex network approach with MEG and DWI data in the context of healthy ageing and the associated non-lesional brain changes35 (see Fig. 1). We used the multiplex participation coefficient as an indicator of the similarity of connectivity between brain structure and function: a high level of this coefficient corresponded to a similarity of connectivity patterns between these modalities whereas a low level corresponded to a divergence of connectivity patterns between these modalities. We investigated age-related changes in brain structure and function in young and older healthy participants from the Cam-CAN database (Cambridge Centre for Aging and Neuroscience36,37). This database includes multimodal neuroimaging data (MEG, MRI and DWI) as well as cognitive performance evaluation for each individual. Our objectives were twofold: (i) To investigate changes in the interaction between structural integrity levels and synchronised functional networks between young and old individuals, with the underlying hypothesis that a decrease in white matter integrity could negatively impact brain function. (ii) To study the impact of such structure–function relationship on participants’ cognitive performance, where we expected that these changes would be associated with cognitive performance and reveal unique individual differences therein. Compensatory adjustments or maintenance of brain function at the same level as young adults would result in the preservation of cognitive performance. Such results could clarify and better characterise maladaptive and compensatory brain communication changes in the presence of ageing structural networks.
This multiplex network was built with two layers: one representing functional connectivity (FC) from MEG data, either PLV or TE data; the other layer representing structural connectivity (SC) from DWI (anisotropic fraction) data, i.e., FA data. MEG magnetoencephalography, DWI diffusion-weighted imaging, PLV phase locking value, TE transfer entropy, FC functional connectivity, SC structural connectivity.
Results
Two groups of participants (22–29 years for the younger group and 60–69 years for the older group) were formed from the Cam-CAN36,37 database. Connectivity analyses were performed on MEG data, and in particular, two measures were studied: phase locking value (PLV), which measures synchrony between regions, and transfer entropy (TE), which measures the directionality of the coupling between brain regions. TE is complementary to the synchrony measure (PLV) as it provides an estimation of directed connectivity. These measures have already been used in previous works investigating age-related changes28,29. The data from these two measures were combined with DWI data to form two multiplex structure–function networks (see Fig. 1). From these networks, the multiplex participation coefficient could be calculated. This coefficient was then studied to determine the level of similarity of connectivity between the two layers (structural and functional) of the network. The different phases of data processing, creation of multiplex networks and statistical analysis are described in the materials and methods section.
The positive association between multiplex participation coefficients and cognitive performance in older adults
We first studied a multiplex network composed of PLV and DTI data. Our main objective was to study the effect of healthy ageing on structural and functional connectivity and its association with cognitive abilities (measured with neuropsychological tests assessing working and short-term memory, reasoning ability, executive functions and general cognitive functions; see materials and methods for more information). Thus, we determined which region and which frequency bands age-related changes in multiplex participation coefficient could be associated with cognitive performance. First, we identified the regions and frequency bands that differed between age groups and were associated with cognition: the left temporal and right parietal regions in the alpha frequency band (these two regions showed, respectively, a decrease or an increase in participation in the older individuals compared to the younger). For other regions and frequency bands showing differences, they were not associated with cognitive performance, see Fig. S1 in supplementary. We found that, for both of these regions, increased multiplex participation coefficient levels were positively associated with cognitive performance in older adults (left temporal/MMSE test, r = 0.313, p = 0.034; right parietal/MMSE test, r = 0.393, p = 0.007; Fig. 2). No association was found in young adults.
a Distribution of the young and old groups in the left inferior temporal region (t-test) for the multiplex participation coefficient in the alpha frequency band for the measure of synchrony (PLV) and positive association between this level of multiplex participation coefficient and MMSE score. b Distribution of the young and old groups in the right parietal region (t-test) for the multiplex participation coefficient in the alpha frequency band for the measure of synchrony (PLV) and positive association between this level of multiplex participation coefficient and MMSE score in older adults. The level of education was controlled as a covariate. All results were adjusted for multiple comparisons using FDR corrections at q < 0.05. n = 46 participants per group. The black vertical line represents the standard error of the mean. *p < 0.05 **p < 0.01.
Maintaining a lower level of multiplex participation coefficient than younger adults is positive for the older population
To further analyse these results, subgroup analyses were performed for these two regions. To do this, participants were grouped according to the level of participation coefficient in each region, forming two groups of older individuals. The older subgroups (i.e., Low participation, High participation; see Table S1 to Table S4 in supplementary data for the characteristics of each subgroup) did not differ on any measure (e.g., age, gender ratio, level of education and general cognitive performance) other than the level of multiplex participation coefficient (left temporal and right parietal regions). For the left temporal region, young adults differ from both older subgroups, and both subgroups also significantly differ from each other: the level of the participation coefficient was significantly higher for the High participation subgroup than the younger group (p = 0.009). The Low participation subgroup showed lower multiplex participation levels than both younger individuals and the High participation subgroup (p < 0.001 for both comparisons). The Low participation subgroup showed better cognitive performance on the VSTM test than the High participation subgroup (r = 0.584, p = 0.009; Fig. 3a). For the right parietal region, young adults differ from the High participation subgroup, but not with the Low participation subgroup. We observed that the Low participation subgroup (with similar low participation as younger individuals, p = 0.962) showed better cognitive performance on the VSTM test (r = 0.475, p = 0.040; no association with cognition for the high participation older subgroup; Fig. 3b). Replication of the main findings using wPLI ((weighted Phase Lag Index analyses) see Fig. S2) (Fig. 3). Multiplex participation coefficient level differences between young and older subgroups and association with cognition.
a Distribution of young adults and older adults’ subgroups for the multiplex participation coefficient in the left temporal region for the measure of synchrony (PLV) in the alpha frequency band. The positive association between participation in the left temporal region and VSTM scores for the Low participation subgroup (regression test; no association with cognition for the High participation older subgroup). b Distribution of the young adults and older adults’ subgroups for the multiplex participation coefficient in the right parietal region in the alpha frequency band. The positive association between participation in the right parietal region and VSTM scores for the Low participation older subgroup (regression test; no association with cognition for the high participation older subgroup). c Distribution of young adults and older adults’ subgroups for the multiplex participation coefficient in the right parietal region in the alpha frequency band for the measure of directionality (TE). The positive association between the participation of the right parietal region and VSTM scores for the Low participation subgroup (regression test; negative association with cognition for the High participation older subgroup: r = −0.491, p = 0.033). The level of education was controlled as a covariate. All results were adjusted for multiple comparisons using FDR corrections at q < 0.05. n = 46 participants per group. The black vertical line represents the standard error of the mean. *p < 0.05; **p < 0.01; ***p < 0.001.
Age-related changes in network coupling directionality are positively associated with cognitive performance
Following these results, we examined ageing effects and individual differences in these regions using directed functional couplings. For this, we used the multiplex network formed from TE and DTI data. For the right parietal region only, in the alpha band, we observed an increase in inward directionality (i.e., directed towards the right parietal region) in older individuals compared to younger individuals (t-test, p = 0.038; Fig. 4a). See Supplementary Fig. S3 for consistent results involving gamma frequency bands. This increased participation in the inward direction for the right parietal region with ageing was positively associated with performance in the VSTM test (r = 0.314, p = 0.034; Fig. 4c).
a Increased inward directionality (i.e., directed towards the right parietal region) in older adults relative to younger adults (t-test) for the right parietal region in the alpha frequency band. b Preserved outward direction (i.e., directed towards other regions of the network) in older adults relative to the younger group for the right parietal region in the alpha frequency band. c Positive association between the increased multiplex participation coefficient in the inward direction for the right parietal region in an alpha frequency band and VSTM test scores (regression test) in the older group. The level of education was controlled as a covariate. All results were adjusted for multiple comparisons using FDR corrections at q < 0.05. n = 46 participants per group. The black vertical line represents the standard error of the mean.*p < 0.05.
To further analyse these results, we investigated differences in the same subgroups as in the first part (PLV/DWI) of the results.
We observed that the Low participation subgroup, showing increased inward-directed couplings in the right parietal region, also showed better cognitive performance on the VSTM test (r = 0.463, p = 0.046; Fig. 3c) than the High participation subgroup. Supporting these results, the High participation older subgroup showed lower cognitive performance on the VSTM test (r = −0.491, p = 0.033; Fig. S3 in Supplementary data).
The respective contribution of each network layer in younger and older adults
Degree analyses (number of connections) were performed on the respective contribution of each layer and suggest that the structural layer makes the largest contribution to the reported results, as the degree was larger in the structural layer (DWI) than in the functional layer (PLV/TE) for the right parietal region (difference between DWI/PLV and DWI/TE layers, p = 0.001; see Fig. S4 in Supplementary data). The left temporal region follows this trend as well (difference between DWI/PLV layers, p = 0.086; difference between DWI/TE layers, p = 0.001).
Interestingly, we examined the contribution of the different layers of connectivity within both older subgroups compared to the younger group for alpha temporal and parietal functional activity (see Fig. S5). We observed that the older subgroup that showed lower cognitive performance (High participation) did show the difference in contribution between the two functional layers (differences between PLV and TE, p < 0.001), in contrast to the Low older subgroup that did show better associations with cognitive performance (p < 0.05). These results were found only for the left temporal region.
Unique detection of subgroups relative to unimodal network analyses
Finally, we performed unimodal analyses (DWI and MEG) to determine the added value of multiplex analyses relative to functional or structural network investigations (see Fig. S6). Regarding the structural layer, we replicated the significant difference in white matter integrity between young and old groups (p < 0.001) on global connectivity data. Regarding the functional layer, we did not find a significant difference between younger and older adults at the global matrix level in the alpha frequency band. At the nodal level, no difference between subgroups was observed in functional or structural networks, in contrast with multilayer analyses.
Discussion
In this study, we have shown the importance of integrating structural and functional information together to better understand ageing effects. Our objectives were twofold: to investigate changes in the brain structure-function association with age and to determine the impact of changes in this association on cognitive performance in older individuals. Our approach relied on a two-layer multiplex network, with a structural layer based on DWI data and another layer based on resting-state MEG data, to identify changes between younger and older healthy individuals from the Cam-CAN repository and to further understand maintenance8 and compensation9 phenomena observed in ageing. Two aspects of functional network connectivity were studied: phase synchrony and directed connectivity. We showed the existence of inter-individual variability at the functional level in older individuals at rest that was associated with cognitive performance. Low structure/function multiplex participation coefficient for structure/synchrony and structure/information transfer in temporal and parietal regions in the alpha frequency band, similar to young adults in the parietal region, was associated with preserved cognitive performance in older individuals.
The multiplex participation coefficient can be considered as an indicator of co-dependence between modalities: a high level of this coefficient would indicate a high similarity of connectivity between brain structure and function, whereas a low coefficient would indicate a dissociation of structure and function connectivity. Subgroup analyses based on this coefficient allowed the detection of heterogeneity within cognitively healthy older individuals. First, we showed that lower levels of structure/synchrony participation relative to younger adults might be beneficial for cognitive performance. Second, using multiplex structure/directed connectivity network analyses, we showed that low levels of participation in the inward direction (i.e., corresponding to couplings directed towards a given region), to a similar level than young adults, for the regions investigated was beneficial for cognitive performance. In contrast, an increase in this coefficient was found to be negatively associated with cognitive performance. These subgroups were not found in unimodal analyses.
The inferior temporal and supramarginal parietal gyri are both considered to be brain structural cores38. They are also both part of the default mode network39,40 (DMN), a network activated at rest and whose activity has been associated with memory and executive performance41. Moreover, the alpha frequency band is involved in the structuring of neural rhythms and has notably been associated with attention allocation and the inhibition of couplings not required for the task42,43. By assessing the interaction between brain structure and the alpha frequency band, the present results contribute to existing frameworks about this central brain rhythm42, as they did not consider such an association. Thus, the disengagement of the DMN, as well as the posterior alpha reduction, are critical for cognition and are impacted by aging44,45. Results reveal that, at the scale of our study (i.e., early structural damage and small individual differences in microstructural integrity), certain regions and certain frequency bands are more affected than others46. Recent work47 show that structure–function coupling is heterogeneous according to brain region and frequency band. Structure–function coupling was found to be greater in the slower and intermediate frequency bands than in the faster frequency bands. Moreover, the alpha band is a central frequency band that previously showed significant age-related changes30. Alpha oscillations may play a role in the activation or deactivation of the DMN48, and Jann et al.49 showed that the BOLD correlates of alpha-band synchronisation in the resting state were localised in brain regions involving the DMN. Age-related structural changes would be central to these changes and would impact brain function. Our results could indicate that in the presence of fine changes in brain architecture, some older individuals will show a lower level of participation coefficient (i.e., a dissociation of connectivity patterns between brain structure and function) than others, which may be due to compensatory functional readjustments involving the alpha frequency band. These changes would enable better cognitive performance than individuals who will not make these functional readjustments, with higher levels of participation coefficient (i.e., a stronger association of connectivity patterns between brain structure and function). Future, longitudinal investigations remain important to further clarify this association.
Our results also reveal that the subgroup of older individuals who showed lower structure/function multiplex participation coefficient and for whom these changes were positively associated with cognitive performances showed no difference in contribution (calculated by measuring connectivity levels in each layer) between the phase synchrony and information transfer layers. Conversely, an increase in the contribution of the phase synchrony layer compared to information transfer was found for the group without association with cognition. These results were only observed in the left inferior temporal region. These results could indicate inefficient connectivity in these individuals (i.e., synchronised couplings with little to no information exchange). The observation of synchronised activity may, therefore, be related to cognitive function but may also be dissociated from it. Thus, considering synchrony in association with information transfer seems important to clarify age-related changes and to distinguish efficient communications from inefficient/maladaptive network couplings. These communications are highly dependent on the integrity of the underlying structural network, and investigating the respective contribution of structure and function through a multiplex network could also allow distinguishing these functional connectivity patterns in pathologies. Indeed, an increase in neuronal synchrony can be observed in neurodegenerative pathologies and has been considered as maladaptive changes (for a review, see50). Further investigations of this distinction could lead to the identification of new markers of subsequent decline and progression of neurodegenerative pathologies.
Taken together, these results demonstrate the importance of the relationship between brain structure and function, particularly with advancing age. Thus, in a population of healthy older individuals, alterations in white matter fibres appear to influence the stability of the functional networks they underlie. These functional changes would then influence an individual’s cognitive performance. The concept of maintenance (8) would, therefore, be characterised by a relative preservation of white matter fibres, with no changes in cerebral function and a relative preservation of cognitive performance. In that case, the link between brain structure and function would, therefore, remain similar to that of younger individuals. Conversely, cognitive decline would be related to fine alterations of white matter fibres without a reorganisation of functional networks. The relationship between brain structure and function is altered and tends towards a stronger similarity, in contrast to younger individuals. Finally, compensation (9) would occur when white matter fibres are altered, and compensatory functional reorganisation takes place. This reorganisation of functional networks would then enable individuals to maintain their cognitive performance. The link between structure and function is thus different, leading to greater dissimilarity in connectivity patterns.
Several methodological considerations should be discussed regarding the reported results. First, the study of resting-state activity partly limits the direct investigation of the neural bases of cognitive processes, as it might be less directly associated with cognitive functioning than task-related activity51. Second, the use of phrase synchrony measures (phase locking value or phase lag index) could potentially be impacted by volume conduction, although the main results were replicated across methods. Furthermore, transfer entropy measures are not affected by volume conduction52 and provide converging findings to phase synchrony. In addition, multilayer analyses investigate local levels of similarity in connectivity between structure and function for each region of the atlas. Third, the analysis of layer contributions only showed results for the left inferior temporal region, which does not allow us to generalise our interpretations to the entire brain. Thus, the pattern of layer contributions may be different in other regions and frequency bands2, although the reported changes were central in the context of healthy ageing. Longitudinal studies could further validate our interpretations and improve our knowledge of other brain regions. Fourth, the reduced size of the subgroups may lead to spurious correlations. Complementary analyses using the participation coefficient as a continuous variable have, however, enabled replication of the results observed in the older subgroup analyses. Fifth, the age groups studied do not allow us to see the changes in the structure/function link that may occur in middle age. However, these comparisons between young and old groups are in line with previous studies on healthy ageing. Further longitudinal studies of this link are needed to complete our results. Sixthly, in addition to the use of measures such as phase synchrony and entropy transfer with MEG, other methods are also relevant for studying the dynamics of brain connectivity, in particular with fMRI53. Seventh, this study, like the previous ones, is still based on correlational evidence, particularly in the link between cerebral connectivity and cognition. However, our approach allows us to better consider the complexity of the relationship between structure and function. Eighth, in this study, we used sLoreta to determine source locations. Although alternative source reconstruction methods could have been used, previous work from the team showed consistent results across methods54. Finally, these results provide a better understanding of the relationship between brain structure and function, highlighting the influence of fine structural alterations on functional connectivity changes with ageing. Although it seems less consistent with the literature7, the opposite may also be possible (i.e., an alteration in white matter fibres following a decrease in functional connectivity in certain regions).
Several questions remain about the association between brain structure and function2,7. Indeed, this relationship undergoes crucial changes throughout the lifespan, as well as following several pathologies. The structure–function coupling also appears to fluctuate both over time and regionally. Although structural changes appear to drive changes in coupling between regions, brain functions are not solely determined by brain structure. Decreased integrity seems to have an impact on neuronal synchrony and information exchange, and these changes are distinctly associated with cognitive performance in individuals. Although this would be less consistent with age-related changes on structural network55, this causality can also be reversed, with reduced synchrony and information exchange impacting white matter integrity. Here, we defined multiplex structure-function models in the context of healthy brain ageing to better understand the heterogeneity of these changes across individuals (see Fig. 5 for a schematic representation of this model). In particular, we show its impact on cognitive performance, which improves our knowledge of different theoretical models of ageing, such as concepts of cognitive maintenance8 and compensation9. Maintenance would thus be characterised by an imbalance in the contribution of phase synchrony and transfer information, with a higher level of contribution from PLV than from TE. Moreover, the level of similarity of connectivity between brain structure and function would be very low. The cognitive decline would also be associated with an imbalance in the contribution of phase synchrony and transfer of information. However, in contrast to maintenance, the level of similarity of connectivity between brain structure and function would be very high. Finally, Compensation would be characterised by a balance in the contribution of phase synchrony and transfer information. The level of similarity of connectivity between structure and brain function would be very low, in the same way as in the maintenance concept. Indeed, a dissociation of connectivity patterns between structure and function has been associated with the preservation of cognitive performance. Importantly, these individual markers were not found in unimodal analyses. This new approach might yield a better understanding of the brain, which could be useful in clinical applications to better understand certain pathologies such as neurodegenerative diseases and, more generally, to further our understanding of the link between structure and function in the brain.
a Level of contribution for PLV and TE. b Participation coefficient for PLV/DWI and TE/DWI multiplex network. c Summary of the relation between the level of similarity of contribution from PLV/TE, participation coefficient and concepts of ageing. DWI diffusion tensor imaging, PLV phase locking value, TE transfer entropy, FC functional connectivity, SC structural connectivity.
Methods
Participants
We selected only individuals in the 20–30 years and 60–70 years age groups who had completed all the neuropsychological tests and brain imaging acquisitions, resulting in 46 individuals per group. All participants aged 20–30 years and 60–70 years were selected from the Cam-CAN database36,37, in line with the demographic characteristics of individuals recruited in previous works19,56. Thus, we analysed data from 46 young (29 women and 17 men; aged 22–29 years) and 46 older healthy adults (29 women and 17 men; aged 60–69 years) whose MEG data have already been published57 (Table 1). All participants were right-handed, showed normal cognitive functioning58 (Montreal Cognitive Assessment (MoCA) score >26), and had no neurological or psychiatric conditions. Participants had no depression problems measured with the Hospital Anxiety and Depression Scale (HADS59) and self-report (see Fig. S7 in supplementary for a detail of the selection process).
This study is conducted in compliance with the Helsinki Declaration and has been approved by the local ethics committee, Cambridgeshire 2 Research Ethics Committee (reference: 10/H0308/50). Each participant contributed to the study after written informed consent. All ethical regulations relevant to human research participants were followed.
Behavioural measures
A detailed description of behavioural measures can be found in supplementary materials (see also refs. 36,37). Cognitive performance was assessed with the mini-mental state evaluation60 (MMSE) as a measure of general cognitive functioning, the accuracy of the visual short-term memory61 (VSTM) as a test of short-term memory and working-memory maintenance, the Cattel test62 measuring reasoning ability, and the Hotel Test63 assessing executive functions (notably planning abilities). Despite significant differences between the two groups, all participants had normal cognitive functions. These variables were added as covariates in statistical analyses.
MEG, structural MRI and DWI data acquisition
Resting MEG activity was measured for 10 min, eyes closed (sampling rate: 1 kHz, bandpass filter: 0.03–330 Hz) with a 306-channel MEG system. Participants’ 3D-T1 MRI images were acquired on a 32-channel 3 T MRI scanner. The following parameters were used: repetition time = 2250 ms; echo time = 2.99 ms; inversion time = 900 ms; flip angle = 9°; field of view = 256 mm × 240 mm × 192 mm; voxel size = 2 mm isotropic; GRAPPA acceleration factor = 2; acquisition time = 4 min and 32 s. DWI data were obtained with the following parameters: repetition time = 9100 ms; echo time = 104 ms; inversion time = 900 ms; field of view = 192 mm × 192 mm; 66 axial slices; voxel size = 2 mm isotropic; B0 = 0.1000/2000 s/mm2; acquisition time = 10 min and 2 s, readout time 0.0684 (echo spacing = 0.72 ms, EPI factor = 96). See https://camcan-archive.mrc-cbu.cam.ac.uk/dataaccess/ for more information.
MEG data pre-processing
The Elekta Neuromag MaxFilter 2.2 has been applied to MEG data (temporal signal space separation (tSSS): 0.98 correlation, 10 s window; bad channel correction: ON; motion correction: OFF; 50 Hz + harmonics (mains) notch). Afterwards, artefact rejection, filtering (0.3–100 Hz bandpass), temporal segmentation into epochs, averaging and source estimation were performed using Brainstorm64. In addition, physiological artefacts (e.g., blinks and saccades) were identified and removed using spatial space projection of the signal. In order to improve the accuracy of the source reconstruction, the FreeSurfer65 software was used to generate cortical surfaces and automatically segment them from the cortical structures from each participant’s T1-weighted anatomical MRI. The advanced MEG model was obtained from a symmetric boundary element method (BEM model66; OpenMEEG67), fitted to the spatial positions of each sensor68. A cortically constrained sLORETA procedure was applied to estimate the cortical origin of the scalp MEG signals. The estimated sources were then smoothed and projected into standard space (i.e., ICBM152 template) for comparisons between groups and individuals while accounting for differences in anatomy (i.e., grey matter). This procedure was applied for the entire recording duration.
Connectivity analyses
Phase-locking value analyses69 (PLV) were used to determine the functional synchrony between regions of interest. PLV estimates the variability of phase differences between two regions over time. If the phase difference varies little, the PLV is close to 1 (this corresponds to high synchronisation between the regions), while the low association of phase difference across regions is indicated by a PLV value close to zero. To ensure PLV results did not reflect volume conduction artefacts, additional control analyses were conducted using phase lag index (weighted PLI analyses) replicated our main subgroups analyses results (see Fig. S2). Because PLV is an undirected measure of functional connectivity and to investigate brain dynamics with complementary metrics, analyses of transfer entropy (TE) have also been conducted. TE measures how a signal can predict subsequent changes in another signal70. It thus provides a directed measure of a coupling’s strength. If there is no coupling between regions, then TE is close to 0, while TE is close to 1 if there is a strong coupling between two regions. This method quantifies the flow of information between brain regions, which is why we will refer to it as information transfer for the rest of this article. This information transfer enables us to determine the functional role of a brain region, specifying whether it is a transmitter (i.e., the direction of information flow from this region to other brain regions) or a receiver (i.e., the direction of information flow to this region from other brain regions)34. Moreover, this complementary measure is not influenced by volume conduction52.
PLV and TE were computed using these processes selected in Brainstorm and followed the same processing steps. The range of each frequency band was based on the frequency of the individually observed alpha peak frequency (IAF), measured as the average of peaks detected from both occipitoparietal magnetometers and gradiometers. In line with previous work71 the following frequency bands were considered: Delta (IAF-8/IAF-6), Theta (IAF-6/IAF-2), Alpha (IAF-2/IAF + 2), Beta (IAF + 2/IAF + 14), Gamma (IAF + 15/IAF + 80). The Hilbert transformation was used for the time-frequency decomposition. The number of cycles per frequency was determined based on IAF (see Table S5). To reduce the dimensionality of the data and to preserve the phase of the time series, the first component of the principal component analysis (PCA) decomposition of the time course of activation in each of the 68 regions of interest (ROI) from the Desikan–Killiany brain atlas72. The first component, rather than the average activity, was chosen to reduce signal leakage and volume conduction effects73. PLV and TE were computed following these processes in Brainstorm64.
DWI data pre-processing
Pre-processing of the diffusion data was performed using ExploreDTI74 and included the following steps: (a) images were corrected for eddy current distortions and participant motion; (b) a non-linear least squares method was applied for diffusion tensor estimation, and (c) deterministic DWI tractography was applied using the following parameters: uniform resolution of 2 mm, fractional anisotropy (FA) threshold of 0.2 (limit: 1), angle threshold of 45°, and fibre length range of 50–500 mm. The network analysis tools in ExploreDTI were used to quantify the FA value of the fibres connecting the regions of the Desikan atlas to obtain similar matrices to MEG data, using Freesurfer’s individual cortical parcellation.
Multiplex network construction and measures
Using BRAPH75 software (http://braph.org/), a multiplex network was defined for each subject, with two layers: one “structural” layer with DWI tract FA data and one “functional” layer with PLV or TE MEG data (in this study, a simplification of TE was used to determine whether a region was a receiver or sender). TE analyses were performed on each region and distinguished coupling directed from the network towards a given region (i.e., the inward direction) or from a given region towards the rest of the network (i.e., the outward direction). Frequency bands were analysed separately to investigate their respective associations with the structural layer, in line with previous work showing heterogeneous association across frequency bands76,77. In each layer, brain regions from the Desikan–Killiany atlas78 are represented by nodes connected by edges (see a method’s summary in Fig. 1). A binary multiplex matrix was calculated from the individual matrices of DWI and MEG data of each participant. Matrices were binarized according to the minimum density observed for DWI in the older adult group (22%). The density, or number of connections for all matrices, was the same across layers. Auto-correlations between regions were excluded from the analyses.
To evaluate across-layer integration, the multiplex participation coefficient79 was investigated, allowing the quantification of the connectivity similarity of a node across the different layers. The multiplex participation coefficient of a node i is defined as79: \({p}_{i}=\frac{M}{M-1}\left[1-{\sum }_{\propto =1}^{M}{\left(\frac{{k}_{i}^{\left[\propto \right]}}{{o}_{i}}\right)}^{2}\right]\) where M is the number of layers, \({k}_{i}^{\left[\propto \right]}\) the degree of node i at the \(\propto -{th}\) layer and \({o}_{i}\) is the overlapping degree of node i, \({o}_{i}={\sum }_{\propto }{k}_{i}^{\left[\propto \right]}\,\). This coefficient measures how similar the connectivity patterns are in both layers of the multiplex network. Values range between 0 and 1. In particular, a value of 1 means that the node makes the exact connections in both layers, while a value of 0 means that the node’s connections in both layers are different from each other. A large participation value indicates that the node may be central or a hub. To determine which layer is driving the observed results, the degree (i.e., number of connections of each layer of the multiplex network for a given region) was also calculated for each group as: \({d}^{[\alpha ]}={\sum }_{j=1}^{N}{a}_{{ij}}^{[\alpha ]}\); where \({a}_{{ij}}^{[\alpha ]}\) is the link between node i and j in layer α.
Statistics and reproducibility
To assess differences between age groups in multiplex participation for different brain regions, t-tests were applied using the Jamovi software (https://www.jamovi.org/; version 1.6.23). Regression analyses were performed in the older adults’ group to assess whether the level of participation coefficient for a region was associated with cognitive performance. Afterwards, participants were grouped according to the level of participation coefficient for each region. Two subgroups were then formed: one corresponding to individuals with a high participation coefficient called “High participant group” and another with a low participation coefficient called “Low participant group”. The median individuals (four from each group) were removed from subgroup analyses to reduce median split bias. As a result, each subgroup was composed of 19 individuals. Subgroups were also found in young adults but due to the large variability in young individuals, were considered as a single group. T-tests were also performed to determine differences between subgroups. Non-parametric correlations were used when the values were non-continuous (for the MMSE, for example). Level of education, grey matter and total intracranial volume were used as a covariate to control it in the various statistical analyses. Results were FDR corrected for multiple comparisons80 at each step of the analysis, including t-tests between age groups, between different frequency bands and regions, and for regressions with cognition. Original degrees of freedom and corrected p-values are reported.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The datasets analysed in this study are available from the Cambridge Centre for Ageing and Neuroscience (http://www.mrc-cbu.cam.ac.uk/datasets/camcan/). Numerical source data for figures and plots can be found in the Supplementary data file.
Code availability
The analyses are based on open-source Matlab toolboxes: Brainstorm (https://neuroimage.usc.edu/brainstorm/) for MEG data analysis and BRAPH (http://braph.org/) for graph analysis. Brain region representations were created using the ENIGMA toolbox (https://github.com/MICA-MNI/ENIGMA.git), also an open-source toolbox. All the codes can be found on their respective site. No code has been generated internally.
References
Buzsáki, G. Rhythms of the Brain. (Oxford University Press, 2006). https://doi.org/10.1093/acprof:oso/9780195301069.001.0001.
Liu, Z.-Q. et al. Time-resolved structure-function coupling in brain networks. Commun. Biol. 5, 1–10 (2022).
Sporns, O. Structure and function of complex brain networks. Dialogues Clin. Neurosci. 15, 247–262 (2013).
Park, H.-J. & Friston, K. Structural and functional brain networks: from connections to cognition. Science 342, 1238411 (2013).
Bullmore, E. & Sporns, O. Complex brain networks: graph theoretical analysis of structural and functional systems. Nat. Rev. Neurosci. 10, 186–198 (2009).
Honey, C. J. et al. Predicting human resting-state functional connectivity from structural connectivity. Proc. Natl Acad. Sci. USA 106, 2035–2040 (2009).
Suárez, L. E., Markello, R. D., Betzel, R. F. & Misic, B. Linking structure and function in macroscale brain networks. Trends Cogn. Sci. 24, 302–315 (2020).
Nyberg, L., Lövdén, M., Riklund, K., Lindenberger, U. & Bäckman, L. Memory aging and brain maintenance. Trends Cogn. Sci. 16, 292–305 (2012).
Cabeza, R. et al. Maintenance, reserve and compensation: the cognitive neuroscience of healthy ageing. Nat. Rev. Neurosci. 19, 701–710 (2018).
Uddin, L. Q., Supekar, K. S., Ryali, S. & Menon, V. Dynamic reconfiguration of structural and functional connectivity across core neurocognitive brain networks with development. J. Neurosci. 31, 18578–18589 (2011).
Baum, G. L. et al. Development of structure–function coupling in human brain networks during youth. Proc. Natl Acad. Sci. USA 117, 771–778 (2020).
Medaglia, J. D. et al. Functional alignment with anatomical networks is associated with cognitive flexibility. Nat. Hum. Behav. 2, 156–164 (2018).
Burzynska, A. Z. et al. White matter integrity supports BOLD signal variability and cognitive performance in the aging human brain. PLoS ONE 10, e0120315 (2015).
Webb, C. E., Rodrigue, K. M., Hoagey, D. A., Foster, C. M. & Kennedy, K. M. Contributions of white matter connectivity and BOLD modulation to cognitive aging: a lifespan structure-function association study. Cereb. Cortex 30, 1649–1661 (2020).
Hinault, T., Larcher, K., Bherer, L., Courtney, S. M. & Dagher, A. Age-related differences in the structural and effective connectivity of cognitive control: a combined fMRI and DTI study of mental arithmetic. Neurobiol. Aging 82, 30–39 (2019).
Uddin, L. Q. Bring the noise: reconceptualizing spontaneous neural activity. Trends Cogn. Sci. 24, 734–746 (2020).
Untergehrer, G., Jordan, D., Kochs, E. F., Ilg, R. & Schneider, G. Fronto-parietal connectivity is a non-static phenomenon with characteristic changes during unconsciousness. PLoS ONE 9, e87498 (2014).
Deslauriers-Gauthier, S. et al. White matter information flow mapping from diffusion MRI and EEG. NeuroImage 201, 116017 (2019).
Hinault, T., Kraut, M., Bakker, A., Dagher, A. & Courtney, S. M. Disrupted neural synchrony mediates the relationship between white matter integrity and cognitive performance in older adults. Cereb. Cortex 30, 5570–5582 (2020).
Hinault, T. et al. Age-related differences in network structure and dynamic synchrony of cognitive control. NeuroImage 236, 118070 (2021).
Vaiana, M. & Muldoon, S. F. Multilayer brain. Netw. J. Nonlinear Sci. 30, 2147–2169 (2020).
Battiston, F., Guillon, J., Chavez, M., Latora, V. & De Vico Fallani, F. Multiplex core–periphery organization of the human connectome. J. R. Soc. Interface 15, 20180514 (2018).
Brookes, M. J. et al. A multi-layer network approach to MEG connectivity analysis. NeuroImage 132, 425–438 (2016).
Canal-Garcia, A. et al. Multiplex connectome changes across the alzheimer’s disease spectrum using gray matter and amyloid data. Cereb. Cortex 32, 3501–3515 (2022).
Guillon, J. et al. Loss of brain inter-frequency hubs in Alzheimer’s disease. Sci. Rep. 7, 10879 (2017).
Battiston, F., Nicosia, V., Chavez, M. & Latora, V. Multilayer motif analysis of brain networks. Chaos Interdiscip. J. Nonlinear Sci. 27, 047404 (2017).
Breedt, L. C. et al. Multimodal multilayer network centrality relates to executive functioning. Netw. Neurosci. 7, 299–321 (2023).
van Lingen, M. R. et al. The longitudinal relation between executive functioning and multilayer network topology in glioma patients. Brain Imaging Behav. https://doi.org/10.1007/s11682-023-00770-w (2023).
Yu, M. et al. Selective impairment of hippocampus and posterior hub areas in Alzheimer’s disease: an MEG-based multiplex network study. Brain 140, 1466–1485 (2017).
Courtney, S. M. & Hinault, T. When the time is right: Temporal dynamics of brain activity in healthy aging and dementia. Prog. Neurobiol. 203, 102076 (2021).
Tóth, B. et al. Frontal midline theta connectivity is related to efficiency of WM maintenance and is affected by aging. Neurobiol. Learn. Mem. 114, 58–69 (2014).
Ariza, P. et al. Evaluating the effect of aging on interference resolution with time-varying complex networks analysis. Front. Hum. Neurosci. 9, 255 (2015).
Xu, N., Doerschuk, P. C., Keilholz, S. D. & Spreng, R. N. Spatiotemporal functional interactivity among large-scale brain networks. NeuroImage 227, 117628 (2021).
Moon, J.-Y., Lee, U., Blain-Moraes, S. & Mashour, G. A. General relationship of global topology, local dynamics, and directionality in large-scale brain networks. PLoS Comput. Biol. 11, e1004225 (2015).
Park, D. C. & Reuter-Lorenz, P. The adaptive brain: aging and neurocognitive scaffolding. Annu. Rev. Psychol. 60, 173 (2009).
Shafto, M. A. et al. The Cambridge Centre for Ageing and Neuroscience (Cam-CAN) study protocol: a cross-sectional, lifespan, multidisciplinary examination of healthy cognitive ageing. BMC Neurol. 14, 204 (2014).
Taylor, J. R. et al. The Cambridge Centre for Ageing and Neuroscience (Cam-CAN) data repository: structural and functional MRI, MEG, and cognitive data from a cross-sectional adult lifespan sample. NeuroImage 144, 262–269 (2017).
Hagmann, P. et al. Mapping the structural core of human cerebral cortex. PLOS Biol. 6, e159 (2008).
Andrews-Hanna, J. R., Smallwood, J. & Spreng, R. N. The default network and self-generated thought: component processes, dynamic control, and clinical relevance. Ann. N. Y. Acad. Sci. 1316, 29–52 (2014).
Uddin, L. Q., Yeo, B. T. T. & Spreng, R. N. Towards a universal taxonomy of macro-scale functional human brain networks. Brain Topogr. 32, 926 (2019).
Andrews-Hanna, J. R. et al. Disruption of large-scale brain systems in advanced aging. Neuron 56, 924–935 (2007).
Bonnefond, M., Kastner, S. & Jensen, O. Communication between brain areas based on nested oscillations. eNeuro 4, 0153–16.2017 (2017).
Sadaghiani, S. & Kleinschmidt, A. Brain networks and α-oscillations: structural and functional foundations of cognitive control. Trends Cogn. Sci. 20, 805–817 (2016).
Anderson, B. A., Folk, C. L. & Courtney, S. M. Neural mechanisms of goal-contingent task disengagement: response-irrelevant stimuli activate the default mode network. Cortex 81, 221–230 (2016).
Poza, J. et al. Phase-amplitude coupling analysis of spontaneous EEG activity in Alzheimer’s disease. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2017, 2259–2262 (2017).
Mišić, B. et al. Network-level structure-function relationships in human neocortex. Cereb. Cortex 26, 3285–3296 (2016).
Liu, Z.-Q., Shafiei, G., Baillet, S. & Misic, B. Spatially heterogeneous structure-function coupling in haemodynamic and electromagnetic brain networks. NeuroImage 278, 120276 (2023).
Knyazev, G. G., Slobodskoj-Plusnin, J. Y., Bocharov, A. V. & Pylkova, L. V. The default mode network and EEG alpha oscillations: an independent component analysis. Brain Res. 1402, 67–79 (2011).
Jann, K. et al. BOLD correlates of EEG alpha phase-locking and the fMRI default mode network. NeuroImage 45, 903–916 (2009).
Jauny, G., Eustache, F. & Hinault, T. T. M/EEG dynamics underlying reserve, resilience, and maintenance in aging: a review. Front. Psychol. 13, 861973 (2022).
Grigg, O. & Grady, C. L. Task-related effects on the temporal and spatial dynamics of resting-state functional connectivity in the default network. PLoS ONE 5, e13311 (2010).
Wibral, M., Vicente, R., Triesch, J. & Pipa, G. Using transfer entropy to measure the patterns of information flow though cortex: application to MEG recordings from a visual Simon task. BMC Neurosci. 10, P232 (2009).
Fong, A. H. C. et al. Dynamic functional connectivity during task performance and rest predicts individual differences in attention across studies. NeuroImage 188, 14–25 (2019).
Hinault, T., Baillet, S. & Courtney, S. M. Age-related changes of deep-brain neurophysiological activity. Cereb. Cortex 33, 3960–3968 (2023).
Westlye, L. T. et al. Life-span changes of the human brain white matter: diffusion tensor imaging (DTI) and volumetry. Cereb. Cortex N. Y. N. 1991 20, 2055–2068 (2010).
Coquelet, N. et al. The electrophysiological connectome is maintained in healthy elders: a power envelope correlation MEG study. Sci. Rep. 7, 1–10 (2017).
Jauny, G., Eustache, F. & Hinault, T. Connectivity dynamics and cognitive variability during aging. Neurobiol. Aging 118, 99–105 (2022).
Nasreddine, Z. S. et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc. 53, 695–699 (2005).
Zigmond, A. S. & Snaith, R. P. The hospital anxiety and depression scale. Acta Psychiatr. Scand. 67, 361–370 (1983).
Folstein, M. F., Folstein, S. E. & McHugh, P. R. “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 12, 189–198 (1975).
Vogel, E. K., Woodman, G. F. & Luck, S. J. Storage of features, conjunctions, and objects in visual working memory. J. Exp. Psychol. Hum. Percept. Perform. 27, 92–114 (2001).
Horn, J. L. & Cattell, R. B. Refinement and test of the theory of fluid and crystallized general intelligences. J. Educ. Psychol. 57, 253–270 (1966).
Shallice, T. & Burgess, P. W. Deficits in strategy application following frontal lobe damage in man. Brain 114, 727–741 (1991).
Tadel, F., Baillet, S., Mosher, J. C., Pantazis, D. & Leahy, R. M. Brainstorm: a user-friendly application for MEG/EEG analysis. Comput. Intell. Neurosci. 2011 (2011).
Fischl, B. FreeSurfer. NeuroImage 62, 774–781 (2012).
Kybic, J. et al. A common formalism for the Integral formulations of the forward EEG problem. IEEE Trans. Med. Imaging 24, 12–28 (2005).
Gramfort, A., Papadopoulo, T., Olivi, E. & Clerc, M. OpenMEEG: opensource software for quasistatic bioelectromagnetics. Biomed. Eng. OnLine 9, 45 (2010).
Huang, M. X., Mosher, J. C. & Leahy, R. M. A sensor-weighted overlapping-sphere head model and exhaustive head model comparison for MEG. Phys. Med. Biol. 44, 423 (1999).
Lachaux, J.-P., Rodriguez, E., Martinerie, J. & Varela, F. J. Measuring phase synchrony in brain signals. Hum. Brain Mapp. 8, 194–208 (1999).
Ursino, M., Ricci, G. & Magosso, E. Transfer entropy as a measure of brain connectivity: a critical analysis with the help of neural mass models. Front. Comput. Neurosci. 14, 45 (2020).
Toppi, J. et al. Different topological properties of EEG-derived networks describe working memory phases as revealed by graph theoretical analysis. Front. Hum. Neurosci. 11, 637 (2017).
Brkić, D. et al. The impact of ROI extraction method for MEG connectivity estimation: practical recommendations for the study of resting state data. NeuroImage 284, 120424 (2023).
Sato, M., Yamashita, O., Sato, M.-A., & Miyawaki, Y. Information spreading by a combination of MEG source estimation and multivariate pattern classification. PLoS ONE 13, e0198806 (2018).
Leemans, A., Jeurissen, B., Sijbers, J. & Jones, D. K. ExploreDTI: a graphical toolbox for processing, analyzing, and visualizing diffusion MR data. (2009).
Mijalkov, M. et al. BRAPH: a graph theory software for the analysis of brain connectivity. PLoS ONE 12, e0178798 (2017).
Kulik, S. D. et al. Structure-function coupling as a correlate and potential biomarker of cognitive impairment in multiple sclerosis. Netw. Neurosci. 6, 339–356 (2022).
Liu, Z.-Q., Shafiei, G., Baillet, S. & Misic, B. Spatially heterogeneous structure-function coupling in haemodynamic and electromagnetic brain networks. NeuroImage 278, 120276 (2023).
Desikan, R. S. et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage 31, 968–980 (2006).
Battiston, F., Nicosia, V. & Latora, V. Structural measures for multiplex networks. Phys. Rev. E 89, 032804 (2014).
Benjaminit, Y. & Hochberg, Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. B 57, 289–300 (1995).
Acknowledgements
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. The authors declare no competing interests. We would like to thank the reviewers for their advice. Data collection and sharing for this project were provided by the Cambridge Centre for Ageing and Neuroscience (Cam-CAN). Cam-CAN funding was provided by the UK Biotechnology and Biological Sciences Research Council (grant number BB/H008217/1), together with support from the UK Medical Research Council and the University of Cambridge, UK.
Author information
Authors and Affiliations
Contributions
G.J.: Investigation, Analysis, Writing; M.M.: Methodology, Software, Review; A.C.G.: Methodology, Software, Review; G.V.: Methodology, Software, Review; J.B.P.: Methodology, Software, Review; F.E.: Supervision, Review; T.H.: Conceptualisation, Methodology, Supervision, Review.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Biology thanks Mahmoud Hassan, Deniz Kumral and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editors: Joao Valente. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jauny, G., Mijalkov, M., Canal-Garcia, A. et al. Linking structural and functional changes during aging using multilayer brain network analysis. Commun Biol 7, 239 (2024). https://doi.org/10.1038/s42003-024-05927-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s42003-024-05927-x
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.