Abstract
Unprecedented discoveries during the past decade have unearthed the ubiquitous presence of biomolecular condensates (BCs) in diverse organisms and their involvement in a plethora of biological functions. A predominant number of BCs involve coacervation of RNA and proteins that demix from homogenous solutions by a process of phase separation well described by liquid-liquid phase separation (LLPS), which results in a phase with higher concentration and density from the bulk solution. BCs provide a simple and effective means to achieve reversible spatiotemporal control of cellular processes and adaptation to environmental stimuli in an energy-independent manner. The journey into the past of this phenomenon provides clues to the evolutionary origins of life itself. Here I assemble some current and historic discoveries on LLPS to contemplate whether BCs are extant biological hubs or evolving microcompartments. I conclude that BCs in biology could be extant as a phenomenon but are co-evolving as functionally and compositionally complex microcompartments in cells alongside the membrane-bound organelles.
Similar content being viewed by others
Introduction
The phenomenon of liquid-liquid phase separation (LLPS) in aqueous solutions has been known for quite some time, especially in the fields of polymer science and process engineering1,2,3. The idea of the demixing of liquids occurring in biology was first postulated by Bugenberg de John and Kurt in 19294 and shortly after by Oparin in his book called The Origin of Life in 19385. However, it was not until eight decades later in 2009 that unequivocal evidence in support of LLPS and the formation of membraneless organelles (MLOs) in living cells was brought to light by Brangwynne and Hyman6. A cornucopia of equally significant discoveries following Brangwynne and Hyman’s in the last decade has now unearthed the near-ubiquitous involvement of MLOs in cellular processes across many organisms. Together, these discoveries have not only opened an unprecedented scientific quest to understand the mechanisms of spatiotemporal control in biological processes but also help glean the origins of life itself. MLOs are spatially separated mesoscale compartments devoid of a membrane barrier observed in membrane-bound organelles such as mitochondria or lysosomes. A variety of different cellular processes involve MLOs, which are also referred to as biomolecular condensates (BCs)7. In BCs, biomolecules such as proteins and nucleic acids coacervate to demix from the bulk solution by the process that is best described by LLPS (detailed below in the next section). One of the earliest discovered membraneless organelles in eukaryotes is the nucleolus within the nucleus, which is the location of ribosomal synthesis8. Since then, more than 20 MLOs have come to the limelight in mammalian cells, and the numbers are rising at a staggering rate9,10. The near ubiquity of the phenomenon in a wide range of cellular processes begs the question of whether the coacervation of proteins and RNA toward the formation of BCs and MLOs have come into existence by Darwinian evolution of natural selection for acquiring spatiotemporal control on biochemical processes or are they relics of cellular origins on earth. Given the prevalence and functional integration of this phenomenon in all kingdoms of life, it is important to dwell in some historical, as well as current research perspectives on biomolecular condensates and take a philosophical dive into the possible origins and evolution of BCs. which is likely to kindle intriguing thoughts about this phenomenon in the biological world.
Physical chemistry of LLPS and condensate formation
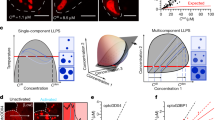
A homogeneous liquid containing a binary mixture of solute and solvent can exist in a fully miscible single phase or undergo LLPS into a demixed, inhomogeneous state containing two or more phases. If the energy of interactions between solute and solvent is greater than that of solute and solute, a single phase will exist but on the other hand, if the energy of solute-solute interactions is greater than that of the solute-solvent interactions, the system will demix and co-exist in two distinct liquid phases11,12,13. For complex multi-component solutions containing two or more biopolymers, the ability of biopolymers containing opposite charges to engage in complex coacervation involving weak, transient, multivalent, and nonstoichiometric interactions comprising of, but not limited to, electrostatic, π-π or cation-π forces, will determine whether they undergo liquid-liquid demixing into two distinct phase regimes14,15,16. LLPS is a density transition with a concentration threshold called saturation concentration (Csat) above which the system undergoes LLPS. Csat defines the phase boundary of a particular system16,17,18. Although widely referred to as LLPS, many of the coacervating biomolecules both in vitro and in vivo show viscoelastic properties meaning that the condensates show liquid-like behavior as a function of time and length scales. However, numerous models developed for LLPS adequately describe the macroscopic behavior of the coacervating biomolecules and BCs, and therefore, one may note that within the biological timescales, BCs show liquid-like behavior. Nevertheless, in demixed state, the dense phase is enriched, and the dilute phase is depleted in biopolymers creating a concentration gradient across the phase boundary19. The formation of condensates depends on the mixture’s composition and other parameters such as temperature and ionic strength. In non-equilibrium states like those in the cells, the phase boundary can be shifted depending on the diffusion and fluxes of molecules and regulators. The demixed state can therefore be reversibly dissolved or formed based on the environmental cues. Demixing of liquids into inhomogeneous two or more component systems by LLPS-like process presents three fundamental advantages in cellular functions: a) It offers spatial control via compartmentalization and confinement of biomolecules without having to actively or passively transport them into specialized, membrane-bound organelles7,10,20, b) it enables to achieve an increase in the effective concentrations of coacervating molecules within the dense phase and thereby providing a temporal control over the rates of the reactions21,22,23, and c) MLOs thus formed as distinct heterogeneous phases can be diffused back to a single homogenous phase by a variety of different mechanisms depending on the environment and stimuli in an ATP-independent manner. These advantages predispose the cellular machinery to adopt phase separation as a cost-effective and need-based mechanism to optimize their functions.
RNA and pre-biotic world
It is now well established that RNA molecules are the earliest- biomolecules formed on the planet and were able to sustain primordial life their ability to that catalyze their own self-replication24,25,26. This remarkable discovery of the origins of life on Earth was made by systematically uncovering the complex cellular forms layer by layer to reveal the conserved molecules and machinery across the phylogenic tree. Discoveries such as self-splicing introns in the form of ribozymes27, and auto-regulatory riboswitches28 to name a few, showcase the versatility of RNA molecules. One of the important properties of RNA molecules is the ability self-replicate and a significant body of evidence points toward the evolution of RNA replicase, which is an RNA molecule that is capable of acting as a template for its own replication and the storage of genetic information, and as an RNA polymerase that is able to catalyze its own production28,29 Although RNAs are versatile molecules that are capable to replicate, catalyze and multiply, these molecules by themselves cannot accomplish more complex functions without achieving some level of selectivity and spatial compartmentalization.
Evolution of RNA-protein condensates
One of the most intriguing questions in the transformation of primordial biological reactions occurring in protocells is the evolution of compartmentalization. Despite the self-replicating ability of RNA molecules, an important step toward the evolution of prebiotic molecules to initiate life requires an increase in the effective concentrations of the reactants. It would be necessary for the heterogenous mixture of RNAs and the molecules they interacted with, to confine themselves within microcompartments that can enhance the rate, efficiency, and selectivity. Theories diverge in how protocells evolved in achieving this but based on the evidence one can safely conclude that before the evolution of both lipid-based membrane compartments that furthered into the current day complex eukaryotic cells, simpler non-lipid-based protocells could have evolved to achieve optimization and selectivity for the reactions necessary30. If so, what were the methods by which such confinement of molecules especially with RNA was adopted? Evidence indicates that many such membraneless microcompartments could have existed in the prebiotic world in the form of liquid droplets made of organic molecules and oils31, silica-based inorganic microcells32,33, aqueous two-phase systems34, peptide coacervates32,35, polyester microdroplets from α-hydroxy acids30, and anhydride compartmentalization mechanisms34. Although it will be difficult to track the timeframes of origins for these membraneless microcompartments, parsimoniously one can conclude that they could have coevolved with membrane-bound protocells and compartments. Yet, one of the compelling arguments for the membraneless compartments to have evolved earlier than the membrane-bound ones is the simplicity by which the ions and other molecules can reversibly diffuse in and out of the droplets without spending valuable energy or having to engage active transporters to accomplish sequestration and confinement. In addition, the ease and reversibility of formation and dissolution provide a great deal of temporal and spatial control for the reactions within membraneless microcompartments. One of the pivotal transformations during the compartmentalization period of the prebiotic evolution in gaining spatiotemporal control of biological reactions was the evolution of RNA to protein or the coevolution of RNA-protein complexes, which likely followed the ‘RNA-only’ world36,37,38,39 (Fig. 1). In either case, the compatibility of RNA to interact with amino acids and peptides was key to organizing into efficient reaction hubs. The highly negatively charged anionic phosphates in monomeric or oligomeric RNA molecules and their 3-dimensional structural plasticity make them highly suitable for electrostatic coacervation with counter-ionic molecules for phase separation, especially cationic amino acids. This complex coacervation phenomenon drives phase separation and the formation of RNA-peptide condensates. During the coevolution of RNA and proteins, evidence indicates that both these molecules interacted extensively in the prebiotic world mainly via such counter-ionic complex coacervation mechanisms40,41. In the prebiotic world, cationic depsipeptides, those that contain both ester and amide linkages formed from hydroxy acids under mild conditions, have been known to be abundant42,43,44. Furthermore, it has recently been identified that cationic amino acids such as lysine, Lys; arginine, Arg; histidine, His; ornithine, Orn; and diamino propionic acid, Dap, interacted with RNA molecules extensively45. Among these, Lys and Arg were less likely to be present in the prebiotic world although they have been found in meteorites46, it is well known that Orn and Dap were abundant and could have formed the earliest RNA-amino acid coacervates47. The reminiscence of RNA-peptide condensates’ origins is evident when one notices the sheer preponderance of RNA-protein BCs across many organisms9, which provides unambiguous evidence for RNA-peptide coacervates being one of the earliest microcompartments to be formed in the biological world48 (Fig. 1).
The presence of biomolecular condensates across many organisms raises the question of whether they co-evolved with other membrane-bound compartments or are still extant
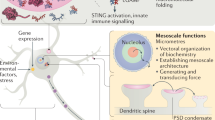
BCs and MLOs are prevalent across many organisms in the phylogenetic tree, and discoveries are being made at a staggering rate in many kingdoms of life49. In addition to mammalian cells, which I will elaborate on further below, BCs and MLOs are observed in plants50, bacteria51, and fungi52. Among these, plants are arguably the species that undergo rather unrelenting and constant environmental and climatic stress than others, and therefore, are likely to adopt coping mechanisms that are both reversible and economical. Recent discoveries indicate that plants do contain many different MLOs including, Auxin Response Factor condensates53, photoreceptor-containing nuclear bodies or “photobodies” that are directly regulated by external light54, dicing bodies (D-bodies) that are membraneless nuclear hubs for mi-RNA biogenesis in plants55, in addition to those found in mammalian ones such as Cajal bodies, p-bodies, stress granules, etc.50 (Fig. 2).
Simplified cartoon of a plant cell with MLOs depicted along with microscopic images of MLOs of a select few (reproduced with permission from50).
Similarly, advanced single-molecule techniques and super high-resolution microscopy have made it possible to detect MLOs in microorganisms such as bacteria and fungi also. In bacteria, several MLOs, in the form of dense foci, are known to form namely BR-bodies, PopZ microdomains, RNA polymerase clusters, polyphosphate granules, etc. In Caulobacter crescentus, BR-bodies are RNA foci assembled by the protein RNase-E that are responsible for mRNA degradation under stress56. Also, in C. crescentus, MLO-like microdomains formed asymmetrically along the poles by the disordered regions in PopZ, are necessary for the selective localization of many proteins eventually resulting in a skewed inheritance of the transcription factor, CtrA-P in the daughter cells57,58. Similarly, RNA polymerases also form liquid-like clusters in E. Coli with the help of NusA, a transcriptional termination factor59. Furthermore, granules containing inorganic polyphosphates have also been shown to form under cellular stress and starvation to control DNA replication60. More recently, phase separation of the bacterial transcription termination factor Rho in B. thetaiotaomicron through its large intrinsically disordered region was found to be key in the survival of the bacteria in the mammalian gut61. Similarly, fungi have also been known to contain several MLOs. For example, S. cerevisiae, such as the P-bodies, stress granules, nuclear heterochromatin compartmentalization, etc. (reviewed in52). The widespread prevalence of BCs across many organisms is probably the consequence of the physiochemical simplicity of the LLPS process which provides a distinctive advantage in sensing and adapting to environmental changes within the cellular milieu without having to expend precious energy. Based on this hypothesis, one could infer that BCs are extant. However, on the other hand, the prevalence of BCs could also suggest their co-evolution along with higher-order organisms containing membrane-bound organelles, as they continue to utilize LLPS and BCs to carry out a multitude of complex functions.
The spatial and functional ubiquity of biomolecular condensates also prods the question about their evolution
As detailed above, MLOs are present in many organisms across prokaryotes and eukaryotes and are involved in diverse functions. I will refocus our attention back on mammalian cells to bring out not only the ubiquity involved in the utility of phase separation in cellular functions but also the complexities of the condensates in terms of their composition, size, and biological functions. Although not a prerequisite, intrinsic disorder in proteins facilitates LLPS62,63,64,65. Based on sequence analysis, it is predicted that disordered proteins are significantly more prevalent in eukaryotes (33%) as compared to prokaryotes (4%)66, and thus many BCs and MLOs have been identified in mammalian cells.
Among the MLOs in eukaryotes, those that are formed between proteins and RNA are predominant. The most prominent ones discovered so far include Cajal bodies, nuclear speckles, PML bodies and nucleoli in the nucleus, and P-bodies, Balbiani bodies, synaptic densities, RNA transport granules, and germ granules in the cytoplasm10,67. In addition to these ubiquitous MLOs, stimuli and condition-dependent ones such as stress granules, U-bodies, metabolic granules, and proteasome storage granules are also formed in various cells68,69 (Fig. 3). Although many associative polymers and biological molecules can coacervate to form BCs, the ones that are formed between RNA and proteins predominate MLOs in eukaryotic cells9. Not only the MLOs are numerous, but they are also equally complex condensates each containing a large number of coacervating biomolecules. The degree of complexity and the ubiquitous nature of BCs can be better appreciated from the following examples which span both physiological and pathological scenarios in eukaryotes.
Shown are the condensates that are ubiquitous (brown hues), cell-type specific ones (green hues), and the condition-dependent ones (red hues). (Reproduced with permission from10).
Ribosome biogenesis
Arguably nucleolus is the first MLO to be observed visually. Being the site of ribosome biogenesis, it is now clear that nucleolus is also a complex, multi-layer condensate containing three compartments of immiscible liquids with specific events of ribosome biogenesis taking place sequentially from the innermost layer to the outermost layer8. The innermost core layer called the fibrillar center (FC) is where the rRNA synthesis begins and as the nascent pre-rRNA emerges, intrinsically disordered Gly-Arg-rich proteins coacervate with them to initiate the formation of the second layer of the nucleolus called the dense fibrillar component (DFC)70,71.
Stress response
These cytoplasmic MLOs are RNA-rich foci that are reversibly formed during cellular stress conditions. The critical role of the stress granules includes the control of translational regulation of mRNA during stress by sequestering the transcripts into the foci72,73,74. They are known to contain more than 25 different proteins, including ribonucleoproteins, those in translationally arrested pre-initiation complex with 40 S ribosomal unit, small RNAs, SG-associated initiation complexes, stress granule nucleators, etc., in addition to mRNA transcripts72,75. Proteomic and genetic screens have identified hundreds of proteins associated with stress granules, which require a well-orchestrated play involving physical and chemical forces that synchronize phase separation and one that can adapt to the environmental cues to provide cellular protection under stress. This multi-component, complex biomolecular condensate formation is a key integral part of stress-coping mechanisms in eukaryotic cells.
Gene regulation
It has been well-established that transcriptional machinery involved many disordered proteins and disordered regions that provide the plasticity needed for long-range and multivalent interactions between the DNA, RNA, and activation factors76,77,78. Recently, the transcriptional control of transcriptional factors and activating domains embryonic stem cell transcription factor OCT4, estrogen receptor, and yeast transcription factor, GCN4 form phase-separated condensate with other co-activating molecules for gene activation79. LLPS-like process has also been observed in key RNA polymerase II transcription80 and in chromatin regulation81. An increasing number of RNA-dependent transcriptional and translational condensate hubs have been discovered82,83,84, making the foci containing biomolecular condensates a key component for organizing and activating transcriptional machinery. Related to transcriptional control, the processing bodies or p-bodies are cytoplasmic hubs enriched in mRNA and also play key roles85,86.
Catalysis
A high degree of specificity and selectivity are the key features of enzyme-catalyzed reactions, and therefore, one could assume that enzymes evolved late to carry out such specific reactions. However, since compartmentalization by phase-separated condensates provides higher effective concentrations, enzymes could take advantage of biomolecular condensate as a way of increasing their efficiency. This seems to be the case as many enzymes are known to exist as assemblies and in condensate states87,88. Partitioning of enzymes in biomolecular condensates not only facilitates enzyme efficiency by providing increased effective concentrations but also by other mechanisms, such as changing conformations and specific activity21,22,23. An increasing number of reports in addition to these examples showcase the ability of enzymes to be efficient and specific by adopting LLPS-like mechanisms and thus help us glean the versatility of the phenomenon in many cellular aspects. In addition to these, BCs and MLOs are known to play a role in other cellular processes, including autophagy89, organizing synaptic density90, and immune response91.
Cellular dysfunction and pathology
One of the obvious consequences of spatial compartmentalization in BCs is the increase in protein concentration in the dense phase. Although this is critical for many physiological processes, BCs become a crucible for many proteins that are prone to form toxic amyloid aggregates92,93. Proteins such as FUS94, TDP4395, and tau96 have been shown to form amyloid aggregates nucleating from the BCs and are implicated in pathologies such as amyotrophic lateral sclerosis (ALS), frontotemporal dementia (FTD), and Parkinson’s disease (PD)72. BCs have also been implicated in cancer97, and in SARS-Cov-2 viral infection98.
Discussion
The afore-described biophysical, spatial, and functional characteristics along with the prevalence of BCs and MLOs in organisms across eukaryotes, prokaryotes, and archaea seem to suggest that the phenomenon of phase separation resembling LLPS is an evolutionarily conserved mechanism for spatial compartmentalization since the primordial life on earth. The most important attribute of phase separation is its simplicity for compartmentalization and spatiotemporal control of coacervating components in a given system as opposed to membrane-bound organelles. LLPS-like process involving simpler two- or three-component systems could have prevailed during pre-biotic and early life processes, but the formation of BCs and MLOs in cellular life forms would have been challenging given the organismal complexity and multi-component systems in a crowded milieu. Yet as described above, widespread BCs are observed in eukaryotes often involved in complex cellular functions and comprising hundreds of components, in some cases containing multiple phases, in a dynamical non-equilibrium system. Ubiquity observed in such complex condensates suggests that cells may have adapted to embrace the tenets of soft matter physics to continue to utilize phenomenologically and energetically simplistic LLPS-like mechanisms rather than the requirement for membrane-bound specialized compartments (Fig. 4). If one were to assume that the phase separation mechanism was out-evolved by the membranous compartmentalization, fewer BCs and MLOs would be observed in eukaryotes and with minimal functional roles, if any. But this does not seem to be the case; not only are BCs prevalent in many cells but also, intriguingly, eukaryotes containing many membrane-bound organelles seem to have integrated phase separation and BCs in their repertoire of biological functions without which the cells may not be able to survive. Some of the complex BCs now observed in many life forms are implicitly dictated and controlled by the sequence and secondary structures of both proteins and RNA –clear evidence for the linked evolution of BCs with the evolution of sequence and structural compositional variance of RNA and protein molecules99,100,101,102,103. In addition, the evolution of protein clusters and structural disorder and sequence low complexity also played a role in the evolution of BCs. However, it is also clear that during the early evolution period, LLPS-based compartmentalization also presented limitations on selectivity and specificity, which the membrane-bound organelles could offer. So there seems to be a trade-off between the energy-efficient, simpler compartmentalization by phase separation and the membrane-bound compartmentalization that offered greater selectivity and specificity but with energy-demanding processes, such as active transport, endo- or exocytosis. Therefore, it seems as if MLOs and membrane-bound organelles have co-evolved compartments instead of out-competing each other. The cells have adapted to utilize both methods of compartmentalization to optimize biological functions depending on the need. Although it would be difficult to assert whether or not BCs are an extant biological relic, or they are evolving alongside the increasing complexities of life forms, it would certainly not be inaccurate to conclude that BCs and MLOs are unlikely to get out-evolved by membrane-bound compartments. It seems as if a synergy has been achieved to utilize both mechanisms to cope and adapt to the increasing complexities of the biological world.
The simplicity and ease of spatiotemporal compartmentalization by LLPS remain the key characteristic of BC formation. However, BCs and MLOs have adapted to achieve high levels of compositional and functional complexity in higher-order organisms that seem to co-evolve with membranous organelles. The dotted arrows indicate the longitudinal adaptation of LLPS in organisms.
Data availability
The article contains no new data.
References
Flory, P. J. & Krigbaum, W. R. Thermodynamics of High Polymer Solutions. Annu. Rev. Phys. Chem. 2, 51–61 (1951).
De Gennes, P. G. Dynamics of fluctuations and spinodal decomposition in polymer blends. J. Chem. Phys. 72, 1996–2000 (1980).
Saeki, S., Kuwahara, N., Nakata, M. & Kaneko, M. Phase separation of poly(ethylene glycol)-water-salt systems. Polymer. 18, 1027–1031 (1977).
de Jong Bungenberg, H. G. & Kruyt, H. R. Chemistry—Coacervation (Partial miscibility in colloid systems). Proc. K. Ned. Akad. Wet. 32, 849–856 (1929).
Oparin A. I. Origin of Life. (Macmillan, 1938).
Brangwynne, C. P. et al. Germline P granules are liquid droplets that localize by controlled dissolution/condensation. Science (1979) 324, 1729–1732 (2009).
Banani, S. F., Lee, H. O., Hyman, A. A. & Rosen, M. K. Biomolecular condensates: Organizers of cellular biochemistry. Nat. Rev. Mol. Cell Biol. 18, 285–298 (2017).
Lafontaine, D. L. J., Riback, J. A., Bascetin, R. & Brangwynne, C. P. The nucleolus as a multiphase liquid condensate. Nat. Rev. Mol. Cell Biol. 22, 165–182 (2021).
Gomes, E. & Shorter, J. The molecular language of membraneless organelles. J. Biol. Chem. 294, 7115–7127 (2019).
Spannl, S., Tereshchenko, M., Mastromarco, G. J., Ihn, S. J. & Lee, H. O. Biomolecular condensates in neurodegeneration and cancer. Traffic. 20, 890–911 (2019).
Harmon, T. S., Holehouse, A. S. & Pappu, R. V. To Mix, or To Demix, That Is the Question. Biophys. J. 112, 565–567 (2017).
Choi, J. M., Holehouse, A. S. & Pappu, R. V. Physical Principles Underlying the Complex Biology of Intracellular Phase Transitions. Annu Rev. Biophys. 49, 107–133 (2020).
Brangwynne, C. P. et al. Polymer physics of intracellular phase transitions. Nat. Phys. 11, 899–904 (2015).
Das, R. K. & Pappu, R. V. Conformations of intrinsically disordered proteins are influenced by linear sequence distributions of oppositely charged residues. Proc. Natl Acad. Sci. USA 110, 13392–13397 (2013).
Martin, E. W. et al. Valence and patterning of aromatic residues determine the phase behavior of prion-like domains. Science (1979) 367, 694–699 (2020).
Pak, C. W. et al. Sequence Determinants of Intracellular Phase Separation by Complex Coacervation of a Disordered Protein. Mol. Cell 63, 72–85 (2016).
Posey, A. E., Holehouse, A. S. & Pappu, R. V. Phase Separation of Intrinsically Disordered Proteins. Methods in Enzymology 611 (Elsevier Inc., 2018).
Boeynaems, S. et al. Spontaneous driving forces give rise to protein−RNA condensates with coexisting phases and complex material properties. Proc. Natl Acad. Sci. USA 116, 7889–7898 (2019).
Ceballos, A. V., McDonald, C. J. & Elbaum-Garfinkle, S. Methods and Strategies to Quantify Phase Separation of Disordered Proteins. Methods Enzymol. 611, 31–50 (2018).
Weber, C., Michaels, T. & Mahadevan, L. Spatial control of irreversible protein aggregation. Elife 8, e42315 (2019).
Peeples, W. & Rosen, M. K. Mechanistic dissection of increased enzymatic rate in a phase-separated compartment. Nat. Chem. Biol. 17, e42315 (2021).
Huang, W. Y. C. et al. A molecular assembly phase transition and kinetic proofreading modulate Ras activation by SOS. Science (1979) 363, 1098–1103 (2019).
Stroberg, W. & Schnell, S. Do Cellular Condensates Accelerate Biochemical Reactions? Lessons from Microdroplet Chemistry. Biophys. J. 115, 3–8 (2018).
Higgs, P. G. & Lehman, N. The RNA World: Molecular cooperation at the origins of life. Nat. Rev. Genet. 16, 7–17 (2015).
Gilbert, W. Origin of life: The RNA world. Nature 319, 618 (1986).
Crick, F. H. C. The origin of the genetic code. J. Mol. Biol. 38, 367–379 (1968).
Kruger, K. et al. Self-splicing RNA: Autoexcision and autocyclization of the ribosomal RNA intervening sequence of tetrahymena. Cell 31, 147–57 (1982).
Mandal, M. & Breaker, R. R. Gene regulation by riboswitches. Nat. Rev. Mol. Cell Biol. 5, 451–463 (2004).
Bartel, D. P. & Unrau, P. J. Constructing an RNA world. Trends in Biochemical Sciences 9, 9–13 (1999).
Jia, T. Z. et al. Membraneless polyester microdroplets as primordial compartments at the origins of life. Proc. Natl Acad. Sci. USA 116, 15830–15835 (2019).
Fallah-Araghi, A. et al. Enhanced chemical synthesis at soft interfaces: A universal reaction-adsorption mechanism in microcompartments. Phys. Rev. Lett. 112, 028301–028306 (2014).
Mann, S. Systems of creation: The emergence of life from nonliving matter. Acc. Chem. Res 45, 2131–2141 (2012).
Li, M., Green, D. C., Anderson, J. L. R., Binks, B. P. & Mann, S. In vitro gene expression and enzyme catalysis in bio-inorganic protocells. Chem. Sci. 2, 1745 (2011).
Pir Cakmak, F. & Keating, C. D. Combining Catalytic Microparticles with Droplets Formed by Phase Coexistence: Adsorption and Activity of Natural Clays at the Aqueous/Aqueous Interface. Sci. Rep. 7, 3215 (2017).
Ghosh, B., Bose, R. & Tang, T. Y. D. Can coacervation unify disparate hypotheses in the origin of cellular life? Curr. Opin. Colloid Interface Sci. 52, 101415 (2021).
Wieczorek, R. D., Dörr, M., Chotera, A., Luisi, P. L. & Monnard, P. A. Formation of RNA Phosphodiester Bond by Histidine-Containing Dipeptides. ChemBioChem 14, 217–223 (2013).
Griesser, H., Bechthold, M., Tremmel, P., Kervio, E. & Richert, C. Amino Acid-Specific, Ribonucleotide-Promoted Peptide Formation in the Absence of Enzymes. Angew. Chem. - Int. Ed. 56, 1224–1228 (2017).
Antifeeva, I. A. et al. Liquid–liquid phase separation as an organizing principle of intracellular space: overview of the evolution of the cell compartmentalization concept. Cell Mol. Life Sci. 79, 251 (2022).
Hansma, H. G. Liquid–liquid phase separation at the origins of life. In Droplets of Life. Academic Press https://doi.org/10.1016/C2020-0-01058-3 (2023).
Vázquez-Salazar, A. & Lazcano, A. Early Life: Embracing the RNA World. Current Biol. 28, 220–222 (2018).
Levy, M. & Ellington, A. D. Peptide-templated nucleic acid ligation. J. Mol. Evol. 56, 607–615 (2003).
Yu, S. S. et al. Elongation of Model Prebiotic Proto-Peptides by Continuous Monomer Feeding. Macromolecules 50, 286–9294 (2017).
Forsythe, J. G. et al. Ester-Mediated Amide Bond Formation Driven by Wet-Dry Cycles: A Possible Path to Polypeptides on the Prebiotic Earth. Angew. Chem. - Int. Ed. 54, 9871–9875 (2015).
Fahnestock, S. & Rich, A. Ribosome-catalyzed polyester formation.Science (1979) 173, 340–343 (1971).
Frenkel-Pinter, M. et al. Selective incorporation of proteinaceous over nonproteinaceous cationic amino acids in model prebiotic oligomerization reactions. Proc. Natl Acad. Sci. USA 116, 16338–16346 (2019).
Cobb, A. K. & Pudritz, R. E. Nature’s starships. I. observed abundances and relative frequencies of amino acids in meteorites. Astrophys. J. 783, 140–151 (2014).
Zaia, D. A. M., Zaia, C. T. B. V. & De Santana, H. Which amino acids should be used in prebiotic chemistry studies? Orig. Life Evol. Biospheres. 38, 469–488 (2008).
Poudyal, R. R., Pir Cakmak, F., Keating, C. D. & Bevilacqua, P. C. Physical Principles and Extant Biology Reveal Roles for RNA-Containing Membraneless Compartments in Origins of Life Chemistry. Biochemistry 57, 2509–2519 (2018).
Vladimir Uversky. Droplets of Life Membrane-Less Organelles, Biomolecular Condensates, and Biological Liquid-Liquid Phase Separation. (Elsevier, 2022).
Emenecker, R. J., Holehouse, A. S. & Strader, L. C. Emerging Roles for Phase Separation in Plants. Dev. Cell 55, 69–83 (2020).
Azaldegui, C. A., Vecchiarelli, A. G. & Biteen, J. S. The emergence of phase separation as an organizing principle in bacteria. Biophys. J. 120, 1123–1138 (2021).
Staples, M. I., Frazer, C., Fawzi, N. L. & Bennett, R. J. Phase separation in fungi. Nat. Microbiol 8, 375–386 (2023).
Powers, S. K. et al. Nucleo-cytoplasmic Partitioning of ARF Proteins Controls Auxin Responses in Arabidopsis thaliana. Mol. Cell 76, 177–190.e5 (2019).
van Buskirk, E. K., Decker, P. V. & Chen, M. Photobodies in light signaling. Plant Physiol. 158, 52–60 (2012).
Liu, Q., Shi, L. & Fang, Y. Dicing bodies. Plant Physiol. 158, 61–6 (2012).
Al-Husini, N. et al. BR-Bodies Provide Selectively Permeable Condensates that Stimulate mRNA Decay and Prevent Release of Decay Intermediates. Mol. Cell 78, 670–682.e8 (2020).
Lasker, K. et al. Selective sequestration of signalling proteins in a membraneless organelle reinforces the spatial regulation of asymmetry in Caulobacter crescentus. Nat. Microbiol. 5, 418–429 (2020).
Chen, Y. E. et al. Spatial gradient of protein phosphorylation underlies replicative asymmetry in a bacterium. Proc. Natl Acad. Sci. USA 108, 1052–7 (2011).
Ladouceur, A. M. et al. Clusters of bacterial RNA polymerase are biomolecular condensates that assemble through liquid-liquid phase separation. Proc. Natl Acad. Sci. USA 117, 1052–1057 (2020).
Ault-Riché, D., Fraley, C. D., Tzeng, C. M. & Kornberg, A. Novel assay reveals multiple pathways regulating stress-induced accumulations of inorganic polyphosphate in Escherichia coli. J. Bacteriol. 180, 1841–1847 (1998).
Krypotou, E. et al. Bacteria require phase separation for fitness in the mammalian gut. Science (1979) 379, 1149–1156 (2023).
Martin, E. W. & Holehouse, A. S. Intrinsically disordered protein regions and phase separation: Sequence determinants of assembly or lack thereof. Emerg. Topics Life Sci. 4, 307–329 (2020).
Garaizar, A., Sanchez-Burgos, I., Collepardo-Guevara, R. & Espinosa, J. R. Expansion of intrinsically disordered proteins increases the range of stability of liquid⇓liquid phase separation. Molecules 25, 4705 (2020).
Zhou, H. X., Nguemaha, V., Mazarakos, K. & Qin, S. Why Do Disordered and Structured Proteins Behave Differently in Phase Separation? Trend Biochem. Sci. 43, 499–516 (2018).
Dignon, G. L., Best, R. B. & Mittal, J. Biomolecular phase separation: From molecular driving forces to macroscopic properties. Ann. Rev. Phys. Chem. 71, 53–75 (2020).
Ward, J. J., Sodhi, J. S., McGuffin, L. J., Buxton, B. F. & Jones, D. T. Prediction and Functional Analysis of Native Disorder in Proteins from the Three Kingdoms of Life. J. Mol. Biol. 337, 635–645 (2004).
Shin, Y. & Brangwynne, C. P. Liquid phase condensation in cell physiology and disease. Science 357, eaaf4382 (2017).
Boeynaems, S. et al. Protein Phase Separation: A New Phase in Cell Biology. Trends Cell Biol. 28, 420–435 (2018).
Hyman, A. A., Weber, C. A. & Julicher, F. Liquid-liquid phase separation in biology. Annu Rev. Cell Dev. Biol. 30, 39–58 (2014).
Berry, J. et al. RNA transcription modulates phase transition-driven nuclear body assembly. Proc. Natl Acad. Sci. USA 112, E5237–45 (2015).
Lafontaine, D. L. J. Birth of Nucleolar Compartments: Phase Separation-Driven Ribosomal RNA Sorting and Processing. Mol. Cell 76, 694–696 (2019).
Wolozin, B. & Ivanov, P. Stress granules and neurodegeneration. Nat. Rev. Neurosci. 20, 649–666 (2019).
Wang, J., Gan, Y., Cao, J., Dong, X. & Ouyang, W. Pathophysiology of stress granules: An emerging link to diseases (Review). Int. J. Mol. Med. 49, 44 (2022).
Asadi, M. R. et al. Stress Granules and Neurodegenerative Disorders: A Scoping Review. Front Aging Neurosci. 13, 650740 (2021).
Aulas, A. et al. Stress-specific differences in assembly and composition of stress granules and related foci. J. Cell Sci. 130, 927–937 (2017).
Liu, J. et al. Intrinsic disorder in transcription factors. Biochemistry 45, 6873–88 (2006).
Garza, A. S., Ahmad, N. & Kumar, R. Role of intrinsically disordered protein regions/domains in transcriptional regulation. Life Sciences 84, 189–193 (2009).
Peng, L., Li, E. M. & Xu, L. Y. From start to end: Phase separation and transcriptional regulation. Biochim. Biophys. Acta Gene Regul. Mech. 1863, 194641 (2020).
Boija, A. et al. Transcription Factors Activate Genes through the Phase-Separation Capacity of Their Activation Domains. Cell 175, 1842–1855.e16 (2018).
Schärfen, L. & Neugebauer, K. M. Transcription Regulation Through Nascent RNA Folding. J. Mol. Biol. 433, 166975 (2021).
Rippe, K. Liquid–Liquid Phase Separation in Chromatin. Cold Spring Harb. Perspect. Biol. 14, a040683 (2022).
Feric, M. & Misteli, T. Function moves biomolecular condensates in phase space. BioEssays 44, e2200001 (2022).
Luo, Y. Y., Wu, J. J. & Li, Y. M. Regulation of liquid-liquid phase separation with focus on post-translational modifications. Chem. Commun. 57, 13275–13287 (2021).
Oses, C., Stortz, M., Verneri, P., Guberman, A. & Levi, V. Pluripotency transcription factors at the focus: The phase separation paradigm in stem cells. Biochem. Soc. Transactions 49, 2871–2878 (2021).
Luo, Y., Na, Z. & Slavoff, S. A. P-Bodies: Composition, Properties, and Functions. Biochemistry 57, 2424–2431 (2018).
Youn, J. Y. et al. Properties of Stress Granule and P-Body Proteomes. Mol. Cell 76, 286–294 (2019).
An, S., Kumar, R., Sheets, E. D. & Benkovic, S. J. Reversible compartmentalization of de novo purine biosynthetic complexes in living cells. Science (1979) 320, 103–6 (2008).
Bahadori, F. & Demiray, M. A Realistic View on ‘the Essential Medicinal Chemistry of Curcumin’. ACS Med. Chem. Lett. 8, 893–896 (2017).
Noda, N. N., Wang, Z. & Zhang, H. Liquid–liquid phase separation in autophagy. Journal of Cell Biology vol. 219, e202004062 (2020).
Chen, X., Wu, X., Wu, H. & Zhang, M. Phase separation at the synapse. Nat. Neurosci. 23, 301–310 (2020).
Du, M. & Chen, Z. J. DNA-induced liquid phase condensation of cGAS activates innate immune signaling. Science (1979) 361, 704–709 (2018).
Aguzzi, A. & Altmeyer, M. Phase Separation: Linking Cellular Compartmentalization to Disease. Trends Cell Biol. 26, 547–558 (2016).
Aguzzi, A. & Rajendran, L. The Transcellular Spread of Cytosolic Amyloids, Prions, and Prionoids. Neuron 64, 783–790 (2009).
Murray, D. T. et al. Structure of FUS Protein Fibrils and Its Relevance to Self-Assembly and Phase Separation of Low-Complexity Domains. Cell 171, 615–627.e16 (2017).
Weskamp, K. et al. Shortened TDP43 isoforms upregulated by neuronal hyperactivity drive TDP43 pathology in ALS. J. Clin. Investig. 130, 1139–1155 (2020).
Chanson, J. B. et al. TDP43-positive intraneuronal inclusions in a patient with motor neuron disease and Parkinson’s disease. Neurodegener. Dis. 7, 260–264 (2010).
Zhu, G. et al. Phase Separation of Disease-Associated SHP2 Mutants Underlies MAPK Hyperactivation. Cell 183, 490–502.e18 (2020).
Iserman, C. et al. Genomic RNA Elements Drive Phase Separation of the SARS-CoV-2 Nucleocapsid. Mol. Cell 80, 1078–1091.e6 (2020).
Reichheld, S. E., Muiznieks, L. D., Keeley, F. W. & Sharpe, S. Direct observation of structure and dynamics during phase separation of an elastomeric protein. Proc. Natl Acad. Sci. USA 114, E4408–E4415 (2017).
Sanchez de Groot, N. et al. RNA structure drives interaction with proteins. Nat. Commun. 10, 3246 (2019).
Langdon, E. M. et al. mRNA structure determines specificity of a polyQ-driven phase separation. Science (1979) 360, 922–927 (2018).
Meyer, M. O., Yamagami, R., Choi, S., Keating, C. D. & Bevilacqua, P. C. RNA folding studies inside peptide-rich droplets reveal roles of modified nucleosides at the origin of life. bioRxiv https://doi.org/10.1101/2023.02.27.530264 (2023).
Vernon, R. M. C. et al. Pi-Pi contacts are an overlooked protein feature relevant to phase separation. Elife 7, e31486 (2018).
Acknowledgements
The author would like to thank Anukool Bhopatkar, Jhinuk Saha, Shailendra Dhakal, and Faqing Huang for scintillating discussions and inputs.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The author declares no competing interests.
Peer review
Peer review information
Communications Biology thanks Sourav Chowdhury and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editors: Krishnananda Chattopadhyay and Manuel Breuer. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rangachari, V. Biomolecular condensates – extant relics or evolving microcompartments?. Commun Biol 6, 656 (2023). https://doi.org/10.1038/s42003-023-04963-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s42003-023-04963-3
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.