Abstract
Quantifying and predicting variation in gross primary productivity (GPP) is important for accurate assessment of the ecosystem carbon budget under global change. Scaling traits to community scales for predicting ecosystem functions (i.e., GPP) remain challenging, while it is promising and well appreciated with the rapid development of trait-based ecology. In this study, we aim to integrate multiple plant traits with the recently developed trait-based productivity (TBP) theory, verify it via Bayesian structural equation modeling (SEM) and complementary independent effect analysis. We further distinguish the relative importance of different traits in explaining the variation in GPP. We apply the TBP theory based on plant community traits to a multi-trait dataset containing more than 13,000 measurements of approximately 2,500 species in Chinese forest and grassland systems. Remarkably, our SEM accurately predicts variation in annual and monthly GPP across China (R2 values of 0.87 and 0.73, respectively). Plant community traits play a key role. This study shows that integrating multiple plant functional traits into the TBP theory strengthens the quantification of ecosystem primary productivity variability and further advances understanding of the trait-productivity relationship. Our findings facilitate integration of the growing plant trait data into future ecological models.
Similar content being viewed by others
Introduction
Gross primary productivity (GPP) is the largest carbon flux in terrestrial ecosystems and thus plays a prominent role in global carbon cycle regulation1. However, accurate prediction of GPP across ecosystems or regions remains challenging2, especially in the context of climate change and the impact of human activities on the carbon balance of the biosphere3. Although much research has been conducted on how environmental changes across space and time affect ecosystem primary productivity1,4,5, there remain fundamental knowledge gaps in terms of accurately capturing spatial or temporal variation in GPP and assessing its drivers. Intuitively, plants, especially its leaves, are the direct organs for photosynthesis and plant biomass production from first principles; hence, as the primary producer, plants contribute most to carbon fluxes across ecosystems1. Thus, plant leaf traits that are closely related to photosynthesis should directly influence ecosystem GPP, in combination with the direct and indirect effects of environmental factors, thereby regulating the global terrestrial carbon cycle and its response to climate change6,7,8. In this sense, previous studies have proposed the ecosystem functional biogeography concept, which integrates the effects of plant traits and environmental conditions in assessing ecosystem function9.
In ecology, the trait-based approaches offer a promising way to generalize predictions across organizational and spatial scales, independent of taxonomy. Accordingly, predicting ecosystem processes and functions such as GPP from functional traits instead of species identity has been considered the “holy grail” of trait-based ecological studies10,11. Although the use of plant traits to capture and predict the variation in ecosystem primary productivity (i.e., GPP) along a broad environmental gradient has aroused widespread interest10,12,13,14,15, a recent study has shown that plant traits alone are poor predictors of ecosystem functions16. Most related studies have established correlative linkages between the means of plant traits at the leaf scale and ecosystem primary productivity per unit land area17,18,19,20. While these studies have provided important insight into the trait-productivity relationship, there remain knowledge gaps18,21. Indeed, there is little to no evidence of causal linkage between the mean trait values characterizing leaf-level photosynthesis and total carbon absorption via the continuous activity of all photosynthetic tissues per unit land area during the growing season18,21. A high photosynthetic rate per unit leaf area or mass provides limited information about the carbon uptake of the entire plant18,22, and ignores the carbon-capture capacity of the ecosystem21. Even at lower values of leaf-level traits (e.g., lower leaf nutrient concentration), a community’s primary productivity per unit land area may still increase23,24. A more robust and mechanistic approach is therefore needed to integrate plant traits to predict variation in ecosystem primary productivity along broad environmental gradients.
Recently, a trait-based productivity (TBP) theory, which scales plant traits to the community level, has been proposed (Fig. 1; Text S1). The TBP theory assumes that ecosystem primary productivity is determined by environmental factors, trait quantity (Traitquantity), trait efficiency (Traitefficiency), and growing season length (GSL). GPP is affected by environmental factors (including growing season temperature, precipitation, and the moisture index) that both power ecosystem carbon-uptake as energy inputs (i.e., affecting net photosynthesis or maintaining respiration) and regulate plant carbon distribution, such as the assimilation of C as a nonstructural compound (i.e., in reserve pools) that represents storage at the expense of organ formation24. Traitquantity, which standardize traits on the unit land area, represents ecosystems carbon uptake capacity21. GSL determines the effective period of carbon absorption in the ecosystem, thus positively influencing GPP25. In addition, environmental factors affect GPP both directly and indirectly, by affecting plant community traits3,13,26. We therefore assume that a large part of their effect on GPP is mediated by plant community traits.
As an analogy to the Production Ecology Equation, we use emergent thinking to elucidate the formation of productivity at the ecosystem level, applying several simple and powerful parameters to predict ecosystem productivity (gross primary productivity, GPP). Here, environmental factors refers to energy input, representing the total supply of resources in an ecosystem; as plant community traits, Traitquantity represents resource uptake and carbon fixation, and Traitefficiency represents the intrinsic efficiency of resource utilization and production; and growing season length represents the period of CO2 absorption by the ecosystem. These three key parameters, reflecting ecosystem attributes, jointly determine ecosystem productivity. We extend this analogous multiplicative framework via structural equation modeling, to distinguish direct and indirect effects. In essence, trait-based productivity (TBP) theory scales traits to the community level, then uses plant community traits to predict GPP.
Integrating multiple functional traits to predict ecosystem primary productivity, rather than simply relying on the type of trait selected, is a major aspect of the TBP theory27. Based on the mass ratio hypothesis, community-weighted mean values of leaf traits (efficiency traits such as leaf nutrient concentration, LNC, and specific leaf area, SLA) are considered to be closely related to ecosystem efficiency13,28, ultimately affecting GPP13. However, although leaf size appears to be a good predictor of GPP at large scales20, leaf nutrient concentration is not a stable predictor18,19,22, even though both are based on community-weighted means. These knowledge gaps require a more reliable method to integrate multiple traits to predict GPP, with more convincing clear explanations. Meanwhile, “synthetic traits” (i.e., quantity traits) providing more contextual information (i.e., relating to phenotypic, environmental, and biogeographic context) are better predictors of ecosystem function18,21. Synthesizing these concepts, we propose a conceptual GPP model that integrates multiple traits based on TBP theory, representing the proposed hypothesis that the environment, plant community traits (quantity and efficiency traits), and GSL jointly determine GPP (Fig. 2a; Text S1).
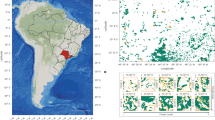
Conceptual model depicting linkages among the environmental factors and plant community traits affecting the variation in GPP (a) and geographic distribution of study sites (b).Traitquantity and Traitefficiency: quantity and efficiency traits, respectively; GSL: growing-season length (months). The map of sample distribution was created using ArcGIS software v10.8.
We systematically applied a high-quality dataset of plant traits and GPP, spanning broad environmental gradients, to verify our conceptual model based on the TBP theory. We surveyed 72 typical natural ecosystems across China, with high biodiversity and GPP gradients, measuring multiple leaf traits that are closely related to photosynthesis24,29, and using more than 13,000 plant samples and ca. 2,500 species (Fig. 2b). We asked three primary research questions: 1) How well can structural equation modeling based on TBP theory predict the observed yearly and monthly GPP along broad environmental gradients? 2) How do environmental factors and traits directly and indirectly affect GPP variation? 3) What is the relative importance of environmental factors and traits in determining the variation in GPP?
Results
Overall, our structural equation modelling (SEM), based on TBP theory, significantly captured GPP variation along broad environmental gradients (R2 = 0.87; Fig. 3a). Even after removing the effect of GSL as a phenological trait (GPPyearly divided by GSL to obtain GPPmonthly), the overall prediction ability was still strong (R2 = 0.73; Fig. 3b). Intriguingly, Traitquantity had the highest direct effect on GPPyearly (βstd = 0.33; Fig. 4a), while MIgs exerted the highest indirect effect on GPPyearly (βstd = 0.48; Fig. 4a). As expected, a large proportion of the impact (71%; Fig. 4b) of the environmental factors [growing season moisture index (MIgs), growing season monthly mean temperature (Tgs), and Soilpc1] on GPPyearly was due to indirect effects on GPPyearly via their effects on plant community traits.
Bayesian piecewise structural equation models exploring the direct and indirect effects of environmental factors and traits on gross primary productivity (GPP) across all sites: (a) yearly GPP (GPPyearly) and (b) monthly GPP (GPPmonthly). PC 1 and PC 2: the first two principal components (PCs) of the corresponding variables; Traitquantity and Traitefficiency: quantity and efficiency traits, respectively; GSL: length of the growing season (months); βstd: standardized path coefficients; black and red arrows: positive and negative relationships, respectively; solid and dotted lines: significant and non-significant effects, respectively; arrow width is proportional to the strength of the relationship; LOOIC: leave-one-out cross-validation information criterion; ELPD, expected log predictive density.
Panels a and b represent the effect on yearly GPP; c and d represent the effect on monthly GPP; Panels b and d also additionally represent the ratio of direct and indirect effects of environmental factors on GPP. PC 1 and PC 2: first two principal components (PCs) of the corresponding variables; Traitquantity and Traitefficiency: quantity and efficiency traits, respectively; GSL: length of the growing season (months). MIgs and Tgs: growing season moisture index and temperature, respectively.
Our random forest trait model revealed that the quantity traits predicted the variation in GPP well (Text S2; Fig. S1). The quantity traits (leaf area index, LAI; leaf biomass per unit area, LMI; and total leaf nitrogen and phosphorus per unit land area, LNI and LPI, respectively), were significantly positively associated with GPPyearly, and leaf area (LA) and leaf dry mass (LM) were important as efficiency traits (Fig. S1a). For GPPmonthly, all four quantity traits (LAI, LMI, LNI, and LPI) had robust predictive power, considerably outperforming the efficiency traits (Fig. S1b). Our random forest modeling and forecasting revealed that GPPyearly (Slope, 1.12 ± 0.10, RMSE = 262.41) and GPPmonthly (Slope, 1.16 ± 0.16, RMSE = 39.79) were well-predicted, based on TBP theory (Fig. S2).
We applied independent effects analysis (IEA), an important complementary analysis to SEM, to assess the independent effects of each variable on GPP. Based on the GPPyearly model, trait factors accounted for 69% of all the variables’ influence on GPPyearly, while environmental variables accounted for 31% (Fig. 5). Based on the GPPmonthly model, trait factors accounted for 70% of all the variables’ influence on GPP, while environmental factors accounted for 30% (Fig. 5). Irrespective of the modeled response variable, the key results did not change when the models were run separately for each vegetation type (Fig. 5).
GPPyearly: yearly GPP model (GPPyearly ~ MIgs + Tgs + Soilpc1 + Traitquantity + Traitefficiency + GSL). GPPmonthly: monthly GPP model (GPPmonthly ~ MIgs + Tgs + Soilpc1 + Traitquantity + Traitefficiency). PC 1 and PC 2: first two principal components (PCs) of the corresponding factors; Traitquantity and Traitefficiency: quantity and efficiency traits, respectively; GSL: growing season length (months). MIgs and Tgs: growing season moisture index and temperature, respectively. n values in brackets: number of observations for the corresponding group.
Discussion
The primary goal of this study was to test whether this conceptual model based on TBP theory can be used to capture the spatial variation in ecosystem GPP along a wide environmental gradient, at both yearly and monthly scales. Our model successfully captured the spatial variation in both yearly and monthly productivity. Our TBP theory explains clearly how four key controlling elements—environmental factors (Tgs and MIgs), Traitquantity, Traitefficiency, and GSL—capture the spatial variation in GPP.
Tgs and MIgs regulate plant structural growth by mediating their energy and resource (water and nutrient) inputs24,30 and carbon allocation24, thereby collectively affecting GPP. Here, environmental factors significantly impacted GPP, both directly and indirectly. Previous studies have assumed such a trait-based response-and-effect framework26, in which plant traits mediate the effects of environmental factors on ecosystem function. Nonetheless, our study is one of few to present empirical evidence of the irreplaceable mediatory role of plant traits.
Our findings for Traitquantity, the product of mass-based leaf traits and leaf biomass per unit area (LMI), were consistent with our expectations; this variable substantially affected GPP, even more so than Traitefficiency. This is consistent with the carbon economy theory, which predicts that the plant relative growth rate is determined by the biomass allocated to photosynthetic tissues22,31. Compared with Traitefficiency, Traitquantity represents the vegetation nutrient stocks of the whole ecosystem and thus can be better used to predict GPP. Although the plant nutrient pool is not a direct measure of ecosystem function (i.e. ecosystem fluxes of energy and matter)32, it is an important attribute that determines ecosystem function (e.g., decomposition, carbon sequestration, nitrification and nutrient recycling)33,34,35,36,37. Numerous studies have shown that plant nutrient pool is particularly relevant to the long-term net ecosystem balance of energy and matter34,37. Higher plant nutrient pool values mean that, for each unit area of land, the vegetation has better resource utilization capacity, indicating that more productive species are selected (such as leaf area index38). Plant nutrient pool is thus considered a driver of ecosystem production-related function21,39.
Traitefficiency, a conventionally used community-weighted trait mean trait, was positively related to GPP; this relationship was primarily driven by LA (see Fig. 5). LA, representing leaf size, is closely related to plant energy balance (including energy uptake and conversion) and is a reliable indicator of GPP on a large scale20. A recent global-scale study revealed a strong relationship between LA and canopy size (including canopy height, diameter, and tree height), which reflects the total photosynthetic capacity of the whole tree40. While this explains why LA can predict large-scale variation in GPP, this coupling does not necessarily reflect causality. The efficiency traits leaf nutrient concentration (LNC) and leaf phosphorus concentration (LPC), for instance, reflect the photosynthetic rate per unit leaf area or unit leaf mass; however, they do not always link well causally with GPP, because of the absence of other community context information, such as leaf biomass allocation or total leaf area18,22. The choice of efficiency traits is therefore critical for predicting ecosystem function27. In contrast, as quantity traits, nutrient concentration and leaf size per unit land area predict variation in GPP well, by standardizing the unit land area21, making the effect of trait choice less important. GSL plays an important role in shaping GPP by affecting the period of photosynthetic activity6,25,41.
Notably, Traitquantity and Traitefficiency pc2 (mainly representing SLA), played key roles at the monthly scale (Fig. 4b), indicating that once the vegetation growth of a particular ecosystem starts under normal resource supply conditions, its efficiency, and especially its capacity to capture resources, becomes more important. SLA, an important component of the plants’ relative growth rate model42, may further affect monthly ecosystem productivity by affecting specific primary productivity43. More than 90% of the spatial variation in annual GPP is determined by the CO2 uptake period (i.e., GSL) and the ecosystem’s CO2 uptake capacity25, which are closely related to vegetation community structural variables such as total leaf area and above-ground biomass8. Here, trait quantity, which represents ecosystem CO2 uptake capacity, reflects the structural characteristics of the vegetation community21. Therefore, GPP at the monthly scale (GPPyearly/GSL) was most affected by Traitquantity. Plant growth peak sampling to measure plant functional traits ignores the temporal variation of plant functional traits, which is an important reason that only part of GPP variation can be captured. Seasonal sampling of vegetation to measure plant functional traits combined with dynamic flux observations will help us capture the variation of GPP more accurately. Diversity can enhance total leaf area (i.e., Traitquantity) and light interception due to increased canopy packing, thus increasing the likelihood of overyielding44.
A large component of the impact of environmental factors on ecosystem primary productivity was indirect, via the trait variables (Fig. 5). While there is no doubt that environmental factors significantly affect GPP4, several studies have proposed quantifying the pathways whereby abiotic factors (environmental factors) contribute to GPP through biotic factors1,15,41. Understanding this impact has great significance for quantifying the impact of environmental change on ecosystem carbon cycles3, especially in the context of biodiversity loss due to global change. Here, particularly at the monthly scale, environmental factors strongly affected GPP, via their effects on traits (Fig. 4b).
These findings have important implications for future research. Much of the impact of environmental factors on GPP is mediated by traits, including Traitquantity, Traitefficiency, and phenology (growing season length). Plant traits directly affect ecosystem productivity, from first principles. In studies in which traits are not standardized by unit land area7,17, the indirect effects of environmental factors on GPP are difficult to detect or are greatly weakened. Indeed, “synthesis traits”, such as Traitquantity, provide extra information on the total amount of photosynthetic tissue; such traits are needed to improve the capture of multi-dimensional variation in plant function22. Here, soil nutrients had no direct effects on GPP, possibly due to scale effects45, whereas they acted indirectly via their effects on plant traits.
Global changes, such as increased atmospheric CO2 concentration and nitrogen deposition, alter plant biochemical traits (e.g., leaf nutrient concentration)46 and biomass allocation characteristics23, which in turn have an important impacts on ecosystem carbon uptake23. Our study demonstrates that such plant traits can effectively predict GPP. Therefore, detecting changes in traits will help to elucidate, even predict, potential changes in ecosystem carbon balance, as highlighted in the trait-based response-and-effect framework26. The effects of plant functional traits on GPP are not limited to leaf traits. Root traits, such as fine-root nutrients or biomass38, could potentially influence GPP, especially if they are not coupled or coordinated with leaf traits47. Phenological trait datasets require updating. As this study was limited to large-scale data, we could only roughly estimate the length of the growing season. It is better to use first-hand observational data, based on ecological field stations48. Further studies are required to identify interactions among the various types of plant community traits and/or across resource gradients. As such, we could better understand or clarify whether Traitquantity and Traitefficiency always act synergistically, and the circumstances under which trade-offs occur. Standardizing traits by unit land area (i.e., Traitquantity), and further examining their relationships to ecosystem function, deserves further attention, especially given that most ecosystem functions are quantified on a unit land area basis. This will improve our ability to explain and predict the responses of terrestrial ecosystems to global change at different scales.
Methods
Plant traits and soil data
The dataset of plant functional traits was collected during a large-scale field survey in 72 typical natural ecosystems using a unified sampling standard from 2013–2019. These sites include evergreen broadleaf forests, deciduous broadleaf forests, evergreen coniferous forests, deciduous coniferous forests, shrublands, meadows, steppes, and sparse grasslands, spanning broad environmental gradients and with high environmental heterogeneity. Standardized sampling and measurement protocols were applied to each vegetation and soil surveys. Specifically, the surveyed sites extended from 18.74°N to 53.33°N and 78.47°E to 128.89°E, with mean annual temperatures ranging from −3.8 °C to 22.2 °C, and mean annual precipitation ranging from 32–1942 mm. Plant samples were collected using the quadrat method (30 m × 40 m for the forest, 10 m × 10 m for shrubland, and 1 m × 1 m for grassland) to investigate the community structure during the plant growth peak period from July to August (see Text S3 for more information on the sampling protocol). In each plot within a site, key plant community structure variables were measured, including species identity, species number, plant height, diameter at breast height (DBH; basal stem diameter for shrubs) for all woody plants with DBH ≥ 1 cm, and aboveground biomass for herbaceous species. The measured individual-level functional traits for woody and herbaceous plants included leaf area (LA, cm2), leaf dry mass (LM, g), specific leaf area (SLA, cm2/g), leaf nitrogen concentration (LNC, mg/g), and leaf phosphorus concentration (LPC, mg/g), closely related to plant photosynthesis and growth29,49 (Text S4). Functional traits were divided into size traits, reflecting plant size and light competitiveness, and economic traits, reflecting leaf photosynthetic capacity and nutrient economic40,50. All of these traits selected in this study are closely related to the plant light competitiveness and ecosystem photosynthetic capacity. Soil samples from the 0–10 cm soil layer were collected via auger boring for analysis of total soil carbon (%), nitrogen (%), phosphorus (%), and soil pH (Text S5). For further details regarding plot setting, plant trait measurement, and soil analysis, see Text S2–4, and other sources published by this team51,52,53.
Climate and length of the growing season
Monthly mean temperature (MMT) and precipitation (MMP) data were downloaded from Climatologies at High resolution for the Earth’s Land Surface Areas (CHELSA, https://chelsa-climate.org/)54,55. Monthly potential evapotranspiration data were downloaded from the Global Potential Evapo-Transpiration Climate Database (http://www.csi.cgiar.org). The moisture index (MI) was calculated as MMP/PET to represent the monthly water balance of the sample sites56. All consecutive months meeting the following two conditions were determined as months of plant growth: (1) MMT ≥ 5 °C and (2) moisture index (MI) ≥ 0.0557. We determined growing season length (GSL) as number of months of plant growth, and further averaged and summed the MI, MMT, and MMP of the growing months (MIgs, Tgs, and Pgs).
Plant community traits and gross primary productivity
As introduced by He, et al.21,39, we linked GPP by scaling individual-level traits to the community level in two ways:
where Pi is the relative biomass of the ith species in the community (%), and Traiti represents the leaf nitrogen and phosphorus concentrations of the ith species observed in the plot
where n is the number of species in the community, Traiti represents the leaf N and P concentrations of the ith species observed in the plot, LMIi is the leaf mass per land area of the ith species in a specific community (kg m−2), and LMIT is the total leaf mass per land area in a specific community (kg m−2).
Site-specific annual GPP data for 2000–2016 were extracted from a global GPP raster dataset with a moderate spatial resolution (500 m), validated against 113 eddy covariance flux towers across the globe58. We also calculated monthly GPP (GPPmonthly = GPPyearly/GSL).
Statistics and reproducibility
To simplify the analysis and avoid overfitting (Fig. S3), a principal component (PC) analysis was performed on all factors (soil factor, trait efficiency, and trait capacity) containing more than three variables (Fig. 6; Table S2). We applied Kaiser’s rule to retain PC axes whose eigenvalue is greater than 1, and where the cumulative variance explained by the variables reaches more than 80%, which meets the default variance capture threshold (≥ 70%)59. The first two PC axes for the community-weighted means of LA, LM, SLA, LNC, and LPC were used as the Traitefficiency and captured 53% (Traitefficiency pc1) and 30% (Traitefficiency pc2) of the variation in these traits, respectively. Traitefficiency pc1 primarily explained variability in LM (27%), LA (26%), LNC (22%), and LPC (21%). Traitefficiency pc2 best explained variability in SLA (35%). The first PC axis for leaf area index (LAI, m2/m2), leaf mass index (LMI, g/m2), and total leaf N and P per unit land area (LNI and LPI, g/m2) was used as the Traitquantity, and captured 98% of the variation in these traits explaining the variability in the plant community traits evenly: LMI (25%), LAI (25%), LNI (25%), and LPI (25%). The traits were log-transformed before analysis to eliminate size-dependent trait biases60. The first PC axis of soil variables (including total soil carbon, nitrogen, phosphorus content, and soil pH) explained 61% of the variation (Table S2). As high collinearity can distort model estimation, collinearity was checked by first calculating the variance inflation factor: this was >5 for Pgs, which was therefore discarded from the main analysis.
a and b Biplots resulting from the principal component analysis for efficiency trait (Traitefficiency, a) and capacity trait (Traitquantity, b). Grey points: different sites. c Bar plots of the contributions; LA, leaf area (cm2); LM, leaf dry mass (g); SLA, specific leaf area (cm2/g); LNC, leaf nitrogen concentration (mg/g); LPC, leaf phosphorus concentration (mg/g); LAI, leaf area index (m2/m2); LMI, leaf mass index (g/m2); LNI, total leaf nitrogen per unit land area (g/m2); and LPI, total leaf phosphorus per unit land area (g/m2).
To distinguish the direct and indirect effects of environmental factors and traits on GPP and test our pathway hypothesis (Text S6), Bayesian piecewise structural equation modeling (SEM)61,62 was used (Fig. 2b). The SEM models fitted in this study were created in the Stan computational framework (http://mc-stan.org/) accessed using the brms package63 and run with two Markov chain Monte Carlo (MCMC) chains, 10,000 iterations, and a warm-up of 1000 runs. Model convergence was assessed by visually examining trace plots and using \(\hat{R}\) values (the ratio of the effective sample size to the overall number of iterations, with values close to one indicating convergence). All \(\hat{R}\) values were below 1.01, and effective sample sizes were > 5000 for all coefficient estimates (Supplementary Figs. 2–5). The significance of the coefficient estimates assumes that the credible interval does not include zero. All variables were standardized (mean = 0, standard deviation = 1) before analysis to ensure that standardized path coefficients (hereafter βstd) were obtained. The indirect effect was the product of the direct effects.
LOOIC (leave-one-out cross-validation [LOO] information criterion) and ELPD (expected log predictive density)64 were used for model verification, using the loo package (for LOOIC and ELPD, smaller and larger values indicate a better fit, respectively)65. Posterior prediction checks were performed using the bayesplot package66. The validation results for all the models are provided in Supplementary Figs. 2–5. The Pareto shape k is used to diagnose abnormal observation points64. Although some of the observations had k estimates reflecting abnormality, the results were not substantially different after removing the outliers (Supplementary Data 1). Considering that the site trait data was obtained via standard protocol field surveys, and the need for caution when deleting outliers, we included all sites and analyses in the main text. The model results without outliers are listed in the attachment (Supplementary Data 1). We repeated the main analysis after dividing the observations into woody and herbaceous communities (Supplementary Data 1), and using a single trait to represent the quantity and efficiency traits, respectively (Supplementary Data 2).
Complementary to the SEM analysis, we used independent effects analysis (IEA) in the R package hier.part to examine the independent contribution of each explanatory variable in predicting GPP67. This approach quantifies the contribution of each predictor in explaining total variance in GPP by comparing the fit of all models containing a particular variable to the fit of all nested models lacking that variable, a process referred to as hierarchical partitioning68. Considering the correlation between environmental factors and plant traits, and the ability of IEA to robustly partition the independent contributions of correlated predictors, this analysis is highly appropriate and effective for determining the relative importance of the environmental factors and plant traits in our study68.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The data used in this study are available via the Figshare repository (https://doi.org/10.6084/m9.figshare.22081634.v1)69.
Code availability
All the R packages used are described in the methods section. No new code (or function) was created.
References
Beer, C. et al. Terrestrial gross carbon dioxide uptake: global distribution and covariation with climate. Science 329, 834–838 (2010).
Badgley, G., Field, C. B. & Berry, J. A. Canopy near-infrared reflectance and terrestrial photosynthesis. Sci. Adv.3, e1602244 (2017).
Chapin, F. S. III Effects of plant traits on ecosystem and regional processes: a conceptual framework for predicting the consequences of global change. Ann. Bot. 91, 455–463 (2003).
Chu, C. et al. Does climate directly influence NPP globally? Global Chan. Biol. 22, 12–24 (2016).
Yao, Y. et al. Spatiotemporal pattern of gross primary productivity and its covariation with climate in China over the last thirty years. Global Chan. Biol. 24, 184–196 (2018).
Fang, J., Lutz, J. A., Wang, L., Shugart, H. H. & Yan, X. Using climate-driven leaf phenology and growth to improve predictions of gross primary productivity in North American forests. Global Chan. Biol. 26, 6974–6988 (2020).
Fernández-Martínez, M. et al. The role of climate, foliar stoichiometry and plant diversity on ecosystem carbon balance. Global Chan. Biol. 26, 7067–7078 (2020).
Migliavacca, M. et al. The three major axes of terrestrial ecosystem function. Nature 598, 468–472 (2021).
Reichstein, M., Bahn, M., Mahecha, M. D., Kattge, J. & Baldocchi, D. D. Linking plant and ecosystem functional biogeography. Proc. Natl. Acad. Sci. 111, 13697–13702 (2014).
Funk, J. L. et al. Revisiting the Holy Grail: using plant functional traits to understand ecological processes. Biol. Rev. 92, 1156–1173 (2017).
Lavorel, S. & Garnier, É. Predicting changes in community composition and ecosystem functioning from plant traits: revisiting the Holy Grail. Fun. Ecol. 16, 545–556 (2002).
Enquist, B. J. et al. in Advances in Ecological Research 52 (eds Samraat P, Guy W, & Anthony I. D) 249–318 (Academic Press, 2015).
Garnier, E. et al. Plant functional markers capture ecosystem properties during secondary succession. Ecology 85, 2630–2637 (2004).
Enquist, B. J. et al. Assessing trait-based scaling theory in tropical forests spanning a broad temperature gradient. Global Ecol. Biogeogr. 26, 1357–1373 (2017).
Fyllas, N. M. et al. Solar radiation and functional traits explain the decline of forest primary productivity along a tropical elevation gradient. Ecol. Lett. 20, 730–740 (2017).
Van der Plas, F. et al. Plant traits alone are poor predictors of ecosystem properties and long-term ecosystem functioning. Nat. Ecol. Evol. 4, 1602–1611 (2020).
Ali, A., Yan, E.-R., Chang, S. X., Cheng, J.-Y. & Liu, X.-Y. Community-weighted mean of leaf traits and divergence of wood traits predict aboveground biomass in secondary subtropical forests. Sci. Total Environ. 574, 654–662 (2017).
Yang, J., Cao, M. & Swenson, N. G. Why Functional Traits Do Not Predict Tree Demographic Rates. Trend Ecol. Evol. 33, 326–336 (2018).
Šímová, I. et al. The relationship of woody plant size and leaf nutrient content to large-scale productivity for forests across the Americas. J. Ecol. 107, 2278–2290 (2019).
Li, Y. et al. Leaf size of woody dicots predicts ecosystem primary productivity. Ecol. Lett. 23, 1003–1013 (2020).
He, N. et al. Ecosystem Traits Linking Functional Traits to Macroecology. Trend. Ecol. Evol. 34, 200–210 (2019).
Rubio, V. E., Zambrano, J., Iida, Y., Umaña, M. N. & Swenson, N. G. Improving predictions of tropical tree survival and growth by incorporating measurements of whole leaf allocation. J. Ecol. 109, 1331–1343 (2021).
Drake, J. E. et al. Increases in the flux of carbon belowground stimulate nitrogen uptake and sustain the long-term enhancement of forest productivity under elevated CO2. Ecol. Lett. 14, 349–357 (2011).
Hilty, J., Muller, B., Pantin, F. & Leuzinger, S. Plant growth: the What, the How, and the Why. New Phytol. 232, 25–41 (2021).
Xia, J. et al. Joint control of terrestrial gross primary productivity by plant phenology and physiology. Proc. Natl. Acad. Sci. 112, 2788–2793 (2015).
Suding, K. N. et al. Scaling environmental change through the community-level: A trait-based response-and-effect framework for plants. Global Chan. Biol. 14, 1125–1140 (2008).
Liu, C., Li, Y., Yan, P. & He, N. How to Improve the Predictions of Plant Functional Traits on Ecosystem Functioning? Front. Plant Sci. 12, 622260 (2021).
Reich, P. B., Walters, M. B. & Ellsworth, D. S. From tropics to tundra: global convergence in plant functioning. Proc. Natl. Acad. of Sci. 94, 13730–13734 (1997).
Reich, P. B. The world-wide ‘fast–slow’ plant economics spectrum: a traits manifesto. J. Ecol. 102, 275–301 (2014).
Monteith, J. L. Climate and the efficiency of crop production in Britain. Philosophical Transactions of the Royal Society of London. B. Biol. Sci. 281, 277–294 (1977).
Garnier, E. Resource capture, biomass allocation and growth in herbaceous plants. Trend. Ecol. Evol. 6, 126–131 (1991).
Farnsworth, K. D., Albantakis, L. & Caruso, T. Unifying concepts of biological function from molecules to ecosystems. Oikos 126, 1367–1376 (2017).
Zhang, R. et al. Biodiversity alleviates the decrease of grassland multifunctionality under grazing disturbance: A global meta-analysis. Global Ecol. Biogeogr. 31, 155–167 (2022).
Jing, X. et al. The links between ecosystem multifunctionality and above-and belowground biodiversity are mediated by climate. Nat. Commun. 6, 1–8 (2015).
Peters, M. K. et al. Climate–land-use interactions shape tropical mountain biodiversity and ecosystem functions. Nature 568, 88–92 (2019).
Hu, W. et al. Aridity-driven shift in biodiversity–soil multifunctionality relationships. Nat. Commun. 12, 1–15 (2021).
Jing, X. et al. The influence of aboveground and belowground species composition on spatial turnover in nutrient pools in alpine grasslands. Global Ecol. Biogeogr. 31, 486–500 (2022).
Jing, X. et al. Above-and belowground complementarity rather than selection drives tree diversity-productivity relationships in European forests. Funct Ecol. 35, 1756–1767 (2021).
He, N. et al. Predicting ecosystem productivity based on plant community traits. Trend. Plant Sci. 28, 43–53 (2023).
Maynard, D. S. et al. Global relationships in tree functional traits. Nat. Commun. 13, 1–12 (2022).
Michaletz, S. T., Kerkhoff, A. J. & Enquist, B. J. Drivers of terrestrial plant production across broad geographical gradients. Global Ecol. Biogeogr. 27, 166–174 (2018).
Shipley, B. Net assimilation rate, specific leaf area and leaf mass ratio: which is most closely correlated with relative growth rate? A meta-analysis. Funct. Ecol. 20, 565–574 (2006).
Violle, C. et al. Let the concept of trait be functional! Oikos 116, 882–892 (2007).
Jucker, T., Bouriaud, O. & Coomes, D. A. Crown plasticity enables trees to optimize canopy packing in mixed-species forests. Funct. Ecol. 29, 1078–1086 (2015).
McGill, B. J. Matters of Scale. Science 328, 575 (2010).
Penuelas, J. et al. Increasing atmospheric CO2 concentrations correlate with declining nutritional status of European forests. Communi. Biol. 3, 1–11 (2020).
Weemstra, M. et al. Towards a multidimensional root trait framework: a tree root review. New Phytol. 211, 1159–1169 (2016).
Oehri, J., Schmid, B., Schaepman-Strub, G. & Niklaus, P. A. Biodiversity promotes primary productivity and growing season lengthening at the landscape scale. Proc. Natl. Acad. Sci. 114, 10160–10165 (2017).
Wright, I. J. et al. The worldwide leaf economics spectrum. Nature 428, 821–827 (2004).
Diaz, S. et al. The global spectrum of plant form and function. Nature 529, 167–171 (2015).
Liu, Y. et al. The optimum temperature of soil microbial respiration: Patterns and controls. Soil Biol. Biochem. 121, 35–42 (2018).
Zhao, N. et al. Coordinated pattern of multi-element variability in leaves and roots across Chinese forest biomes. Global Ecol. Biogeogr. 25, 359–367 (2016).
Zhang, J. et al. C: N: P stoichiometry in China’s forests: From organs to ecosystems. Funct. Ecol. 32, 50–60 (2018).
Karger, D. N. et al. Climatologies at high resolution for the earth’s land surface areas. Sci. Data 4, 1–20 (2017).
Dirk Nikolaus, K. et al. Climatologies at high resolution for the earth’s land surface areas. EnviDat. https://doi.org/10.16904/envidat.332 (2021).
Kerkhoff, A. J., Enquist, B. J., Elser, J. J. & Fagan, W. F. Plant allometry, stoichiometry and the temperature-dependence of primary productivity. Global Ecol. Biogeogr. 14, 585–598 (2005).
Wright, I. J. et al. Global climatic drivers of leaf size. Science 357, 917–921 (2017).
Zhang, Y. et al. A global moderate resolution dataset of gross primary production of vegetation for 2000-2016. Sci. Data 4, 170165 (2017).
Jolliffe, I. T. & Cadima, J. Principal component analysis: a review and recent developments. Philos. Trans. Soc. A Math. Phys. Eng. Sci. 374, 20150202 (2016).
Wieczynski, D. J. et al. Climate shapes and shifts functional biodiversity in forests worldwide. Proc. Natl. Acad. Sci. 116, 587–592 (2019).
Lefcheck, J. S. piecewiseSEM: Piecewise structural equation modelling in r for ecology, evolution, and systematics. Method Ecol. Evol. 7, 573–579 (2016).
Bürkner, P.-C. Advanced bayesian multilevel modeling with the R Package brms. R J. 10, 395–411 (2018).
Bürkner, P.-C. brms: An R package for Bayesian multilevel models using Stan. J. Stat. Software 80, 1–28 (2017).
Vehtari, A., Gelman, A. & Gabry, J. Practical Bayesian model evaluation using leave-one-out cross-validation and WAIC. Stat. Comput. 27, 1413–1432 (2017).
Vehtari, A. et al. loo: Efficient leave-one-out cross-validation and WAIC for Bayesian models. R package version 2, 1003 (2019).
Gabry, J. & Mahr, T. bayesplot: Plotting for Bayesian models. R package version 1 (2017).
Mac Nally, R. & Walsh, C. J. Hierarchical partitioning public-domain software. Biodivers. Conserv. 13, 659 (2004).
Murray, K. & Conner, M. M. Methods to quantify variable importance: implications for the analysis of noisy ecological data. Ecology 90, 348–355 (2009).
Yan, P., He, N., Yu, K., Xu, L. & Van Meerbeek, K. Integrating multiple functional traits to predict ecosystem productivity. figshare (2023). Dataset. https://doi.org/10.6084/m9.figshare.22081634.v1.
Acknowledgements
We thank all the members who have participated in field investigation over the eight years to assist us in collecting the data. This study was financially supported by the National Key R&D Program of China (2022YFF080210102), the National Natural Science Foundation of China (42141004, 31988102), by CAS Project for Young Scientists in Basic Research (YSBR-037), and by National Science and Technology Basic Resources Survey Program of China (2019FY101300).
Author information
Authors and Affiliations
Contributions
N.H. and P.Y. designed the research. P.Y., and N.H. conducted the research (collected the datasets and analyzed the data). P.Y. wrote the manuscript. K.V.M., K.Y., L.X., and N.H. commented on the details of the manuscript drafts.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Biology thanks the anonymous reviewers for their contribution to the peer review of this work. Primary Handling Editors: Shouli Li and Luke R. Grinham. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yan, P., He, N., Yu, K. et al. Integrating multiple plant functional traits to predict ecosystem productivity. Commun Biol 6, 239 (2023). https://doi.org/10.1038/s42003-023-04626-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s42003-023-04626-3
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.