Abstract
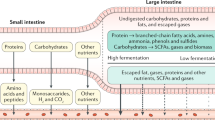
Ingestible sensors are potentially a powerful tool for monitoring human health. Sensors have been developed that can, for example, provide pH and pressure readings or monitor medication, but capsules that can provide key information about the chemical composition of the gut are still not available. Here we report a human pilot trial of an ingestible electronic capsule that can sense oxygen, hydrogen, and carbon dioxide. The capsule uses a combination of thermal conductivity and semiconducting sensors, and their selectivity and sensitivity to different gases is controlled by adjusting the heating elements of the sensors. Gas profiles of the subjects were obtained while modulating gut microbial fermentative activities by altering their intake of dietary fibre. Ultrasound imaging confirmed that the oxygen-equivalent concentration profile could be used as an accurate marker for the location of the capsule. In a crossover study, variations of fibre intake were found to be associated with differing small intestinal and colonic transit times, and gut fermentation. Regional fermentation patterns could be defined via hydrogen gas profiles. Our gas capsule offers an accurate and safe tool for monitoring the effects of diet of individuals, and has the potential to be used as a diagnostic tool for the gut.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Kalantar-Zadeh, K., Ha, N., Ou, J. Z. & Berean, K. J. Ingestible Sensors. ACS Sens. 2, 468–483 (2017).
Mowat, A. M. & Agace, W. W. Regional specialization within the intestinal immune system. Nat. Rev. Immunol. 14, 667–685 (2014).
Bettinger, C. J. Materials advances for next-generation ingestible electronic medical devices. Trends Biotechnol. 33, 575–585 (2015).
Gora, M. J. et al. Tethered capsule endomicroscopy enables less invasive imaging of gastrointestinal tract microstructure. Nat. Med. 19, 238–240 (2013).
Gibson, G. R., Probert, H. M., Van Loo, J., Rastall, R. A. & Roberfroid, M. B. Dietary modulation of the human colonic microbiota: updating the concept of prebiotics. Nutr. Res. Rev. 17, 259–275 (2004).
Mathur, R. et al. Methane and hydrogen positivity on breath test is associated with greater body mass index and body fat. J. Clin. Endocrinol. Metab. 98, E698–E702 (2013).
Ong, D. K. et al. Manipulation of dietary short chain carbohydrates alters the pattern of gas production and genesis of symptoms in irritable bowel syndrome. J. Gastroenterol. Hepatol. 25, 1366–1373 (2010).
Ou, J. Z. et al. Human intestinal gas measurement systems: in vitro fermentation and gas capsules. Trends Biotechnol. 33, 208–213 (2015).
Levitt, M. D. & Bond, J. H. Volume, composition, and source of intestinal gas. Gastroenterol 59, 921–929 (1970).
Carbonero, F., Benefiel, A. C. & Gaskins, H. R. Contributions of the microbial hydrogen economy to colonic homeostasis. Nat. Rev. Gastroenterol. Hepatol. 9, 504–518 (2012).
Levitt, M. D. Volume and composition of human intestinal gas determined by means of and intestinal washout technic. N. Engl. J. Med. 284, 1394–1398 (1971).
Nicholson, J. K. et al. Host-gut microbiota metabolic interactions. Science 336, 1262–1267 (2012).
Tuohy, K. M., Probert, H. M., Smejkal, C. W. & Gibson, G. R. Using probiotics and prebiotics to improve gut health. Drug Discov. Today 8, 692–700 (2003).
King, T. S., Elia, M. & Hunter, J. O. Abnormal colonic fermentation in irritable bowel syndrome. Lancet 352, 1187–1189 (1998).
Braden, B., Lembcke, B., Kuker, W. & Caspary, W. F. C-13-breath tests: Current state of the art and future directions. Dig. Liver Dis. 39, 795–805 (2007).
Major, G. et al. Colon hypersensitivity to distension, rather than excessive gas production, produces carbohydrate-related symptoms in individuals with irritable bowel syndrome. Gastroenterol 152, 124–133 (2017).
Rumessen, J. J. & Gudmandhoyer, E. Functional bowel-disease - malabsorption and abdominal distress after ingestion of fructose, sorbitol, and fructose-sorbitol mixtures. Gastroenterol 95, 694–700 (1988).
Shin, W. Medical applications of breath hydrogen measurements. Anal. Bioanal. Chem. 406, 3931–3939 (2014).
David, L. A. et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 505, 559–563 (2014).
Gibson, G. R. & Roberfroid, M. B. Dietary modulation of the human colonic microbiota - introducing the concept of prebiotics. J. Nutr. 125, 1401–1412 (1995).
Roberfroid, M. et al. Prebiotic effects: metabolic and health benefits. Br. J. Nutr. 104, S1–S63 (2010).
Suarez, F., Furne, J., Springfield, J. & Levitt, M. Insights into human colonic physiology obtained from the study of flatus composition. Am. J. Physiol. Gastrointest. Liver Physiol. 272, G1028–G1033 (1997).
Rotbart, A. et al. Designing an in-vitro gas profiling system for human faecal samples. Sens. Actuat. B Chem. 238, 754–764 (2017).
Tomlin, J., Lowis, C. & Read, N. W. Investigation of normal flatus production in healthy-volunteers. Gut 32, 665–669 (1991).
Kalantar-Zadeh, K. et al. Intestinal gas capsules: a proof-of-concept demonstration. Gastroenterol 150, 37–39 (2016).
Ou, J. Z. et al. Potential of in vivo real-time gastric gas profiling: a pilot evaluation of heat-stress and modulating dietary cinnamon effect in an animal model. Sci. Rep. 6, 33387 (2016).
Jensen, B. B. & Jorgensen, H. Effect of dietary fiber on microbial activity and microbial gas-production in various regions of the gastrointestinal-tract of pigs. Appl. Environ. Microbiol. 60, 1897–1904 (1994).
Walsh, C. J., Guinane, C. M., O’Toole, P. W. & Cotter, P. D. Beneficial modulation of the gut microbiota. FEBS Lett. 588, 4120–4130 (2014).
Barsan, N. & Weimar, U. Conduction model of metal oxide gas sensors. J. Electroceram. 7, 143–167 (2001).
Ou, J. Z. et al. Physisorption-Based Charge Transfer in Two-Dimensional SnS2 for Selective and Reversible NO2 Gas Sensing. ACS Nano 9, 10313–10323 (2015).
Kobayashi, Y. et al. Sonographic detection of a patency capsule prior to capsule endoscopy: case report. J. Clin. Ultrasound 42, 554–556 (2014).
Steggerda, F. R. Gastrointestinal gas following food consumption. Ann. NY Acad. Sci. 150, 57–66 (1968).
Lemarchand, L., Wilkens, L. R., Harwood, P. & Cooney, R. V. Use of breath hydrogen and methane as markers of colonic fermentation in epidemiologic studies - circadian patterns of excretion. Environ. Health Perspect. 98, 199–202 (1992).
Berean, K. J. et al. 2D MoS2 PDMS Nanocomposites for NO2 Separation. Small 11, 5035–5040 (2015).
Berean, K. J. et al. Enhanced gas permeation through graphene nanocomposites. J. Phys. Chem. C. 119, 13700–13712 (2015).
Nour, M. et al. Silver nanoparticle/PDMS nanocomposite catalytic membranes for H2S gas removal. J. Membr. Sci. 470, 346–355 (2014).
Nadeau, P. et al. Prolonged energy harvesting for ingestible devices. Nat. Biomed. Eng. 1, 0022 (2017).
Fernandes, J., Su, W., Rahat-Rozenbloom, S., Wolever, T. M. S. & Comelli, E. M. Adiposity, gut microbiota and faecal short chain fatty acids are linked in adult humans. Nutr. Diabetes 4, e121 (2014).
Minekus, M. et al. A standardised static in vitro digestion method suitable for food - an international consensus. Food Funct. 5, 1113–1124 (2014).
Guarner, F. & Malagelada, J. R. Gut flora in health and disease. Lancet 361, 512–519 (2003).
Henao-Mejia, J. et al. Inflammasome-mediated dysbiosis regulates progression of NAFLD and obesity. Nature 482, 179–185 (2012).
Bhattacharyya, A., Chattopadhyay, R., Mitra, S. & Crowe, S. E. Oxidative stress: an essential factor in the pathogenesis of gastrointestinal mucosal diseases. Physiol. Rev. 94, 329–354 (2014).
Aminov, R. I. et al. Molecular diversity, cultivation, and improved detection by fluorescent in situ hybridization of a dominant group of human gut bacteria related to Roseburia spp. or Eubacterium rectale. Appl. Environ. Microbiol. 72, 6371–6376 (2006).
Wilfart, A., Montagne, L., Simmins, H., Noblet, J. & van Milgen, J. Digesta transit in different segments of the gastrointestinal tract of pigs as affected by insoluble fibre supplied by wheat bran. Br. J. Nutr. 98, 54–62 (2007).
Roberfroid, M. Dietry fiber, inulin, and oligofructose - a review comparing their physiological effects. Crit. Rev. Food Sci. Nutr. 33, 103–148 (1993).
vonSchonfeld, J., Evans, D. F. & Wingate, D. L. Effect of viscous fiber (Guar) on postprandial motor activity in human small bowel. Dig. Dis. Sci. 42, 1613–1617 (1997).
Simren, M. & Stotzer, P. O. Use and abuse of hydrogen breath tests. Gut 55, 297–303 (2006).
Kuo, B. et al. Generalized transit delay on wireless motility capsule testing in patients with clinical suspicion of gastroparesis, small intestinal dysmotility, or slow transit constipation. Dig. Dis. Sci. 56, 2928–2938 (2011).
Jenkins, D. J. A., Kendall, C. W. C., Axelsen, M., Augustin, L. S. A. & Vuksan, V. Viscous and nonviscous fibres, nonabsorbable and low glycaemic index carbohydrates, blood lipids and coronary heart disease. Curr. Opin. Lipidol. 11, 49–56 (2000).
Bermak, A., Belhouari, S. B., Shi, M. & Martinez, D. in Encyclopedia of Sensors Vol. 10 (eds C. A. Grimes, E. C. Dickey, & M. V. Pishko) 1-17 (American Scientifc Publishers, 2006).
Kermit, M. & Tomic, O. Independent component analysis applied on gas sensor array measurement data. IEEE Sens. J. 3, 218–228 (2003).
Tran, K., Brun, R. & Kuo, B. Evaluation of regional and whole gut motility using the wireless motility capsule: relevance in clinical practice. Ther. Adv. Gastroenterol. 5, 249–260 (2012).
Höög, C. M. et al. Capsule retentions and incomplete capsule endoscopy examinations: An analysis of 2300 examinations. Gastroenterol. Res. Pract. 2012, 518718 (2012).
High Fiber Diet (University of Michigan, Michigan Medicine, USA, 2011). http://www.med.umich.edu/1libr/MBCP/HighFiberDiet.pdf.
Acknowledgements
The authors acknowledge the Australian Centre for Ecogenomics for sequencing and bioinformatics advice, Queensland, Australia. The authors also thank the National Health and Medical Research Council (NHMRC), Australia and Department of Business, Australia for the financial support of the project via a Development grant and an Acceleration Commercialisation (AC) grant, respectively.
Author information
Authors and Affiliations
Contributions
K.K.-Z. and P.R.G. initiated the concept. K.K.-Z., J.Z.O. and K.J.B. designed the trial. N.H. designed and fabricated the capsules with some help from A.F.C. and K.X. K.K.-Z., K.J.B., C.K.Y., D.G., J.Z.O., K.M.T., R.E.B., P.R.G. and J.G.M. organized the human trials on volunteers. D.G. and R.B. carried out the metabolomics analysis. K.K.-Z., N.P., J.Z.O. and J.L.C. conducted the in vitro tests. All authors participated in analysis of data and authorship of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figures 1–8, Supplementary Tables 1–4, Supplementary Notes, Supplementary Methods, and Supplementary References.
Rights and permissions
About this article
Cite this article
Kalantar-Zadeh, K., Berean, K.J., Ha, N. et al. A human pilot trial of ingestible electronic capsules capable of sensing different gases in the gut. Nat Electron 1, 79–87 (2018). https://doi.org/10.1038/s41928-017-0004-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41928-017-0004-x
This article is cited by
-
End-to-end design of ingestible electronics
Nature Electronics (2024)
-
A stress test for bioelectronics
Nature Electronics (2024)
-
Sub-1.4 cm3 capsule for detecting labile inflammatory biomarkers in situ
Nature (2023)
-
A swallowable X-ray dosimeter for the real-time monitoring of radiotherapy
Nature Biomedical Engineering (2023)
-
Colloidal robotics
Nature Materials (2023)