Abstract
Mounting data hints that the gut microbiota's role may be pivotal in understanding the emergence of psoriasis. However, discerning a direct causal link is yet elusive. In this exploration, we adopted a Mendelian randomization (MR) strategy to probe the prospective causal interplay between the gut's microbial landscape and the predisposition to psoriasis. Genetic markers acting as instrumental variables for gut microbiota were extrapolated from a genome-wide association study (GWAS) encompassing 18,340 individuals. A separate GWAS yielded summary data for psoriasis, which covered 337,159 patients and 433,201 control subjects. The primary analysis hinged on inverse variance weighting (IVW). Additional methods like the weighted median approach and MR-Egger regression were employed to validate the integrity of our findings. Intriguing correlations emerged between psoriasis risk and eight specific bacterial traits. To illustrate: Mollicutes presented an odds ratio (OR) of 1.003 with a 95% confidence interval (CI) spanning 1.001–1.005 (p = 0.016), while the family. Victivallaceae revealed an OR of 0.998 with CI values between 0.997 and 0.999 (p = 0.023). Eubacterium (coprostanoligenes group) revealed an OR of 0.997 with CI values between 0.994 and 0.999 (p = 0.027). Eubacterium (fissicatena group) revealed an OR of 0.997 with CI values between 0.996 and 0.999 (p = 0.005). Holdemania revealed an OR of 1.001 with CI values 1–1.003 (p = 0.034). Lachnospiraceae (NK4A136 group) revealed an OR of 0.997 with CI values between 0.995 and 0.999 (p = 0.046). Lactococcus revealed an OR of 0.998 with CI values between 0.996 and 0.999 (p = 0.008). Tenericutes revealed an OR of 1.003 with CI values between 1.001 and 1.006 (p = 0.016). Sensitivity analysis for these bacterial features yielded congruent outcomes, reinforcing statistically significant ties between the eight bacterial entities and psoriasis. This comprehensive probe underscores emerging evidence pointing towards a plausible causal nexus between diverse gut microbiota and the onset of psoriasis. It beckons further research to unravel the intricacies of how the gut's microbial constituents might sway psoriasis's pathogenesis.
Similar content being viewed by others
Introduction
Psoriasis is a chronic immune-mediated inflammatory skin disease triggered by various environmental and endogenous factors in genetically susceptible individuals1. Clinically, it typically presents as well-defined scaly erythematous plaques and, in rare cases, can lead to life-threatening generalized erythroderma2. The histologic features of psoriasis include hyperproliferation and hyperkeratosis of epidermal keratinocytes, dilated microvessels in the superficial dermis and an associated inflammatory response. With a better understanding of the pathophysiologic mechanisms involved, various treatments have been investigated. Previously, psoriasis was considered a proliferative skin disease, and treatment efforts were primarily focused on antiproliferative approaches3. However, more recent studies revealing elevated levels of interleukin-17 (IL-17) in psoriasis lesions have shifted the treatment focus towards helper T-cell 17 (Th17) cells4.
In recent years, research on the gut flora has intensified, providing increasing evidence of a link between the gut-skin axis. Dysbiosis in the gut can affect systemic immune function, leading to dysregulation of homeostasis and impaired skin function, which, in turn, may contribute to developing skin disorders. Growing evidence now suggests that in patients with psoriasis, the gut flora plays a role in establishing intestinal immunity5. On the other hand, severe intestinal malnutrition is observed in psoriasis patients, resulting in low diversity and altered relative abundance of specific intestinal flora6.
Although gut flora has been linked to psoriasis, the exact causal relationship remains uncertain. One statistical method that can help infer potential causality from observed associations is Mendelian randomization (MR) analysis7. MR capitalizes on genetic variants correlated with the target exposure, using these as instrumental variables to discern associations between the exposure proxy and the outcome8. The adoption of MR techniques to explore possible causal links between gut microbiota and disease susceptibility genes has recently surged9,10,11. This trend underscores the pressing imperative to scrutinize the plausible causal connection between gut microbial composition and psoriasis susceptibility.
In this study, we conducted a two-sample MR analysis based on pooled data from Genome-Wide Association Studies (GWAS). The purpose was to investigate the potential causal relationship between gut microbiota and psoriasis and identifying specific taxa of pathogenic bacteria involved in the condition.
Materials and methods
Outcome data sources
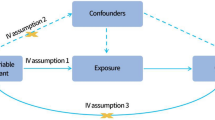
Figure 1 offers a graphic depiction of this study's overarching design. Succinctly, we derived genetic summary metrics for psoriasis from a GWAS encompassing 337,159 cases and 433,201 controls, all of the European lineage. This GWAS amalgamates data sourced from both the UK Biobank.
To be included in the study, all patients with psoriasis were required to satisfy two criteria: item 5 and any of the first four items listed below: (1) Ordinary psoriasis: Characterized by erythematous, scaly lesions accompanied by punctate haemorrhages and the thin film phenomenon. (2) Psoriasis nail damage: Approximately 50% of psoriasis patients exhibit nail abnormalities, such as furrowed indentations, desquamation, and blotchy or oily changes. (3) Psoriatic arthritis: Approximately 30% of psoriatic arthritis patients also have psoriatic arthritis, which presents as joint pain, swelling, and movement limitations. (4) Hospital events with ICD-10 diagnostic codes: Statistical data on hospital events related to inpatient or day patient hospitalizations with a clinical diagnosis of psoriasis were considered using ICD-10 codes. (5) Histopathologic biopsy confirmation: The diagnosis of psoriasis was confirmed by histopathologic biopsy12.
This study utilized human gut microbiome summary statistics from the most recent GWAS meta-analysis, which integrated data from 18,340 participants spanning 24 distinct cohorts13. A more thorough delineation of this study can be found in another publication. To encapsulate, the research synchronized 16S rRNA gene sequencing patterns with genotyping data drawn from multiple international cohorts, including nations such as the United States, Canada, Germany, and others in Northern Europe. Association assessments were conducted, controlling for age, gender, technical covariates, and genetic principal components. Since our study relied on publicly accessible aggregated data, there was no mandate for further ethical clearance or participant consent. The particulars of the datasets employed in our MR exploration are itemized in Table 1.
Selection of instrumental variables
Firstly, we removed 15 bacterial traits that did not have specific names, resulting in 196 remaining bacterial characteristics, which included nine species, 16 orders, 20 families, 32 genera, and 119 genera. Next, we selected instrumental variables (IVs) with a p < 1.0 × 10–5 significance level. To obtain site-independent IVs, we utilized the "TwoSampleMR" package and set the linkage disequilibrium (LD) threshold to R2 < 0.001. The clustering distance was also set to 10,000 kb, using 1000 genomic EUR data. We clustered the 196 bacterial traits, and for each cluster, we retained the SNP (single nucleotide polymorphism) with the lowest p-value among the associated traits. In total, we identified 2,699 independent SNPs that were associated with the 196 bacterial characteristics 12.
Statistical analysis
This study assessed the potential causal relationship between gut microbiota and psoriasis using the fixed/random effects inverse variance weighted (IVW) method. The IVW method was chosen as the primary analysis due to its accurate effect estimation and its widespread utilization as the primary analysis in almost all MR studies14,15,16.
Incorporating many variants into MR analysis not only augments statistical potency but also risks ushering in pleiotropic genetic variants that could render instrumental variables questionable17. We implemented weighted median and MR-Egger regression strategies to navigate and offset pleiotropy. The MR-Egger regression stands resilient against dubious instruments. It recognizes unbalanced pleiotropy by instating a parameter designed to adjust for bias, capitalizing on summary data effect estimates drawn from multiple disparate variants18. The regression's gradient encapsulates the causal effect estimate, whereas the y-intercept offers insight into the overarching horizontal pleiotropic effect across the variants.
Meanwhile, the weighted median estimator yields a reliable causal effect projection, even when as much as half the input originates from potentially questionable genetic variants. Notably, this weighted median estimator upholds enhanced precision in its derivation. We pinned statistical significance at a P-value threshold below 0.05 across all tests in our investigation.
Correlations between the intricacies of human gut microbiota and the predisposition to psoriasis were quantified using odds ratios (ORs) bolstered by their corresponding 95% confidence intervals (CIs). The rigorous Bonferroni method was invoked to rectify multiple comparisons spanning diverse taxonomic echelons. Varied significance cut-offs were calibrated to the bacterial trait count within each gut microbiota tier for phylum, class, order, family, and genus. When p-values straddled between the demarcated significance bar and 0.05 signalled the potential for a causal association. All MR computations were orchestrated using R (version 3.6.3). To facilitate these evaluations, we deployed the "Mendelian Randomization" and "TwoSampleMR" packages, both of which are openly accessible.
Heterogeneity and sensitivity test
To evaluate the heterogeneity among SNPs, we utilized Cochran's Q-statistics and I2 statistics19,20.
Results
The main results of the 196 bacterial traits with the risk of psoriasis
The f-statistics for the 196 bacterial traits were all greater than 10, indicating a low likelihood of weak instrumental bias. notably, using the IVW method (Fig. 2), we observed suggestive evidence of an association between 8 bacterial characteristics and psoriasis risk. Characteristics of the genetic variants associated with eight bacterial that have been identified to be related to the risk of psoriasis can be found in Table S1. The IVs employed for these eight bacterial characteristics can be found in Table 2. In our study, we made several significant findings regarding the association between specific bacterial characteristics and the risk of psoriasis: using the IVW method, genetic predictions highlighted that mollicutes positively correlated with the risk of psoriasis (OR: 1.003; 95% CI: 1.001–1.005; p = 0.016) (See Fig. 3). The weighted median approach corroborated these findings (OR: 1.004; 95% CI 1.0007–1.007; p = 0.016). The MR-Egger regression analysis showed no inclination towards a directional pleiotropic influence (intercept p-value = 0.016) (Refer to Fig. 4), while the funnel plot demonstrated symmetry (Fig. 5).
Forest plot of the associations between genetically determined 8 bacterial traits with the risk of psoriasis. The results is displayed in the form of odds ratio (OR) accompanied by their 95% confidence interval (CI). An odds ratio below 1 indicates a reduction in the risk of urolithiasis, while one above 1 indicates an increased likelihood of contributing to the development of the disease. SNP single nucleotide polymorphism.
Scatter plots for the causal association between gut microbiota and psoriasis. The scatter plot shows that Mollicutes (A), Holdemania (E), Tenericutes (H) are positively correlated with the risk of psoriasis, while genus Victiallaceae (B), Eubacterium (coprostanoligenes group) (C), Eubacterium (fissicatena group) (D), Lachnospiraceae (NK4A136 group) (F) and Lactococcus (G) are negatively correlated with the risk of psoriasis. SNP single nucleotide polymorphism, MR Mendelian randomization.
Leave-one-out plots show that the causal relationship between Mollicutes (A), genus Victiallaceae (B), Eubacterium (coprostanoligenes group) (C), Eubacterium (fissicatena group) (D), Holdemania (E), Lachnospiraceae (NK4A136 group) (F), Lactococcus (G) and Tenericutes (H) was not driven by any single SNP. SNP single nucleotide polymorphism, MR Mendelian randomization.
Similarly, Holdemania positively correlated with psoriasis susceptibility when assessed via the IVW approach (OR: 1.001; 95% CI 1.000–1.003; p = 0.034) (Refer to Fig. 3). Consistent outcomes were gleaned from the weighted median method (OR: 1.001; 95% CI 0.998–1.003; p = 0.033). The MR-Egger regression further validated the absence of horizontal pleiotropy (intercept p-value = 0.033) (See Fig. 4), with the funnel plot maintaining symmetry (Fig. 5).
Tenericutes, as discerned from the IVW technique, showcased a positive tilt with the psoriasis risk profile (OR: 1.003; 95% CI 1.001–1.006; p = 0.016) (Refer to Fig. 3). Sensitivity analyses via the weighted median method mirrored these observations (OR: 1.00; 95% CI 0.98–1.007; p = 0.007). Moreover, MR-Egger regression presented scant evidence of a directional pleiotropic thrust (intercept p-value = 0.012) (See Fig. 4), with funnel plots maintaining their unbiased stance (Fig. 5).
Contrastingly, negative correlations between five bacterial attributes and psoriasis susceptibility emerged from the IVW method, notably for the following taxa: Victivallaceae, Eubacterium (coprostanoligenes group), Eubacterium (fissicatena group), another unidentified Genus, and Lactococcus (details provided in the original text) (See Fig. 3). Nonetheless, it's pertinent to highlight that the weighted median approach offered a more ambivalent stance on these perceived causal associations.
Discussion
Psoriasis, also known as psoriasis vulgaris, is a chronic autoimmune disease characterized by red patches of skin covered with silvery-white scales. In 2019, there were 4,622,594 cases of psoriasis globally (95% uncertainty interval or UI 4,458,904–4,780,771). The incidence rate of psoriasis in 2019 was 57.8 cases per 100,000 individuals (95% UI 55.8–59.7). This represents a decrease of 20.0% (95% UI − 20.2 to − 19.8) compared to 1990. Stratifying by gender, the incidence rates for males [57.8 (95% UI 55.8–59.8) per 100,000 individuals] and females [57.8 (95% UI 55.8–59.7) per 100,000 individuals] were similar. Compared to 1990, the incidence rates decreased by 19.5% (95% UI − 19.8 to − 19.2) for males and 20.4% (95% UI − 20.7 to − 20.2) for females. It was observed that the incidence rate of psoriasis varied geographically, with the highest rates reported in high-income countries and regions [112.6 (95% UI 108.9–116.1)], followed by middle to high Sociodemographic Index (SDI) countries [69.4 (95% UI 67.1–71.9)], while the lowest rates were reported in low SDI countries [38.1 (95% UI 36.8–39.5)]. Similar trends were found regarding prevalence and disability rates21.
While primarily affecting the skin, psoriasis can also lead to various other health issues, often called comorbidities. Among the comorbidities associated with psoriasis are arthritis, cardiovascular diseases, metabolic syndrome, mental health issues, infections, kidney diseases, osteoporosis, and other autoimmune diseases such as rheumatoid arthritis or systemic lupus erythematosus22. Research indicates that psoriasis (PsO) patients are more likely to be diagnosed with 2–4 comorbidities (28.8% vs 23.8%) and > 5 comorbidities (19.6% vs 12.9%). The specific comorbidity profile of PsO patients reflects several core pathological processes, including autoimmune and systemic inflammatory diseases such as hidradenitis suppurativa (OR 3.55, 95% CI 1.88–7.28) or rheumatoid arthritis (OR 3.01, 95% CI 1.96–4.77), inflammatory bowel disease such as Crohn's disease (OR 2.99, 95% CI 2.20–4.13), pulmonary diseases such as chronic obstructive pulmonary disease (OR 1.81, 95% CI 1.61–2.04), hepatic diseases such as cirrhosis (OR 2.00, 95% CI 1.36–3.00), endocrine disorders such as thyroid dysfunction (OR 1.82, 95% CI 1.30–2.59), psychiatric disorders such as depression (OR 1.72, 95% CI 1.57–1.87), and cardiovascular diseases such as hypertension (OR 1.47, 95% CI 1.41–1.53)23. Dysbiosis of the gut microbiota can also result in several comorbidities, some of which may be related to the pathogenesis of psoriasis, thereby triggering or exacerbating the development of psoriasis. Firstly, dysbiosis of the gut microbiota may lead to immune system abnormalities. The gut microbiota is closely linked to the immune system, and dysregulated microbiota may cause either overactivation or diminished function of the immune system, increasing the risk of autoimmune diseases.
Psoriasis is an immune-mediated disease, and its pathogenesis is closely associated with immune system abnormalities, particularly abnormal activation of T cells24. Therefore, dysbiosis of the gut microbiota may increase the risk of psoriasis occurrence and development by influencing the immune system. Secondly, dysbiosis of the gut microbiota may result in increased inflammatory responses. Some studies indicate that dysbiosis of the gut microbiota may lead to damage and inflammation of the intestinal mucosa, releasing inflammatory mediators such as TNF-α, IL-6, etc. The excessive release of these inflammatory mediators may exacerbate the inflammatory response of psoriasis, promoting abnormal proliferation of skin cells and inflammatory reactions25.
Additionally, dysbiosis of the gut microbiota may also affect nutrient absorption and metabolism. The onset of psoriasis is associated with nutritional deficiencies or metabolic abnormalities. Dysregulated microbiota may lead to inadequate absorption of nutrients or the production of metabolites such as short-chain fatty acids, thereby affecting skin health and the development of psoriasis26.
Diet plays a crucial role in regulating patients' onset and progression of psoriasis. Although the etiology of psoriasis is complex, some studies suggest that adopting appropriate dietary habits can help alleviate symptoms, slow disease progression, and improve patients' quality of life. Certain foods contain anti-inflammatory components, such as fish, which are rich in Omega-3 fatty acids, nuts, seeds, fruits, and vegetables, as well as vitamin C, vitamin E, and other antioxidants26. These foods may help reduce inflammation and alleviate symptoms in psoriasis patients. Obesity is associated with the onset and severity of psoriasis. Therefore, maintaining a healthy weight and adopting proper dietary habits can reduce the release of inflammatory factors in the body, thus helping to alleviate psoriasis symptoms. Some psoriasis patients may be allergic or sensitive to certain foods, such as spicy foods, alcohol, caffeine, and dairy products. Avoiding or limiting these foods in personalized cases may help alleviate symptoms. Antioxidants help eliminate free radicals and reduce inflammatory responses. Increasing the intake of foods rich in antioxidants, such as fruits, vegetables, and tea, may help alleviate symptoms in psoriasis patients. Fluctuations in blood sugar levels may affect the immune system and inflammatory responses. Therefore, choosing foods with a low glycemic index (GI), such as whole grains, legumes, and vegetables, can help maintain stable blood sugar levels27. Deficiency in specific vitamins and minerals, such as vitamin D, vitamin A, zinc, and selenium, is associated with the occurrence and development of psoriasis. Ensuring an adequate intake of these nutrients may help alleviate symptoms28.
Systemic inflammation plays a significant role in regulating the onset and progression of psoriasis in patients. Psoriasis is a chronic inflammatory skin disease, and its pathogenesis is closely associated with immune system abnormalities, inflammatory responses, and abnormal proliferation of skin cells. Systemic inflammation can affect the regulation of the immune system, leading to excessive or aberrant activation of immune cells, thereby exacerbating the inflammatory response in psoriasis29. Inflammatory mediators such as tumor necrosis factor-alpha (TNF-α) and interleukin-17 (IL-17), among others, may play a crucial role in this process. Systemic inflammation can result in increased release of inflammatory mediators in the body, such as TNF-α and IL-17 mentioned above, which directly participate in the pathogenesis of psoriasis. They promote abnormal proliferation of keratinocytes and skin inflammation, thereby worsening the condition of psoriasis. Systemic inflammation may affect inflammatory signaling pathways in psoriasis patients, such as the nuclear factor-kappa B (NF-κB) signaling pathway, the Toll-like receptor (TLR) signaling pathway, etc. The signalling pathways abnormal activation or inhibition is closely associated with the onset and development of psoriasis. Systemic inflammation may affect the function of various immune cells, such as T cells, dendritic cells, etc., thereby regulating the activity of the immune system and affecting the immune-inflammatory response in psoriasis30.
Interleukin-17 (IL-17) and Interleukin-23 (IL-23) are crucial immunoregulatory factors in modulating the immune system's functions. They play pivotal roles in numerous immune responses, including combating infections and autoimmune diseases. IL-17, produced by T cells, particularly a subset known as Th17 cells, is capable of inducing inflammatory responses and plays a significant role in developing autoimmune diseases. IL-23, on the other hand, acts as a stimulant for the differentiation and proliferation of Th17 cells while regulating other different types of immune cells. The antagonistic effect of IL-17 and IL-23 pathways is crucial, particularly in infections such as intestinal nematode infection. Intestinal nematode infections trigger the activation of the host immune system, including the IL-17 and IL-23 pathways. IL-17 may promote inflammatory responses leading to tissue damage, but may also play a role in parasite clearance.
Conversely, IL-23 may play a crucial role in regulating inflammatory responses, but overactivation of the IL-23 pathway could lead to the development of inflammatory diseases. Hence, employing precision medicine approaches to avoid drug ineffectiveness is crucial. Precision medicine entails tailoring treatment plans based on individual characteristics such as genomic profiles, phenotypes, environment, and lifestyle. When dealing with the antagonistic effects of IL-17 and IL-23 pathways, precision medicine can aid in determining the patient's immune status, genetic background, and potential drug responses, thereby selecting the most appropriate treatment strategies31. This may involve selecting specific drug targets, adjusting dosages, or using combination therapies to minimize inflammatory responses, control infections, and reduce drug side effects and resistance risks. In conclusion, IL-17 and IL-23 pathways play critical roles in diseases such as intestinal nematode infection, and understanding and managing the interactions of these pathways are crucial for treatment success.
Both drug- and food-induced photosensitivity can potentially impact the treatment and condition of psoriasis. Firstly, drug-induced photosensitivity refers to adverse reactions that certain medications may cause when the skin is exposed to sunlight. Some medicines used to treat psoriasis patients may increase their sensitivity to sunlight, leading to photosensitivity reactions. For example, the use of photosensitizing drugs such as psoralen plus ultraviolet A (PUVA) therapy or certain oral medications (such as methoxsalen and acitretin) may increase patients' sensitivity to ultraviolet light, resulting in skin burns or other adverse reactions32. Secondly, food-induced photosensitivity refers to interactions between certain foods and sunlight that trigger skin reactions. Some foods' chemical components may increase the skin's sensitivity to ultraviolet light, leading to photosensitivity reactions. Some foods that may induce food-induced photosensitivity in psoriasis patients include celery, lemon, cilantro, fennel, and bergamot oil. Ingredients in these foods may interact with sunlight, causing skin allergic reactions or exacerbating the condition33.
Therefore, when treating psoriasis, doctors typically advise patients to avoid using photosensitizing drugs, especially during periods of intense sunlight, to reduce the risk of photosensitivity reactions. Patients should also be cautious about consuming foods that may induce food-induced photosensitivity, particularly during sun exposure. Considering both drug-induced photosensitivity and food-induced photosensitivity can help psoriasis patients manage their condition more effectively and reduce the occurrence of adverse reactions. Precision medicine approaches can optimize treatment outcomes and prevent drug ineffectiveness. Therefore, research suggests that the widespread use of artificial neural networks may help predict the efficacy of dermatological medications, providing valuable theoretical and clinical insights34.
In this study, we identified eight bacterial taxa with potential associations to the risk of psoriasis. These taxa include Mollicutes, Victivallaceae, Eubacterium (coprostanoligenes group), Eubacterium (fissicatena group), Holdemania, Lachnospiraceae (NK4A136 group), Lactococcus, Tenericutes. Our susceptibility analyses, conducted using different MR methods and restricted IV sets, suggest that these bacterial taxa may be linked to psoriasis risk. Further investigations and validation studies are warranted to confirm and understand the biological implications of these potential associations.
The human intestinal flora constitutes a complex micro-ecosystem comprising approximately 1000 microbial species and 1014 microorganisms. The number of microorganisms gradually increases from the stomach to the end of the colon35. The average human intestinal flora primarily consists of two main bacterial species: the thick-walled phylum and the anaplasma phylum36. Acknowledging that the human gut flora exhibits considerable variation among individuals is vital. Under homeostatic conditions, these bacteria play essential roles in digestion, metabolism, immune defence, and tolerance. A healthy and balanced intestinal flora is essential for maintaining these functions37,38,39.
Given the importance of gut flora, alterations in its composition have been associated with the pathogenesis of numerous acute and chronic diseases, such as diabetes, obesity, inflammatory bowel disease (IBD), Crohn's disease, autism, Alzheimer's disease, Parkinson's disease, anxiety, and depression40,41,42,43. For instance, studies have shown that 7–11% of patients diagnosed with IBD also have psoriasis, primarily linked to gastrointestinal inflammation44. Both diseases share certain common genetic and environmental factors and immune pathways. Notably, Th17 cells and their cytokines are known to play a significant role in developing psoriasis and are involved in the pathophysiology of IBD45. In the pathogenesis of both obesity and psoriasis, inflammation and adipokines play crucial roles, and a mutual promotion exists between the two conditions46. Numerous studies have demonstrated that obesity serves as an independent risk factor for psoriasis. It leads to an imbalance in the expression of pro- and anti-inflammatory adipokines in adipose tissue. It subsequently encourages the release of inflammatory cells and factors associated with psoriasis, thus contributing to cutaneous inflammation in psoriasis.
In addition, research has shown that a high-fat diet (HFD) lasting just one week can bring about significant changes in the fecal metabolome and gut microbiome of rats, and this alteration can persist for two months47. Analyses of the gut microbiome in HFD-induced obese mice have revealed the vigorous growth of a single evolutionary clade in the Mollicutes of Firmicutes (HFD). This study has highlighted a potential causal relationship between Mollicutes and psoriasis, underscoring the significance of Mollicutes in developing psoriasis47.
In a study conducted in China with 350 patients with psoriasis, it was observed that high-frequency alcohol consumption, including various types of alcoholic beverages such as white wine, red wine, and beer, as well as single large amounts of alcohol consumption, are associated with an increased risk of developing psoriasis. Ethanol, found in alcoholic drinks, plays a dual role in promoting psoriasis.
On one hand, ethanol can induce the release of pro-inflammatory cytokines from various cell types, either directly or through intermediate pathways. This can result in sustained systemic inflammation and facilitate the proliferation of lymphocytes48. This mechanism elucidates the potential contributory role of ethanol in inflammatory processes and related conditions. On the other hand, ethanol also influences the growth of intestinal flora. In populations that consume excessive amounts of ethanol, there is a higher relative abundance of Holdemania spp. in the gut microbiome. This, in turn, contributes to and exacerbates psoriasis49. These findings are consistent with the results of our study, which suggests a positive association between high levels of Holdemania and the risk of psoriasis.
Hyperglycemia has been found to impact intestinal epithelial cells, disrupting the intestinal vascular barrier and subsequent development of intestinal inflammation. In type 2 diabetic mice, this condition leads to specific changes in the gut microbiome. Notably, there is an increase in the abundance of Micrococcus warty and Tenericutes (soft-walled bacteria). In contrast, Mycobacterium avium and Sclerotium sclerotiorum (Sclerotium) bacteria experience a decrease in their levels compared to healthy mice50. However, it is essential to note that despite the positive association between Tenericutes and psoriasis risk observed in this study, there is currently no clear research evidence directly linking Tenericutes to psoriasis. Therefore, further investigations are required to explore the underlying biological mechanisms responsible for this potential association between Tenericutes and psoriasis.
The Eubacterium (coprostanoligenes group) and Eubacterium (fissicatena group) are intestinal microorganisms, consisting of several species of Eubacterium36. Eubacterium is an essential bacterium found in the colon of healthy individuals and is considered one of the core genera of the human gut51. It widely colonizes the intestinal tract, oral cavity, and other parts of the population, playing a vital role in nutrient metabolism and maintaining intestinal homeostasis52. Certain strains within the Eubacterium (coprostanoligenes group) possess steroid-metabolizing abilities, participating in cholesterol metabolism and converting cholesterol into the cholesterol degradation product, coprostanol53. This metabolic process is crucial for maintaining cholesterol homeostasis and normal cholesterol metabolism.
Members of the Eubacterium family are pivotal due to their capability to produce short-chain fatty acids, notably butyric acid54. These short-chain fatty acids have multifaceted roles in human health. For instance, they furnish specific nutrients and energy to the intestinal lining, fortify the intestinal mucosal barrier, temper inflammation, and augment gastrointestinal movement55. Various Eubacterium species generate butyrate, instrumental in energy balance, regulating colonic movements, modulating immune responses, and curtailing intestinal inflammation56. Butyrate, a short-chain fatty acid, is a fermentation product of Eubacterium spp. It can upregulate mucin twogene expression by directly acting on cuprates and may induce mucin two secretions through the intermediate product, prostaglandin57. Mucin 2 is the primary active component of the colorectal mucosal barrier, and apart from its non-specific barrier function, its polysaccharide group interferes with the expression of DC inflammatory factors, enhances immune tolerance, and improves intestinal stability by binding to a complex receptor on dendritic cells56.
Studies have shown that butyrate reduces Th17 cell production by promoting regulatory T cell (Treg) differentiation, thereby attenuating intestinal inflammatory responses58. Butyrate provides energy to colonocytes and reduces oxidative stress, conferring immune tolerance by triggering regulatory T cells to exert anti-inflammatory effects beyond the gastrointestinal system59. Several studies have indicated significant differences in the gut microflora of psoriasis patients compared to the healthy population. However, changes in specific bacterial species depend on multiple studies, which have been cross-sectional and unable to determine whether changes in the microbiota are a cause or a consequence. This study shows a potential causal relationship between the Eubacterium (coprostanoligenes group), Eubacterium (fissicatena group), and psoriasis risk.
Victivallaceae and Lachnospiraceae NK4A136 group are common gut microorganisms found in the intestines of humans and other animals60. They play a vital role in food digestion and absorption by breaking down complex polysaccharides, including cellulose and other indigestible plant fibres, which release beneficial nutrients like short-chain fatty acids61. These short-chain fatty acids provide energy to intestinal cells and promote a healthy intestinal mucosa. Additionally, they are crucial for maintaining the balance and diversity of the intestinal flora. Working with other probiotics, Victivallaceae and Lachnospiraceae NK4A136 group create a competitive advantage over harmful bacteria, effectively inhibiting their growth62. This balanced intestinal flora helps prevent the overgrowth of harmful bacteria and contributes to the normal functioning of the intestinal tract63.
Furthermore, the flora, as mentioned above plays a crucial role in regulating the immune system64. The interaction between the intestinal microbiota and the immune system is complex, and research has indicated that Victivallaceae and Lachnospiraceae (NK4A136 group) actively promote normal immune function. Moreover, members of the Lachnospiraceae NK4A136 group can synthesize certain nutrients that benefit the human body65. For instance, they can synthesize vitamin K, specific B vitamins such as folic acid and vitamin B12, which are essential for the normal physiological functioning of the body66. Additionally, a study demonstrated a negative correlation between Victivallaceae and Lachnospiraceae NK4A136 group and the risk of psoriasis.
Lactococcus is a common genus of lactic acid bacteria widely found in various environments, including dairy products and the intestinal tract67. This excellent lactose-fermenting bacteria breaks down lactose to produce lactic acid, which is particularly beneficial for individuals with lactose intolerance, as they lack the enzyme lactase required to digest lactose. By breaking down lactose, Lactococcus helps reduce lactose residue and improves lactose tolerance68. Moreover, Lactococcus produces several antimicrobial substances, including lactic acid, hydrogen peroxide, and lactobacilli, which effectively inhibit the growth of harmful bacteria. They compete for nutritional resources and survival space, further reducing the growth of harmful bacteria and maintaining a balanced intestinal flora69.
Studies have indicated that Lactococcus plays a role in regulating the immune system. It interacts with immune cells, stimulating their activity and enhancing the immune response57. Additionally, Lactococcus helps reduce inflammatory responses by regulating the function of the intestinal mucosal barrier70. The present study suggests a negative correlation between Lactococcus and the risk of psoriasis. However, further research is needed to investigate the underlying biological mechanisms linking the two71.
In conclusion, dysbiosis of the gut microbiota may trigger or exacerbate the development of psoriasis through various pathways, including influencing the immune system, triggering inflammatory responses, and affecting nutrient absorption and metabolism. Therefore, maintaining a healthy balance of gut microbiota is crucial for preventing and treating psoriasis. Methods such as adjusting diet, avoiding the overuse of antibiotics, and appropriately using probiotics and prebiotics may help maintain a healthy balance of gut microbiota, thus reducing the risk of occurrence and development of psoriasis72.
Our study has certain limitations that should be acknowledged. Firstly, we only analyzed bacterial taxa at the genus level and did not investigate more specialized levels, such as the species or strain level. This limitation may have implications for the precision of our results. Secondly, although the majority of participants in this GWAS were of European ancestry, including participants from other ethnicities might have influenced the outcomes. Consequently, the generalizability of our findings to different ethnic groups may be restricted. Thirdly, to ensure a sufficient number of instrumental variables (IVs), we selected gut microbiota IVs with a significance level of p < 1.0 × 10–5, which is more lenient than the traditional genome-wide significance level (p < 5 × 10–8). As a result, the effects of the reported bacterial traits were relatively weak, and the absence of other independent psoriasis GWAS datasets with sufficient sample sizes hindered the validation of our findings. Finally, the lack of information on psoriasis subtypes may necessitate further studies to explore this aspect once such data becomes available.
By acknowledging these limitations, we can provide a more comprehensive assessment of the study's scope and the potential impact on interpreting the results. If you have any further specific improvements or suggestions, please feel free to let me know.
Conclusions
In summary, this study investigated the potential causal relationship between gut flora and the risk of psoriasis. The analysis revealed that specific bacterial taxa, including Mollicutes,Victivallaceae,Eubacterium (coprostanoligenes group),Eubacterium (fissicatena group), Holdemania, Lachnospiraceae (NK4A136 group), Lactococcus, and Tenericutes, were suggestively associated with the risk of psoriasis. These findings offer valuable insights into the pathogenesis of psoriasis and may open up possibilities for novel treatment approaches.
Data availability
The data described in this research have been sourced from two online databases, namely the MiBioGen consortium and the IEU OpenGWAS project. MiBioGen consortiumis accessible at the GCC website: https://mibiogen.gcc.rug.nl/menu/main/home/. The IEU OpenGWAS project is accessible at the GWAS Catalog website: https://gwas.mrcieu.ac.uk/. Corresponding author Feng Jiang will provide the data upon reasonable request.
References
Griffiths, C., Armstrong, A. W., Gudjonsson, J. E. & Barker, J. Psoriasis. Lancet 397(10281), 1301–1315 (2021).
Raharja, A., Mahil, S. K. & Barker, J. N. Psoriasis: A brief overview. Clin. Med. (Lond.) 21(3), 170–173 (2021).
Boehncke, W. H. & Schon, M. P. Psoriasis. Lancet 386(9997), 983–994 (2015).
Tokuyama, M. & Mabuchi, T. New treatment addressing the pathogenesis of psoriasis. Int. J. Mol. Sci. 21(20), 7488 (2020).
Szanto, M. et al. Targeting the gut-skin axis-Probiotics as new tools for skin disorder management?. Exp. Dermatol. 28(11), 1210–1218 (2019).
Zeng, L., Yu, G., Wu, Y., Hao, W. & Chen, H. The effectiveness and safety of probiotic supplements for psoriasis: A systematic review and meta-analysis of randomized controlled trials and preclinical trials. J. Immunol. Res. 2021, 7552546 (2021).
Xu, J. J., Zhang, X. B., Tong, W. T., Ying, T. & Liu, K. Q. Phenome-wide Mendelian randomization study evaluating the association of circulating vitamin D with complex diseases. Front. Nutr. 10, 1108477 (2023).
Jin, P. et al. Diabetes and intervertebral disc degeneration: A Mendelian randomization study. Front. Endocrinol. (Lausanne) 14, 1100874 (2023).
Xu, Q. et al. Causal relationship between gut microbiota and autoimmune diseases: A two-sample Mendelian randomization study. Front. Immunol. 12, 746998 (2021).
Long, Y., Tang, L., Zhou, Y., Zhao, S. & Zhu, H. Causal relationship between gut microbiota and cancers: A two-sample Mendelian randomization study. BMC Med. 21(1), 66 (2023).
Yu, X. H., Yang, Y. Q., Cao, R. R., Bo, L. & Lei, S. F. The causal role of gut microbiota in development of osteoarthritis. Osteoarthr. Cartil. 29(12), 1741–1750 (2021).
Bowden, J., Davey, S. G., Haycock, P. C. & Burgess, S. Consistent estimation in mendelian randomization with some invalid instruments using a weighted median estimator. Genet. Epidemiol. 40(4), 304–314 (2016).
Kurilshikov, A. et al. Large-scale association analyses identify host factors influencing human gut microbiome composition. Nat. Genet. 53(2), 156–165 (2021).
Yavorska, O. O. & Burgess, S. MendelianRandomization: An R package for performing Mendelian randomization analyses using summarized data. Int. J. Epidemiol. 46(6), 1734–1739 (2017).
Larsson, S. C. & Burgess, S. Appraising the causal role of smoking in multiple diseases: A systematic review and meta-analysis of Mendelian randomization studies. Ebiomedicine 82, 104154 (2022).
Mansfield, K. L. et al. First satellite tracks of South Atlantic sea turtle “lost years”: Seasonal variation in trans-equatorial movement. Proc. Biol. Sci. 284(1868), 20171730 (2017).
Hartwig, F. P., Davies, N. M., Hemani, G. & Davey, S. G. Two-sample Mendelian randomization: Avoiding the downsides of a powerful, widely applicable but potentially fallible technique. Int. J. Epidemiol. 45(6), 1717–1726 (2016).
Bowden, J., Davey, S. G. & Burgess, S. Mendelian randomization with invalid instruments: Effect estimation and bias detection through Egger regression. Int. J. Epidemiol. 44(2), 512–525 (2015).
Bowden, J. et al. Assessing the suitability of summary data for two-sample Mendelian randomization analyses using MR-Egger regression: The role of the I2 statistic. Int. J. Epidemiol. 45(6), 1961–1974 (2016).
Higgins, J. P. & Thompson, S. G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 21(11), 1539–1558 (2002).
Damiani, G. et al. The global, regional, and national burden of psoriasis: Results and insights from the global burden of disease 2019 study. Front. Med. (Lausanne) 8, 743180 (2021).
Buja, A. et al. The prevalent comorbidome at the onset of psoriasis diagnosis. Dermatol. Ther. (Heidelb.) 13(9), 2093–2105 (2023).
Buja, A. et al. The prevalent comorbidome at the onset of psoriasis diagnosis. Dermatol. Ther. (Heidelb.) 13(9), 2093–2105. https://doi.org/10.1007/s13555-023-00986-0 (2023).
Mahmud, M. R. et al. Impact of gut microbiome on skin health: Gut-skin axis observed through the lenses of therapeutics and skin diseases. Gut Microbes 14(1), 2096995 (2022).
Myers, B. et al. The gut microbiome in psoriasis and psoriatic arthritis. Best Pract. Res. Clin. Rheumatol. 33(6), 101494 (2019).
Kanda, N., Hoashi, T. & Saeki, H. Nutrition and psoriasis. Int. J. Mol. Sci. 21(15), 5405 (2020).
Adawi, M. et al. The impact of intermittent fasting (ramadan fasting) on psoriatic arthritis disease activity, enthesitis, and dactylitis: A multicentre study. Nutrients 11(3), 601 (2019).
Damiani, G. et al. The impact of ramadan fasting on the reduction of PASI Score, in moderate-to-severe psoriatic patients: A real-life multicenter study. Nutrients 11(2), 277 (2019).
Kocic, H. et al. Dietary compounds as potential modulators of microRNA expression in psoriasis. Ther. Adv. Chronic Dis. 7(10), 2040622319864805 (2019).
Fletcher, J. M., Moran, B., Petrasca, A. & Smith, C. M. IL-17 in inflammatory skin diseases psoriasis and hidradenitis suppurativa. Clin. Exp. Immunol. 201(2), 121–134 (2020).
Tashiro, T. & Sawada, Y. Psoriasis and systemic inflammatory disorders. Int. J. Mol. Sci. 23(8), 4457 (2022).
Daniele, S. G., Eldirany, S. A., Damiani, G., Ho, M. & Bunick, C. G. Structural basis for p19 targeting by anti-IL-23 biologics: Correlations with Short- And Long-Term Efficacy In Psoriasis. JID Innov. 4(2), 100261 (2024).
Damiani, G. et al. Circadian oscillations of minimal erythema dose (MED) are also influenced by diet in patients with psoriasis: A chronomedical study. Dermatol. Ther. (Heidelb.) 13(10), 2229–2246 (2023).
Pacifico, A. et al. Diet-related phototoxic reactions in psoriatic patients undergoing phototherapy: Results from a multicenter prospective study. Nutrients 13(9), 2934 (2021).
Damiani, G. et al. Predicting secukinumab fast-responder profile in psoriatic patients: Advanced application of artificial-neural-networks (ANNs). J. Drugs Dermatol. 19(12), 1241–1246. https://doi.org/10.36849/JDD.2020.5006 (2020).
Wang, L., Cao, Z. M., Zhang, L. L., Li, J. M. & Lv, W. L. L. The role of gut microbiota in some liver diseases: From an immunological perspective. Front. Immunol. 13, 923599 (2022).
Chen, Q. et al. Coptis chinensis Franch polysaccharides provide a dynamically regulation on intestinal microenvironment, based on the intestinal flora and mucosal immunity. J. Ethnopharmacol. 267, 113542 (2021).
Wen, Y. et al. Intestinal flora derived metabolites affect the occurrence and development of cardiovascular disease. J. Multidiscip. Healthc. 15, 2591–2603 (2022).
Zhang, Z., Zhang, Y., Li, J., Fu, C. & Zhang, X. The neuroprotective effect of tea polyphenols on the regulation of intestinal flora. Molecules 26(12), 3692 (2021).
Deng, L. et al. GeGen QinLian decoction alleviate influenza virus infectious pneumonia through intestinal flora. Biomed. Pharmacother. 141, 111896 (2021).
Wang, X. et al. Polysaccharide regulation of intestinal flora: A viable approach to maintaining normal cognitive performance and treating depression. Front. Microbiol. 13, 807076 (2022).
Zhai, Y. et al. Astragaloside IV ameliorates diet-induced hepatic steatosis in obese mice by inhibiting intestinal FXR via intestinal flora remodeling. Phytomedicine 107, 154444 (2022).
Heller, F. & Duchmann, R. Intestinal flora and mucosal immune responses. Int. J. Med. Microbiol. 293(1), 77–86 (2003).
Cheng, Y. et al. Relationship between intestinal flora, inflammation, BDNF gene polymorphism and generalized anxiety disorder: A clinical investigation. Medicine (Baltimore) 101(29), e28910 (2022).
Freuer, D., Linseisen, J. & Meisinger, C. Association between inflammatory bowel disease and both psoriasis and psoriatic arthritis: A bidirectional 2-Sample mendelian randomization study. JAMA Dermatol. 158(11), 1262–1268 (2022).
Hashemi, M., Akbari, M. E., Razavi, S. S., Saadat-Niaki, A. & Hoseini, K. S. Evaluating resident physicians’ knowledge, attitude, and practice regarding the pain control in cancer patients. Iran. J. Cancer Prev. 8(1), 1–10 (2015).
Barros, G., Duran, P., Vera, I. & Bermudez, V. Exploring the links between obesity and psoriasis: A comprehensive review. Int. J. Mol. Sci. 23(14), 7499 (2022).
Shi, Z. et al. Short-Term western diet intake promotes IL-23-mediated skin and joint inflammation accompanied by changes to the gut microbiota in mice. J. Investig. Dermatol. 141(7), 1780–1791 (2021).
Wei, J. et al. Alcohol consumption and smoking in relation to psoriasis: A Mendelian randomization study. Br. J. Dermatol. 187(5), 684–691 (2022).
Miguens, B. J. et al. Longitudinal profiling of the gut microbiome in patients with psoriatic arthritis and ankylosing spondylitis: A multicentre, prospective, observational study. BMC Rheumatol. 4(1), 60 (2020).
Thaiss, C. A. et al. Hyperglycemia drives intestinal barrier dysfunction and risk for enteric infection. Science 359(6382), 1376–1383 (2018).
Song, I. et al. Comparative genomic and physiological analysis against clostridium scindens reveals Eubacterium sp. C-25 as an atypical deoxycholic acid producer of the human gut microbiota. Microorganisms 9(11), 2254 (2021).
Lozupone, C. et al. Identifying genomic and metabolic features that can underlie early successional and opportunistic lifestyles of human gut symbionts. Genome Res. 22(10), 1974–1984 (2012).
Mukherjee, A., Lordan, C., Ross, R. P. & Cotter, P. D. Gut microbes from the phylogenetically diverse genus Eubacterium and their various contributions to gut health. Gut Microbes 12(1), 1802866 (2020).
Blaak, E. E. et al. Short chain fatty acids in human gut and metabolic health. Benef. Microbes 11(5), 411–455 (2020).
Guo, C. et al. Deficient butyrate-producing capacity in the gut microbiome is associated with bacterial network disturbances and fatigue symptoms in ME/CFS. Cell Host Microbe 31(2), 288–304 (2023).
Singh, V. et al. Butyrate producers, “the Sentinel of Gut”: Their intestinal significance with and beyond butyrate, and prospective use as microbial therapeutics. Front. Microbiol. 13, 1103836 (2022).
Cobo, E. R., Kissoon-Singh, V., Moreau, F., Holani, R. & Chadee, K. MUC2 mucin and butyrate contribute to the synthesis of the antimicrobial peptide cathelicidin in response to entamoeba histolytica- and dextran sodium Sulfate-Induced colitis. Infect. Immun. https://doi.org/10.1128/IAI.00905-16 (2017).
Hamer, H. M. et al. Butyrate enemas do not affect human colonic MUC2 and TFF3 expression. Eur. J. Gastroenterol. Hepatol. 22(9), 1134–1140 (2010).
Wen, S. et al. Stigmasterol restores the balance of Treg/Th17 cells by activating the Butyrate-PPARgamma axis in colitis. Front. Immunol. 12, 741934 (2021).
Wang, C. et al. Methyl butyrate alleviates experimental autoimmune encephalomyelitis and regulates the balance of effector t cells and regulatory t cells. Inflammation 45(3), 977–991 (2022).
Wei, Y., Lu, X. & Liu, C. Gut microbiota and chronic obstructive pulmonary disease: A Mendelian randomization study. Front. Microbiol. 14, 1196751 (2023).
Cornejo-Pareja, I. et al. Differential microbial pattern description in subjects with Autoimmune-Based thyroid diseases: A pilot study. J. Personal. Med. 10(4), 192 (2020).
Takeuchi, T. et al. Fatty acid overproduction by gut commensal microbiota exacerbates obesity. Cell Metab. 35(2), 361–375 (2023).
Ma, L. et al. Spermidine improves gut barrier integrity and gut microbiota function in diet-induced obese mice. Gut Microbes 12(1), 1–19 (2020).
Wang, P. et al. Resveratrol-induced gut microbiota reduces obesity in high-fat diet-fed mice. Int. J. Obes. (Lond.) 44(1), 213–225 (2020).
Li, L. et al. Gut microbiota may mediate the influence of periodontitis on prediabetes. J. Dent. Res. 100(12), 1387–1396 (2021).
Wei, Z. et al. Soy protein alleviates malnutrition in weaning rats by regulating gut microbiota composition and serum metabolites. Front. Nutr. 8, 774203 (2021).
Bisanz, J. E. E., Upadhyay, V., Turnbaugh, J. A., Ly, K. & Turnbaugh, P. J. Meta-Analysis reveals reproducible gut microbiome alterations in response to a High-Fat diet. Cell Host Microbe 26(2), 265–272 (2019).
Wels, M., Siezen, R., van Hijum, S., Kelly, W. J. & Bachmann, H. Comparative genome analysis of lactococcus lactis indicates niche adaptation and resolves Genotype/Phenotype disparity. Front. Microbiol. 10, 4 (2019).
Chandran, C. et al. Lactococcus lactis secreting phage lysins as a potential antimicrobial against multi-drug resistant Staphylococcus aureus. PeerJ 10, e12648 (2022).
Kim, S. et al. Live biotherapeutic Lactococcus lactis GEN3013 enhances antitumor efficacy of cancer treatment via modulation of cancer progression and immune system. Cancers (Basel) 14(17), 4083 (2022).
Cervantes-Garcia, D. et al. Lactococcus lactis NZ9000 prevents asthmatic airway inflammation and remodelling in rats through the improvement of intestinal barrier function and systemic TGF-beta production. Int. Arch. Allergy Immunol. 182(4), 277–291 (2021).
Funding
This work was supported by Hainan Province Clinical Medical Center, The Excellent Talent Team of Hainan Province (No.QRCBT202121).
Author information
Authors and Affiliations
Contributions
Feng Jiang, Ni Zeng and Gaihe Chen designed the study. Xiaohuan Hu, Yunlei Bao and Chuyan Wu analyzed and interpreted the data. Yuan Li wrote the first draft of the manuscript. All authors revised the manuscript and approved it for publication.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Li, Y., Chen, G., Hu, X. et al. Assessing causal relationships between gut microbiota and psoriasis: evidence from two sample Mendelian randomization analysis. Sci Rep 14, 8831 (2024). https://doi.org/10.1038/s41598-024-59603-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-59603-5
Keywords
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.