Abstract
In the last few decades, researchers have thoroughly studied the use of plants in Palestine, one of them is Cyclamen persicum Mill. (C. persicum). Cyclamen persicum has been historically cultivated since the 1700s due to its tuber. The tuber is known to stimulate the nasal receptors, thus triggering the sensory neurons. Cyclamen persicum has anti-inflammatory effects, reduces cholesterol levels, treats diabetes, and inhibits tumor growth. In this respect, in-vitro examination of antibacterial and anticancer activities and antioxidative potency of C. persicum ethanolic extract were evaluated. The antioxidative potency of the extracted plant material was determined spectrophotometrically using the DPPH free radical scavenging method and the HPLC–PDA method to evaluate its total phenolic content (TPC) and total flavonoid content (TFC). The experimental results revealed weak antibacterial activity of C. persicum extract against both gram negative (E. coli) and gram positive (Streptococcus aureus and S. aureus) bacterial strains, with the zones of inhibition found to be less than 8 mm. On the other hand, powerful activity against MCF7 breast cancer as well as HT29 colon cancer cell lines was obtained. The findings also revealed potent inhibition of free radicals and the presence of maximal levels of natural products such as phenolic compounds and flavonoids, which supportits biological activities and powerful ability to scavenge free radicals. HPLC results showed the presence of numerous flavonoid and phenolic compounds such as rutin, chlorogenic acid, kaempferol, trans-cinnamic acid, quercetin, sinapic acid, and p-coumaric acid.
Similar content being viewed by others
Introduction
Throughout history, the utilization of plants has been used for medicinal purposes. The oldest records of using plants for medicinal purposes date back almost 5000 years and refer to over 250 different types of plants. Some plants that were recorded thousands of years ago, such as poppy (pain relief), henbane (sedative), and mandrake (an ingredient in making medicine), are still used today for their medicinal properties1. Palestine, with its unique geographical location and rich biodiversity, is known for its medicinal plants, with an estimate 2953 plant species2. Currently, more than10% of plants are used for their medicinal properties and contain alkaloids and terpenoids3. However, due to limited resources and studies conducted, much of the published data does not accurately reflect the actual status of medicinal plants in Palestine or the Palestinian territory4,5.
The study of the anticancer properties of plants dates back centuries. However, instead of a couple hundred anticancer medicinal plants, approximately 35,000 plant species are being studied for their anticancer properties, and 3000 plants have been shown to exhibit consistent anti-cancer effectiveness6. Plant-derived drugs are preferred for anticancer treatment as they are highly efficient and generally well-tolerated by the body, being non-toxic to human cells. However, with people turning away from chemotherapy due to its risky side effects, there is a significant demand for herbal medicine, leading to an increase in the diversity of medicinal plants7,8,9,10. Additionally, many medicinal plants have been found to be more efficacious in curing various infectious diseases and alleviating the side effects of synthetic antimicrobial drugs11,12,13,14,15.
Within the Mediterranean area, and more specifically in Palestine, a widely used plant is C. persicum (Fig. 1). The Cyclamen plant has been used as a medicinal plant for several hundred years, particularly since the nineteenth century16,17. In traditional medicine in Palestine, C. persicum is regularly used to extract its root. Studies have shown that the root of C. persicum is rich in saponins, which are known to reduce cholesterol levels, treat diabetes, and inhibit tumor growth. It has been observed that the root is absorbed through the nasal mucosa but not into the bloodstream18.
The C. persicum flower is indeed extremely common in the Mediterranean region and has been cultivated since the 1700s19. It is recognized by its heart-shaped leaves and tubers, which are the primary reasons for its cultivation . The plant is considered semi-poisonous due to the presence of saponins in its tuber, which can stimulate nasal receptors and result in sensory or pain perception. However, studies have shown that when the saponin of C. persicum, particularly repanoside, is isolated, it can influence the response of human macrophages, thereby mediating inflammatory effects20.
The investigation was focused on C. persicum and its antimicrobial and anticancer properties. The objectives of the study are therefore to investigate the antimicrobial, anti-cancer, and antioxidant activities of the ethanolic extract of C. persicum. Additionally, the study aims to determine the polyphenolic and flavonoid of the extract, as well as identify the specific polyphenolic compounds present, using the HPLC–PDA method.
Methodology
Plant extraction
Cyclamen persicum plant samples were collected from Ramallah, West Bank/Palestine and then identified by Dr. Nidal Jaradat. A herbarium voucher number (Pharm-PCT-260) was assigned to the plant, and it was deposited in the herbarium of An-Najah National University The aerial parts of the plant were dried in the shade until a constant weight was achieved, and then they were pulverized. 10 g of the powdered material were separately soaked in 100 mL of ethanol (100% and 50%) using ultrasonic treatment for 3 h. The mixture was then filtered, and the solvent was concentrated using a rotary evaporator at a temperature of 47 °C). The resulting residue was dissolved in methanol to obtain a concentration of 0.1 g/mL.
Antimicrobial testing
In order to assess the antimicrobial activity of the C. persicum extract, two gram-positive strains (S. aureus and Streptococcus) and one gram-negative strain (E. coli) were used. To create the Mullen-Hinton agar media, 19 g of agar media were combined with 500 mL of distilled water. The mixture was then autoclaved at 121 °C for 15 min. After autoclaving , the Mullen-Hinton agar was allowed to cool and poured into 6 culture Petri dishes. To culture Streptococcus, S. aureus, and E. coli on the petri dishes, pure colonies of each strain were obtained and inoculated into small vials containing distilled water. The McFarland turbidity standard was followed to prepare the inoculum. Using an inoculating loop, samples of inoculum were swabbed and streaked on the surface of the agar media in the 6 petri dishes. To create wells, a 100 µL pipette tip was inserted into the agar media, and the contents of the formed wells were removed to make circular holes. The C. persicum extract was then pipetted into the wells. The Petri dishes were incubated for 24 h at 37 °C to allow for microbial growth and the assessment of antimicrobial activity.
Anti-cancer testing
To assess the cytotoxic activity of the C. persicum extracts, two cancer cell lines were used: HT29 colon cancer and MCF7 breast cancer cell lines, obtained from the American Type Culture Collection (ATCC), USA. Prior to the assessment, both cell lines were harvested and suspended in RPMI medium. The cells were then incubated for 24 h to allow them to settle and adapt to the culture conditions. For each cancer cell line, five tissue culture plates were prepared. The plates were divided into three parts in a ratio of 2:2:1. The first two parts were allocated for 100% and 50% ethanolic extracts of C. persicum, respectively, while the third part served as a negative control using DMSO (Dimethyl Sulfoxide). DMSO-diluted extracts were prepared at volumes of 100 μL and 50 μL, and they were added to the tissue culture plates independently for each cell line in the respective volumes. The tissue culture plates of each cell line were treated with 3 μL of 100% and 50% of DMSO diluted ethanol extracts and then incubated at 37 °C for 72 h.
Activity of DPPH in scavenging free radicals
The DPPH antioxidant assay evaluates whether a test sample can scavenge or neutralize the free radical property of the stable DPPH radical. First, a DPPH solution of 0.062 mM was prepared using 95% methanol as the solvent. Later, a 3.9 mL portion of this solution was added to 0.1 mL of C. persicum, and the mixture was vortexed for 10 s and incubated at room temperature for 30 min. The absorbance of the DPPH solution was measured at 515 nm using 95% methanol as blank sample. A calibration curve was measured using different Trolox concentrations ranging from 20 to 200 ppm. The results were expressed as µmol Trolox per gram (µmol Trolox/g).
Determination of Total Phenolic Content (TPC)
The total content of phenolic compounds in C. persicum plant extracts was determined using the Folin–Ciocalteu colorimetric method. The Folin–Ciocalteu reagent was diluted tenfold with distilled water, and 1.8 mL of this diluted reagent was added to a measured volume of the plant extract. The mixture was set aside for five minutes before adding 1.2 mL of the 7.5% NaHCO3. After 60 min at room temperature, the absorbance of the samples was recorded at 765 nm. The same procedure was repeated for the gallic acid standard. A calibration curve was constructed using aqueous solutions with known gallic acid concentrations ranging from 20 to 500 mg/L. The results were expressed in terms of gallic acid equivalents (GAE) per gram of the sample21.
Determination of the total flavonoids content (TFC)
The total content of flavonoids in C. persicum extract was determined spectrophotometrically using the Aluminum chloride method. The following steps were followed using a test tube: 1 mL of C. persicum extract was mixed with 5 mL of distilled water, 0.3 mL of 5% sodium nitrate solution, and 0.3 mL of 10% aluminum chloride solution.The mixture was allowed to stand at room temperature for 5 min. Then 2 mL of 1M sodium hydroxide was added, and the final volume was adjusted to 10 mL using water. After the last step, the final solution was vortexed, and absorbance was measured spectrometrically at 510 nm. A standard calibration curve was prepared using different concentrations of catechin ranging from 20 to 100 mg/L. The results were expressed as milligrams of catechin equivalents (CEQ) per gram of the sample21.
Phytochemicals HPLC analysis
RP-HPLC conditions
The bioactive compounds were evaluated using a Waters Alliance e2695 HPLC system connected to a 2998 PDA detector. Data acquisition and analysis were performed using Empower-3 chromatography information software. The reversed-phase HPLC method was employed to analyze various polyphenolic compounds as well as flavonoids. A C18 column with a length of 25 cm and an inner diameter of 3.6 µm was used. The mobile phase consisted of a combination of 0.5% solution A (CH3COOH-Acetic acid) and solution B (CH3CN-Acetonitrile), following a linear mobile phase configuration as outlined in Table 1. The flow rate was set at 0.5 mL/min, and the column temperature was maintained at approximately 25 ºC. An injection volume of 20 µL was used for the samples, which were filtered through a 0.45 µm filter prior to injection. During the analysis, a photodiode array (PDA) sensor with a wavelength range of 210–400 nm was utilized to detect and measure the compounds of interest.
Preparation of standard solutions
The following standards were used during the HPLC analysis:rutin, vanillic acid, ferulic acid, isovanillic acid, kaempferol, quercetin, verbacoside, p-coumaric acid, chlorogenic acid, sinapic acid, gallic acid, trans-cinnamic acid, caffeic acid, syringic acid, 3,4-dihydroxybenzoic acid, and 3,4-dihydroxyphenylacetic acid.. To prepare the standards mixture, 5 mg of each compound was dissolved in 5 mL of 20% ethanol. Distilled water was then added to create a volume of 25 mL. The standards mixture was injected (20 µL) into the HPLC chromatograph and analyzed using the aforementioned reversed-phase (RP) technique.
Preparation of plant extract samples for HPLC
A dry extract weighing exactly 100 mg was dissolved in 20 mL of 95% ethanol. Subsequently, 100 mL of distilled water was added to the solution.
Statistical analysis
All experimental data analyses were performed using Microsoft Excel 2010. The reported results were presented as means ± standard deviation (SD). Statistical analyses were conducted using SAS (SAS Institute Inc., Cary, USA, Release 8.02, 2001).
To assess the impact of the extraction solvent on TPC, TFC, and AA, comparisons of means were performed using the General Linear Model (GLM) procedure. The main factor, which is the extraction solvent, was treated separately using one-way analysis of variance (ANOVA).
Plant collection approval
No approval is needed from the authority in Palestine to collect Cyclamen persicum for research purposes. However, landowner approved the collection of Cyclamen persicum for research purposes.
IUCN Policy statement
The collection of plant material complies with relevant institutional, national, and international guidelines and legislation.
Results
Total phenolic content (TPC)
The total phenolic content (TPC) was assessed by Folin Ciocalteu method, and the results were presented as milligrams of gallic acid equivalents (GAE) per gram of sample with reference to a calibration curve. The total phenolic content of the ethanolic extracts is presented in Table 2. For the extract with a 50% ethanol concentration, TPC was found to be 114.3 ± 2.2 mg of gallic acid/g. In contrast, the extract with 100% ethanol concentration contained a total of 102.4 ± 3.1 mg of gallic acid/g phenolics. The results demonstrated that the 50% ethanol extract exhibited greater extracting power for total phenolic compounds compared to the 100% ethanol extract. Statistical analysis confirmed this finding, as indicated by the capital letters A and B in Table 2, indicating that TPC of the 50% ethanolic extract was statistically higher than that of the 100% ethanol extract. The higher levels of polyphenolic substances observed in the 50% ethanol mixed mode, which contains both ethanol and water, may be responsible for this behavior. This difference can be attributed to the polarity of the solvent and its ability to saturate active phytoconstituents in plants.
Total flavonoid content (TFC)
The TFC was measured using Aluminium chloride method and expressed as milligrams of catechin per gram (mg catechin/g) with reference to a standard curve of catechin. The results for 50% and 100% ethanolic extract showed that the TFC was found to be 32.4 ± 2.5 and 22.3 ± 2.4 mg catechin/g, respectively. The TFC was observed to be higher in the 50% ethanolic and lower in the 100% ethanol extract. This difference can be attributed to the polarity of the solvent and the solubility of flavonoids in polar solvents. The higher polarity of the 50% ethanolic solvent may have facilitated better extraction of flavonoids, resulting in a higher TFC compared to the 100% ethanol solvent. These findings provide support for the ethanolic solvent extract of C. persicum having a high phenolic and flavonoid content. Statistical analysis confirmed that TFC of 50% ethanolic extract was statistically higher than that using the 100% ethanol extract).
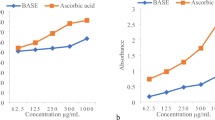
Antioxidant activity (AA)
The AA of the extracts was assessed using the DPPH free radical test which utilizes a stable free radical. The results were reported in terms of micromoles of Trolox per gram (µmol Trolox/g). The findings indicated that the AA of the 50% ethanolic extract was measured at 342.9 ± 4.9 µmol Trolox/g, while the 100% ethanolic extract exhibited a lower AA of 210.7 ± 7.3 µmol Trolox/g. This variation in AA between the two extracts can potentially be attributed to the polarity of the solvents used. This variation in antioxidant activity between the two extracts can potentially be attributed to the polarity of the solvents used. The 50% ethanolic solution, which combines water and ethanol, was more effective in extracting flavonoids and phenolic compounds, known contributors to AA. This trend was consistent with the findings of the total phenolic and total flavonoid content, where the 50% ethanolic solvent demonstrated superior extraction of these compounds compared to the 100% ethanolic solvent.
HPLC examination of flavonoids and polyphenolic compounds
Twenty microliters (20 µL) of the mixture containing the 17 standards were injected into the HPLC chromatograph, following the previously mentioned RP-phase analysis method. A photodiode array detector was used, allowing for detection at multiple wavelengths since each substance has a unique wavelength with maximum absorption (as indicated in Table 3). The chromatograms of the standards mixture were obtained at different wavelengths, specifically 270 nm (a), 290 nm (b), 300 nm (c), and 323 nm (d), as shown in Fig. 2a–d. By using different wavelengths, the 17 compounds were successfully separated, as depicted in Fig. 2a–d. Table 3 provides a list of the retention times for each standard, along with the corresponding peak absorption wavelength for each compound.
Figure 3 displays the RP-HPLC chromatograms of C. persicum analyzed at a wavelength of 300 nm.This wavelength was selected because it showed the highest absorption for the major peaks. The eluted compounds were identified within the elution time range of 21 to 77 min, indicating the presence of both polar and nonpolar substances. Variouscompounds were identified in this extract, including rutin, chlorogenic acid, kaempferol, trans-cinnamic acid, quercetin, sinapic acid, and p-coumaric acid.
Discussion
Comparing the TPC of C. persicum extract with other plant extracts or established standards can provide valuable insights into its potential health-promoting effects. A higher phenolic content is often associated with increased antioxidant activity, which is beneficial for human health. Phenolic compounds have been widely studied for their various biological activities, including antibacterial, anti-inflammatory, and anticancer effects. By understanding the TPC of C. persicum, we can further explore its potential therapeutic benefits and identify it as a potential source of valuable bioactive compounds.
Flavonoids, a subgroup of phenolic compounds, are renowned for their antioxidant and anti-inflammatory properties. Measuring the TPC in C. persicum extract provides valuable information about the presence of these beneficial compounds. Flavonoids have been associated with a range of health benefits, including promoting cardiovascular health, modulating the immune system, and exerting neuroprotective effects. Analyzing the flavonoid content helps in evaluating the potential health implications of consuming or utilizing C. persicum22.
Examining the correlation between the TPC and TFC can unveil the diversity of bioactive compounds present in the extract, highlighting the multifaceted antioxidant potential of C. persicum. A positive correlation between AA and the content of total phenolics/flavonoids validates the crucial role of these compounds in the observed antioxidant effects. The synergistic interactions among different bioactive components contribute to the enhanced antioxidant capacity of C. persicum extract.
In a study conducted by Murat Turan et al.22, the antioxidant and anti-proliferative activities of acetone, methanol, and water extracts from different parts (fresh and underground parts) of Cyclamen were investigated. The study determined the TCP and TFC and tannin contents in the extracts. The maximum values obtained were 3.69 ± 0.13 mg GAE/g-extract for TPC, 18.48 ± 0.12 mg QE/g-extract for TFC, and 41.17 ± 0.44 mg CE/g-extract for tannins. Furthermore, the extracts obtained from C. persicum displayed anti-proliferative activity in Caco-2 colon cancer cells. This suggests that the extracts from C. persicum may have potential therapeutic effects against colon cancer.
In a study by Ayadin et al.21, the phenolic composition and biological activities of various extracts of C. persicum were evaluated. The highest phenolic compound identified in the extracts was cinnamic acid, with a concentration of 411.6 µg/g. The ethanolic extracts demonstrated the highest TPC, measuring 8.99 ± 3.07 mg GAE/mL. Similarly, the ethanolic extracts also exhibited the highest TFC, with a value of 54.66 ± 3.02 mg QE/g. The radical scavenging activity of the extracts varied among the different solvents used for extraction. The tuber acetone extract displayed the highest radical scavenging activity, measuring 336.3 ± 0.02. On the other hand, the tuber ethanol extract showed a lower radical scavenging activity of 129.74 ± 0.02. Moreover, the tuber methanolic extracts exhibited strong inhibition of the growth of Gram-positive S. aureus. The minimum inhibitory concentration (MIC) for the tuber methanolic extract was determined to be 56 ± 0.03 μg/mL, indicating its significant potential as an antibacterial agent against S. aureus.
Antioxidant activity (AA)
Based on Table 2, the AA, measured as DPPH free radical scavenging, was higher in the 50% ethanolic extract compared to the 100% ethanolic extract. The values of 342.9 ± 4.9 µmol Trolox/g for the 50% ethanolic extract and 210.7 ± 7.3 µmol Trolox/g for the 100% ethanolic extract. The presence of polyphenolic substances or flavonoids in the extracts may explain the observed AA. Flavonoids and polyphenolic compounds are well-known for their effectiveness as free radical scavengers, contributing to their antioxidant potential These compounds act as reducing agents, hydrogen donors, and singlet oxygen quenchers, collectively enhancing their antioxidant properties.
Antimicrobial activity
The antibacterial activity of the plant extracts was evaluated using the disk diffusion method against three bacterial strains: S. aureus and Streptococcus (both Gram-positive bacteria), and E. coli (a Gram-negative bacterium). The results indicated that both the 100% ethanol extract and the 50% ethanol extract displayed weak antibacterial activity against all tested bacteria. The zone of inhibition, which indicates the area of bacterial growth inhibition around the disk containing the extract, was less than 8 mm for all bacterial strains, indicating low inhibitory effects.
Anti-cancer activity
In the evaluation of the anti-cancer activity of C. persicum, the effects of the extract were tested on two cancer cell lines: MCF7, representing breast cancer, and HT29, representing colon cancer. The negative control used in the experiment was DMSO (dimethyl sulfoxide), which did not show any significant effect on the cancer cells, as indicated in Fig. 2a. This suggests that the observed effects on the cancer cell lines can be attributed to the C. persicum extract rather than the DMSO control.
MCF7 breast cancer cell line
The results of the study demonstrated that C. persicum extract exhibited significant anti-cancer activity specifically against the MCF7 breast cancer cell line. The assessment involved cell counting and calculation of the percentage of cell inhibition, which indicated a noticeable decrease in cell count when treated with the plant extract compared to the negative control (DMSO). Comparing the 50% and 100% ethanolic extracts to the negative control, a clear decline in the number of cancer cells was observed. The anti-cancer activity was more pronounced at higher concentrations (100 µL) compared to lower concentrations (50 µL). Importantly, there was no significant difference in the activity against the MCF7 cell line between the 100% and 50% ethanolic extracts, as demonstrated in Fig. 4a–e of the study. This suggests that both concentrations of the ethanolic extract have similar efficacy in inhibiting the growth of MCF7 breast cancer cells.
The effect of C. persicum on MCF7: (a). The control samples of the MCF7 cell lines 48 h after culturing with DMSO (b). MCF7 cell lines 48 h after culturing with 100 μL of 50% extract (c). MCF7 cell lines 48 h after culturing with 50 μL of 50% extract (d). MCF7 cell lines 48 h after culturing with 100 μL of 100% extract (e). MCF7 cell lines 48 h after culturing with 50 μL of 100% extract.
Colon cancer cell line (HT29)
The study revealed that the C. persicum extracts exhibited significant anti-HT29 activity, specifically against the HT29 colon cancer cell line. When comparing the activity against HT29, the 100% ethanolic extract of C. persicum showed higher activity compared to the 50% ethanolic extract, as depicted in Fig. 5b–e of the study.
The effect of C. persicum on HT29: (a) The control samples of the HT29 cell lines 48 h after culturing with DMSO (b). HT29 cell lines 48 h after culturing with 100 μL of 100% extract (c). HT29 cell lines 48 h after culturing with 50 μL of 100% extract (d). HT29 cell lines 48 h after culturing with 50 μL of 50% extract (e). HT29 cell lines 48 h after culturing with 100 μL of 50% extract.
Additionally, when comparing the activity of C. persicum extracts against the two cancer cell lines (MCF7 and HT29), it was observed that the extract had a greater effect on HT29 than on MCF7, even when the same amount of extract was used. This suggests that the anti-cancer activity of C. persicum may vary depending on the specific cancer cell line, and HT29 cells may be more sensitive to the effects of the extract compared to MCF7 cells. The results from Fig. 5a–e provide evidence of this differential response between the two cell lines.
Conclusion
Cyclamen persicum, a plant with a longstanding history of medicinal use, possesses nutraceutical, pharmaceutical, and medicinal properties. This can be attributed to it is rich content of polyphenolic substances and flavonoids, which are important secondary metabolites for their diverse biological activities. HPLC analysis of the C. persicum extracts revealed the presence of various flavonoids and phenolic compounds. These include rutin, sinapic acid, chlorogenic acid, kaempferol, quercetin, trans-cinnamic acid, and p-coumaric acid. The presence of these flavonoids and polyphenolic substances contributes to the antioxidant activity and other biological activities exhibited by C. persicum. The plant extract also demonstrates mild inhibitory activity against both Gram-positive and Gram-negative bacterial strains. This suggests its potential as a natural antibacterial agent. Furthermore, C. persicum exhibits notable anti-cancer properties in colon (HT29) and breast (MCF7) cancer cell lines. This indicates its potential as a natural source of anti-cancer compounds. Overall, the diverse biological activities, including antioxidant, antibacterial, and anti-cancer properties, of C. persicum can be attributed to its rich composition of flavonoids and polyphenolic substances. These findings support the traditional medicinal use of C. persicum and highlight its potential for further exploration as a source of therapeutic agents.
Data availability
All data generated or analyzed during this study are included in this published article.
References
Šantić, Ž, Pravdić, N., Bevanda, M. & Galić, K. The historical use of medicinal plants in traditional and scientific medicine. Psychiatr. Danub. 29(Suppl 4), 787–792 (2017).
Abu-Rabia, A. Herbs as a food and medicine source in Palestine. Asian Pac. J. Cancer. Prev. 6(3), 404–407 (2005).
Alibek, K., Bekmurzayeva, A., Mussabekova, A. & Sultankulov, B. Using antimicrobial adjuvant therapy in cancer treatment: A review. Infect. Agent. Cancer 7(1), 33. https://doi.org/10.1186/1750-9378-7-33 (2012).
Leonti, M. & Verpoorte, R. Traditional Mediterranean and European herbal medicines. J. Ethnopharmacol. 199, 161–167 (2017).
Tungmunnithum, D., Thongboonyou, A., Pholboon, A. & Yangsabai, A. Flavonoids and other phenolic compounds from medicinal plants for pharmaceutical and medical aspects: An overview. Medicines (Basel) 5(3), 93. https://doi.org/10.3390/medicines5030093 (2018).
Desai, A. G. et al. Medicinal plants and cancer chemoprevention. Curr. Drug Metab. 9(7), 581–591. https://doi.org/10.2174/138920008785821657FromNLM (2008).
Al-Mazaideh, G. M., Shalayel, M. H., Al-Swailmi, F. K. & Aladaileh, S. H. Vitamin D is a new promising inhibitor to the main protease (Mpro) of COVID-19 by molecular docking. J. Pharm. Res. Int. 33(29B), 186–191. https://doi.org/10.9734/jpri/2021/v33i29B31603 (2021).
Al-Mazaideh, G. M. & Al-Swailmi, F. K. Phytonutrients and antimicrobial activities of methanolic extract from Hafr Al-Batin truffles. J. Pharm. Res. Int. 33(24B), 13–21. https://doi.org/10.9734/jpri/2021/v33i24B31437 (2021).
Mustafa, M. et al. The efficiency of some active ingredients of arum palaestinum as inhibitors to 3CLPRO and NSP15 proteins. Acta Poloniae Pharmaceu 79(5), 657–665. https://doi.org/10.3283/appdr/144970 (2022).
Kennedy, D. O. & Wightman, E. L. Herbal extracts and phytochemicals: Plant secondary metabolites and the enhancement of human brain function. Adv. Nutr. 2(1), 32–50. https://doi.org/10.3945/an.110.000117 (2011).
Al-Mazaideh, G. M. & Al-Quran, S. A. Effect of methanolic extract of Securigera securidaca as antioxidant and antibacterial activities. J. Pharm. Res. Int. 32(11), 10–17. https://doi.org/10.9734/jpri/2020/v32i1130498 (2020).
Khalid, M. et al. Assessment of antimicrobial and anticancer activity of radish sprouts extracts. J. J. Biol. Sci. 13(4), 567–574 (2020).
AldalIn, H. et al. Comparative analysis of phytochemical composition of ethanolic extract of Jordanian Silvia officinalis. Pak. J. Biol. Sci. 23(8), 989–994. https://doi.org/10.3923/pjbs.2020.989.994 (2020).
Al-Zereini, W. A. et al. Identification and antibacterial evaluation of selected Jordanian medicinal plants. Orient. J. Chem. 34(5), 2456–2463. https://doi.org/10.13005/ojc/340530 (2018).
Saranraj, P. & Sivasakthi, S. Medicinal plants and its antimicrobial properties: A review. Global J. Pharmacol. 8(3), 316–327. https://doi.org/10.5829/idosi.gjp.2014.8.3.83194 (2014).
Addam, K. H., Al-Zein, M. S., Bou-Hamdan, M. & Naous, H. A new record: Cyclamen persicum Mill. var. autumnale grey-wilson was added to the native Lebanese flora. Sci. Res. J. Eng. Technol. Sci. 26, 186–194 (2016).
Takamura, T., Cyclamen. Cyclamen persicum Mill. pp 459–478: In NO Anderson (ed.). Flower breeding and genetics; issue, challenges and opportunities for the 21st century. Springer. Dordrecht (The Netherlands). (2007).
Mohammed, G. J., Hameed, I. H. & Kamal, S. A. Anti-inflammatory effects and other uses of cyclamen species: A review. Indian J. Pub. Health Res. Dev. 9(3), 206–211. https://doi.org/10.5958/0976-5506.2018.00210.3 (2018).
Jaradat, N. A. Medical plants utilized in Palestinian folk medicine for treatment of diabetes mellitus and cardiac diseases. Al-Aqsa Univ. J. 9, 1–28 (2005).
Speroni, E. et al. Analgesic and antiinflammatory activity of Cyclamen repandum S. et S. Phytother. Res. 21(7), 684–689. https://doi.org/10.1002/ptr.2145 (2007).
Aydin, C., Mammadov, R. & Davidov, M. Biological activities, phenolic constituents and of various extracts of cyclamen coum tubers and leaves from Turkey. Khimiya Rastitel’nogo Syr’ya 1, 255–263. https://doi.org/10.14258/jcprm.20230111262 (2023).
Turan, M., Seçme, M. & Mammadov, R. Antioxidant and anti-proliferative activities of different parts of Cyclamen cilicium. BAUN Fen Bilimleri Enstitüsü Dergisi 24(42), 436–447. https://doi.org/10.25092/baunfbed.925496 (2022).
Acknowledgements
The authors would like to extend their sincere appreciation to the Researchers Supporting Project, King Saud University, Riyadh, Saudi Arabia for funding this work through the project number (RSP-2024R437)
Funding
This work is financially supported by the Researchers Supporting Project, King Saud University, Riyadh through the Project Number (RSP-2024R437).
Author information
Authors and Affiliations
Contributions
Conceptualization, writing the original draft, reviewing and editing: M.K., F.A., Z.S., M.A.A.Z., S.M.A., S.O.A. Formal analysis, investigations, funding acquisition, reviewing, and editing: F.W., S.K., A.A., F.I.B.A., H.O.A., G.M.A. Resources, data validation, data curation, and supervision: H.-A.N., A.M.S., A.B.M., M.B.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Al-Rimawi, F., Khalid, M., Salah, Z. et al. Anticancer, antioxidant, and antibacterial activity of chemically fingerprinted extract from Cyclamen persicum Mill.. Sci Rep 14, 8488 (2024). https://doi.org/10.1038/s41598-024-58332-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-58332-z
Keywords
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.