Abstract
Medicinal plants can be potential sources of therapeutic agents. Traditional healers use a medicinal plant from Ethiopia, Bersama abyssinica Fresen, to treat various diseases. This study aimed to investigate the phytochemical components and antioxidant and antimicrobial activities of B. abyssinica seed extracts (BASE). Gas chromatography coupled to mass spectroscopy (GC–MS) analysis was used to determine the phytochemical compositions of BASE. The antioxidant activities were assessed by using 2, 2-diphenyl-1-picrylhydrazyl (DPPH) assay, thiobarbituric acid-reactive species (TBARS) assay, ferric chloride reducing assay and hydroxyl scavenging capacity assay. Antimicrobial activity was investigated using the agar well diffusion method. Phytochemical screening showed the presence of saponins, glycosides, tannins, steroids, phenols, flavonoids, terpenoids, and alkaloids. GC–MS analysis revealed the presence of 30 volatile compounds; α-pinene (23.85%), eucalyptol (20.74%), β-pinene (5.75%), d-limonene (4.05%), and o-cymene (5.02%). DPPH-induced free radical scavenging (IC50 = 8.78), TBARS (IC50 = 0.55 µg/mL), and hydroxyl radicals’ scavenging capacities assays (IC50 = 329.23) demonstrated high antioxidant effects of BASE. Reducing power was determined based on Fe3+–Fe2+ transformation in the presence of extract. BASE was found to show promising antibacterial activity against S. aureus, E. coli, and P. aeruginosa (zone of inhibition 15.7 ± 2.5 mm, 16.0 ± 0.0 mm, and 16.7 ± 1.5 mm, respectively), but excellent antifungal activities against C. albican and M. furfur (zone of inhibition 22.0 ± 2.0 mm and 22.0 ± 4.0 mm, respectively). The seeds of B. abyssinica grown in Ethiopia possess high antioxidant potential, promising antibacterial and superior antifungal activity. Therefore, seeds of B. abyssinica provide a potential source for drug discovery.
Similar content being viewed by others
Introduction
The flowering plants demonstrated medicinal value of which 30% of these plants constitute phytochemicals1. In Ethiopia, 60% of plants are supposed to be indigenous with their curative potential2. A medicinal plant from Ethiopia, namely Bersama abyssinica Fresen, has been used by the traditional healer to cure many diseases3. B. abyssinica is a species of medium-sized evergreen shrub and tree that is distributed across sub-Saharan Africa4. Traditionally, all parts of the plants have been used for the treatment of many diseases. The Zulu community uses the leaves of B. abyssinica to cure barrenness, impotence, menstrual pain, and leprosy and to protect charm5. In Kenya, boiled roots and leaves are used for the treatment of sexually transmitted diseases, stomachache, pneumonia, TB, and malaria6.
In Ethiopia, B. abyssinica has various treatment purposes traditionally. Crushed fresh root mixed with cold water is taken orally for treatment of bronchitis and febrile illness7. The fruit and root powder is mixed with honey or butter and applied to cure wound and eczema8. Leaves and bark of roots are employed for the treatment of hypertension, rabies, cough, ascaris, diarrhea, rheumatoid, and wound9. A liquid preparation of buds has been reported as a remedy against dysentery and roundworms, while an infusion prepared from the stem bark is orally taken to treat cancer-like symptoms10. The leaf is boiled and drunk to cure diarrheal diseases11. The Ari and Male communities use the bark and buds for snake bite and liver disease treatment12. Sidama people use the bark by powdering, dissolving, boiling, and drinking small amounts for the treatment of tumors13. Gurage and Silti communities use the root, stem, leaf, and seed of B. abyssinica for curing lung disorders, heart failure, back pain, gonorrhea, intestinal problems, skin diseases, and abnormal menstrual cycles14.
The pathogenesis and prognosis of diseases such as cancer15, wound16, diabetes17 and malaria18 are associated with oxidative stress which traditional healers treat these diseases with extracts from B. abyssinica. Antioxidant therapy may have a positive impact on the course of many diseases, and some available synthetic antioxidants have been reported to cause adverse health-related issues, such as cancer19. Additionally, the shortage of antimicrobial agents is raised from time to time due to emerging and re-emerging infections, emergency drug resistance pathogens, adverse effects, and the high cost of synthetic drug development20,21. Hence, there is a need to search for new antioxidant and antimicrobial agents with better effects, less toxicity, and affordable cost, of which compounds originating from natural medicinal plants are alternatively recommended22.
To the best of our knowledge, analysis of phytochemical composition, and biological activities of seeds extract of B. abyssinica had not been reported so far. This study aimed to investigate the phytochemical composition and antioxidant and antimicrobial properties of the B. abyssinica seed extract (BASE).
Materials and methods
Plant material collection and identification
The seeds of B. abyssinica were collected from Semar village, Sude district in Ethiopia at the latitude of 7° 55′ 48′′ N, longitude of 39° 55′ 12′′ E, and altitude of 2549 m above sea level23. The seeds were gifted from Getaw Alemu who is the owner of the land. The sample was pressed, identified, and authenticated at the Department of Biology, University of Gondar (UoG) by the Botanist Getnet Chekole, and a voucher specimen (01/BA/2022) was deposited in the UoG Herbarium.
Extraction of the seeds of B. abyssinica
The seeds of B. abyssinica were washed with tap water, rinsed with distilled water, and air-dried at room temperature for two weeks under shed24. The seeds were grounded using a mechanical grinder machine (British Cult, Ser.no 15-303/1066). The pounder of the seeds (450 g) of B. abyssinica was soaked in 80% methanol for 72 h and occasional shaking. The procedure was repeated three times. Then, each extract was filtered by Whatman No. 1 filter paper twice and concentrated using a rotatory evaporator (YAMATO CF 301, Japan). The concentrated extract was incubated at 40 °C in an oven for 3 days and placed in a deep freezer at − 140 °C overnight to be lyophilized (LABFREEZ FD-12-MR Vacuum Freeze Dryer, China). Powder of dried seeds of B. abyssinica (100 g) was hydro-distilled for 4 h by using a Clevenger apparatus to isolate the volatile compounds.
Phytochemical screening test
Test for saponin 0.5 g of the plant extract mixed in 10 mL distilled water. The suspension shook in a test tube for 5 min. The formation of the foam indicates the presence of saponin25. Test for flavonoid 1 mL plant extract was added into 2 mL of 2% NaOH solution with a few drops of dilute HCl. An intense yellow color change to colorless indicates flavonoids26. Test for terpenoids Adding 2 mL chloroform in 5 mL plant extract followed by the addition of 3 mL concentrated H2SO4 and boiling in a water bath would result in a grey-colored solution that shows the presence of terpenoid26. Test for phenols 2 mL of plant extract was treated with 5% FCl3. The formation of blue or black color indicated the presence of phenols27. Test for tannins 0.1% FeCl3 was added to crude extracts and the formation of a brownish-green or a blue-black color shows the presence of tannins27. Test for glycosides 0.5 mL glacial acetic acid and 2–3 drops of FeCl3 were mixed with 2 mL of extract. Then, add 1 mL of 98% H2SO4 along the walls of the test tube. A deep blue color was formed at the junction of two liquids showing the presence of glycosides28. Test for steroids A red color was produced at the lower layer when 2 mL of extract was dissolved in 2 mL chloroform and 2 mL of 98% H2SO4 shows the presence of steroids29. Test for alkaloids 0.5 mg of crude extract with 1% HCl was stirred in a water bath for 5 min and was filtered into to test tube. Up on the addition of 1 mL Wagner’s reagent, the formation of a brown/reddish precipitate indicates the presence of alkaloids30.
Gas chromatography–mass spectrometry analysis
GC–MS analysis of BASE was performed on GC coupled to MS (Mass Hunter GC/MS Version B.07.03.2129, Agilent Technologies, USA). The carrier gas was helium flowing at a rate of 1 mL/min. Aliquots of hydro-distillate (1 mL of 1 ppm in hexane) were injected. The injector temperature was 230 ℃. The temperature of the oven was begun at 40 ℃ hold for 5 min and then, it was raised to 250 ℃ hold for 20 min for a total run-time of 60 min. Mass spectra were recorded at 70 eV, scanning the 50–500 m/z range which was connected to a computer Mass Spectra data bank. The components were identified by comparing their retention times with the retention times of authentic standards, and mass spectra with the National Institute of Standards and Technology (NIST 2017). The GC–MS was done in the JIJE LABOGLASS chemistry analysis laboratory, in Addis Ababa, Ethiopia.
In vitro anti-oxidant activity
The antioxidant activity of BASE was evaluated using 2, 2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging assay31, thiobarbituric acid-reactive species (TBARS) assay32, ferric chloride reducing assay33, and hydroxyl radicals’ scavenging capacities34 that are described in the literature with slight modifications. All experiments were performed in triplicate.
In the DPPH radical scavenging assay, the extract was prepared in test tubes containing methanol to give 1000, 500, 250, 125, and 62.5 µg/mL solution. About 4 mL of 0.1 mM methanolic solution of DPPH was dropped into 1 mL solution from each test tube. Ascorbic acid was used as a positive control and was prepared as above without extract. The resulting mixture was placed in an oven at 37 °C for 30 min and subjected to a UV Visible Spectrophotometer (CARY 60, Malaysia) to record absorbance at 517 nm. The blank solution was prepared without a sample or ascorbic acid. The percentage of DPPH Free radical scavenging (FRS) was manipulated by the formula.
where AB and AS are the absorbance values of the blank and test samples, respectively. Free radical scavenging activity was measured as the IC50 value which was defined as the concentration required for 50% inhibition, as compared to the control.
In the TBARS assay, egg homogenate (250 μL, 10% in distilled water, v/v) and 50 μL of extract were mixed in a test tube and the volume was made up to 500 μL, by adding distilled water. Then, 25 μL of 0.07 M ferrous sulfate (FeSO4) was added to the above mixture and incubated for 30 min, to induce lipid peroxidation. Thereafter, 750 μL of 20% acetic acid (pH 3.5) and 750 μL of 0.8% thiobarbituric acid (TBA) (w/v) (prepared in 1.1% sodium dodecyl sulfate) and 25 μL 20% trichloroacetic acid (TCA) was added, vortexed, and then heated in a boiling water bath for 60 min. After cooling, 3 mL of 1-butanol was added to each tube and centrifuged at 3000 revolutions per minute for 10 min. The absorbance of the organic upper layer was measured at 532 nm. For the blank solution, 50 μL of distilled water was used in place of the extract or standard control. Ascorbic acid was used as the positive control and the percent of lipid peroxidation inhibition (I) was calculated by the formula:
where AS is the value absorbance of the test sample and AB is the absorbance value of the blank.
In the ferric chloride reducing assay, 2.5 mL (1 mg/mL) of extract was mixed with 2.5 mL sodium phosphate buffer (pH 6.6, 0.2 mol/L) and 2.5 mL potassium ferricyanide (1%). The reaction mixture was incubated for 20 min at 50 °C. Then, 2.5 mL trichloroacetic acid (10%) was added and the mixture was centrifuged at 650 revolutions per minute for 10 min. After centrifugation, 5 mL supernatant was mixed with 5 mL distilled water and 1 mL of 0.1% ferric chloride. The absorbance was recorded at 700 nm. The absorbance of the reaction mixture would be increased with concentration which indicated reducing power. Ascorbic acid was used as standard.
In hydroxyl radicals’ scavenging capacities, first, 10.40 g of disodium hydrogen phosphate dodecahydrate (Na2HPO4·12H2O) and 1.32 g sodium dihydrogen phosphate dihydrate (NaH2PO4·2H2O) were measured and dissolved in 250 mL distilled water to the phosphate buffer solution. Then, 13.9 mg Ferrous sulfate heptahydrate (FeSO4·7H2O) and 37.23 mg ethylenediamine tetraacetic acid (EDTA) salt were added to distilled water and were made up to a constant volume of 100 mL to obtain the EDTA–Fe2+ solution. Next, 9 mg safranin N was dissolved in the previously prepared phosphate buffer solution to a constant volume of 100 mL. All of the above solutions were fresh and photophobic. Finally, 1 mL of extract or standard control, 0.5 mL of EDTA–Fe2+ solution, 1 mL of potassium phosphate buffer solution, 1 mL of safranin N solution, and 1 mL of 3% hydrogen peroxide (H2O2) solution were added to the order in a test tube. The mixture was incubated for 30 min at 37 °C. The ultraviolet spectroscopy absorption was measured at 520 nm. Ascorbic acid was used as standard. The percentage of hydroxyl radicals’ scavenging capacities (PHRSC) was calculated using the formula
where AS is the absorbance value of the sample and AB is the absorbance value of the blank.
Antimicrobial activity test
The antimicrobial activities were investigated against bacteria and fungi. Bacteria include: Staphylococcus aureus American Type Culture Collection (ATCC43502), Pseudomonas aeruginosa ATCC2785 and Escherichia coli ATCC25922. Fungi include Candida albicans and Malassezia furfur. The bacteria strains were obtained from the microbiology laboratory of the National Clinical Bacteriology and Mycology Reference Laboratory of the Ethiopian Public Health Institution (EPHI), and fungi were clinically isolated from UoG Teaching Hospital. The test organisms were kept on tryptic soy broth supplemented with 20% glycerol at − 80 °C in the microbiology laboratory of UoG.
Inoculum preparation was according to recommended by the Clinical and Laboratory Standards Institute35. All test organisms were grown in Petri dishes containing agar medium specific to each microorganism as a refreshment of each strain for the actual test. Each bacterial strain was incubated for 24 h at 37 °C and each fungus was incubated for 7 days at 25 °C. Standardization was by taking 3–5 inoculums from a fresh, pure culture of the test organism and making a suspension with nutrient broth for bacteria and sabouraud dextrose broth for fungi. Then, these suspensions were diluted with appropriate broth in 1:10 to get 1 × 107 CFU/mL and 1 × 106 spore/mL bacteria and fungi, respectively.
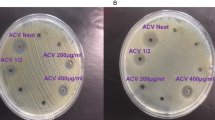
An antimicrobial activity of the plant extracts was conducted using the agar well diffusion method36,37. A sterile cotton swab was dipped into the adjusted suspension and the bacterial inoculum was uniformly spread on a sterile Petri dish Mueller–Hinton agar (the culture media was sterilized for 15 min at 121 °C). This procedure was repeated by streaking two more times, rotating the plate approximately 60° each time to ensure an even distribution of inoculums. The rim of the agar was swabbed. The extracts were diluted in 5% dimethyl sulfoxide (DMSO) to yield concentrations of 50 and 20 mg/mL solution for bacteria and fungi tests respectively. DMSO is used because it cannot inhibit the growth of test organisms at this concentration. 120 μL volume of extracts, positive control, and negative control were added to each of the 3 wells (6 mm diameter holes cut in the agar gel, 20 mm apart from one another). The plates were incubated face up for 24 h at 37 °C under aerobic conditions. A zone of inhibition created around wells was used as an indicator of antimicrobial activities. Ceftriaxone and ketoconazole were positive controls used for bacteria and fungi respectively whereas well-filed with DMSO was negative control38. All tests were done in triplicate.
Data analysis
The antimicrobial inhibition diameter and antioxidant inhibition percentages were reported as the mean ± standard deviation of the three consecutive tests. The statistical differences in the antimicrobial activity of extracts on each microorganism were carried out by employing one-way analysis of variance (ANOVA) followed by Post hoc Tukey’s tests. The IC50 value (50% inhibition) was calculated using regression equations obtained from the graph between percentage (%) inhibition and concentration. The criterion for significance was set at p < 0.05.
Ethical clearance
The research was conducted in accordance with ethical review committee of UoG. Ethical clearance was obtained from Institutional Review Committee School of Medicine, College of Medicine and Health Science, UoG. Plant material collection and use is carried out in accordance with all relevant guidelines to International Convention on the Trade in Endangered Species of Wild Fauna and Flora.
Results
Phytochemical screening
The phytochemical screening test of Bersama abyssinica seed extract (BASE) showed the presence of various phytochemicals (Table 1).
GC–MS analysis
GC–MS analysis led to the identification of 30 volatile compounds from the seed extract of B. abyssinica, representing 100% of the total peak area. Most of the identified compounds belonged to terpene hydrocarbons (65%) which include α-pinene (23.85%), eucalyptol (20.74%), β-pinene (5.75%), and d-limonene (4.05%), aromatic hydrocarbon such as o-Cymene (5.02) and other 2,4-Decadienal, (E, E) (5.50%), Furan, 2-pentyl- (4.81) and Nonanal (4.66) were found to be the main components (Table 2).
In vitro antioxidant activity
DPPH radical scavenging activity of BASE was assessed and compared with the standard ascorbic acid. The sample extract exhibited strong free radical scavenging activities compared to the standard. The percent of free radical inhibition was increased in a concentration-dependent (Table 3).
Inhibition of lipid peroxidation was assessed by TBARS assay. BASE showed lipid peroxidation inhibition in a concentration-dependent (Fig. 1a).
Ferric ion-reducing power was assessed in the ferric chloride-reducing assay. The color change from yellow in the blank sample to different strengths of green in the extract was observed which indicated BASE has reduced capacity of the ferric ion. The reducing power of extracts increased in a concentration-dependent (Fig. 1b).
The hydroxyl radicals’ scavenging capacity BASE was assessed. BASE demonstrated moderate hydroxyl radicals’ scavenging activity. The percentage of hydroxyl radicals’ scavenging capacity was increased in a concentration-dependent (Table 3).
Antimicrobial activity test
The extract showed antimicrobial activity against selected pathogenic bacteria and fungi at a concentration of 50 mg/mL and 20 mg/ mL, respectively. The results are presented in Table 4.
Discussion
The phytochemical screening determined the presence of alkaloids, glycosides, tannins, steroids, phenols, flavonoids, saponins, and Terpenoids which may be attributed to the traditional use of the plant as medicine. This finding is in line with the study on the root of B. abyssinica methanolic extracts39. There is a slight difference with a study on stem bark and leaves of B. abyssinica extracts4,40. A study on Bersama engleriana methanolic leaf extract of the phytochemical screening showed some variation in phytochemical content41. The difference might be due to the differences in genetic factors, oncogenic factors, morphogenetic factors, environmental factors, adaptation to local conditions, interactions with other organisms, and evolutionary history42,43.
GC–MS analysis revealed the presence of 30 volatile compounds. Most of the identified volatile compounds belonged to terpenes (64.38%) among which α-pinene (23.85%), eucalyptol (20.74%), β-β-pinene (5.75%), and d-limonene (4.05%) were found to be the main components. The results obtained from the seed extract were relatively different from the root, stem, and leaf of B. abyssinica GC–MS analysis44. A study done on a leaf of B. abyssinica extracts of the active fraction of GC–MS analysis also provided different phyto-constituents from this study45. Phytochemicals are found in different quantities in the cells, tissues, and organs of various plants. Since phytochemicals are complex and diverse in different parts of medicinal plants, the different secondary metabolites may be synthesized through special regulatory pathways and special transport routes in certain parts of the plants43. The specific tissues as well as the developmental stages, impact the pattern of gene expression related to phytochemical biosynthesis42. The α-pinene, eucalyptol, and β-pinene showed antimicrobial, antioxidant, and anti-inflammatory activities46,47. d-limonene, o-cymene, nonanal, furan,2-pentyl,2,4- and decadienal, (E, E) which have been reported by other studies with activities of antioxidants, antimicrobial, antifungal, and anti-inflammatory that was also existed in seeds of B. abyssinica48,49,50.
Multiple antioxidant assays were conducted to evaluate the antioxidant properties of BASE: DPPH radical scavenging assay, TBARS assay, OH radicals’ scavenging capacities assay, and ferric chloride assay on B. abyssinica seeds extract. Antioxidants are used to scavenge free radicals, prevent lipid peroxidation, and inhibit free-radical damage to biological systems so that they protect the body from oxidative stress. Nowadays, natural antioxidants from plants are recommended as preventive and treatments for many diseases51.
DPPH-induced free radical scavenging activity has been anticipated to be the principal method for determining the antioxidant properties of extracts52. This study results for the DPPH radical scavenging assay showed IC50 values of 8.77 µg/mL which is strong free radical scavenging activities compared to the ascorbic acid (IC50 = 75.44 µg/mL). Our study results coincide with the previous studies on roots, stem bark, and leaves of B. abyssinica. A previous study found the DPPH scavenging potential of solvent extracts of stem bark B. abyssinica with marked activity being exhibited by the methanol extract, followed by the water extract and ethyl acetate extract4. In another study, leaves of B. abyssinica showed the scavenging potential to scavenge DPPH free radicals53. The DPPH scavenging activity of B. abyssinica leaves and twigs has been reported54. In early studies, the root, stem, and leaves of B. abyssinica are rich in polyphenols, flavonoids, terpenes, alkaloids, and saponins compounds4,44,53 that the extracts possess hydrogen donating and radical scavenging ability.
Lipid peroxidation is a free radical-induced oxidative chain reaction by which one lipid molecule oxidation initiates the next lipid molecule up to the maximum possible amount forming lipid peroxide55. Ferrous ion was a potent initiator of lipid peroxidation56. Lipid peroxidation is terminated when the amount of ferrous iron oxidized is limited by the peroxyl radical reacted with antioxidants57. The absorbance values obtained by the TBARS method show the whole peroxide values formed by the oxidation of polyunsaturated acid58. The incubation of the egg homogenate in the presence of Fe2+ caused a significant increase in the malonaldehyde (MDA)59. This study result showed that BASE decreased MDA production. The IC50 values of the extract (0.55 µg/mL), and ascorbic acid (1.10 µg/mL) were shown to have a strong inhibitory effect significantly (p < 0.05) on Fe2+ induced lipid peroxidation in egg-yolk homogenates. The decrease in the Fe2+-induced lipid peroxidation in the egg-yolk homogenates in the presence of the extract could be due to the capacity of the extracts to scavenge free radicals and/or chelate ferrous ions.
In the ferric chloride assay, A reducing power assay was used to measure the free radical scavenging antioxidant activities of the crude extracts. The reducing agents in the sample reduce the ferric ion to the ferrous form. Higher reducing agents’ concentration means a large amount of ferrous ions60. Reduction of ferric ions and/or iron chelation would cause a decrease in free radicals formation61. In this study, the iron-reducing capacity of BASE was estimated from its ability to reduce the ferricyanide (Fe3+) to the ferrous (Fe2+) form by donating an electron. The extract showed a good reducing power capacity, which was concentration-dependent. This study’s results are consistent with the data published previously on methanolic leaf extract of the B. abyssinica. The antioxidant activity and reducing power capacity of the extracts were likely due to the presence of polyphenols and flavonoids, which can act as free radicals scavengers by donating hydrogen or electrons.
Hydroxyl radicals are the most harmful reactive oxygen species which are major contributors to oxidative damage in many biological systems62. In vitro, assays for the hydroxyl radical scavenging capacity are usually based on the scavenging activity of hydroxyl radicals. The extract showed good hydroxyl radical scavenging activity. The polyphenolic compounds especially terpenoids and flavonoids ability to quench hydroxyl radicals might directly relate to the prevention of lipid peroxidation63,64.
Because of the rise in antimicrobial-resistant microorganisms, the use of compounds extracted from medicinal plants may be helpful in the development of antimicrobial agents65. This study result showed promising antibacterial activities of the BASE against S. aureus, E. coli, and P. aeruginosa, with a zone of inhibition 15.7 ± 2.5 mm, 16.0 ± 0.0 mm, and 16.7 ± 1.5 mm, respectively. The positive standard, ceftriaxone, gave the zones of inhibition for S. aureus (33.3 ± 2.5 mm), E. coli (31.1 ± 2.1 mm), and P. aeruginosa (28.8 ± 0.2 mm) were in line with the published zones of inhibitions; 25–31 mm, 29–35 mm, and 18–22 mm, respectively66. The extract exhibited good antibacterial activities against S. aureus and E. coli when compared with a previous study39. Further comparison with literature reported for the stem bark extract that reported zone inhibition diameter of 15 mm and 10 mm S. aureus and P. aeruginosa respectively67. However, this study’s results show better antimicrobial activities compared to the methanolic stem bark extracts of B. abyssinica. The study found better antibacterial activity at lower concentrations against similar bacterial strains compared with the root, stem, and leaf extract of the same plant.
BASE showed zones of inhibition of 24.7 ± 1.5 mm and 22.0 ± 4.4 mm against C. albican and Malassezia spp., respectively whereas ketoconazole gave zones of inhibitions C. albican (22.0 ± 2.0 mm) and M. furfur (22.0 ± 4.0 mm) which is consistence with other studies68,69. Methanolic stem extract of B. abyssinica showed a 5.5 mm zone of inhibition for C. albican67 whereas this study results showed 24.7 ± 1.5 mm for C. albican. The difference in antimicrobial activities with other studies might be possibly due to the difference in concentration and types of antimicrobial agents in different parts of the study plant, environmental factors, and the laboratory method.
Conclusion
In this study, the seeds of B. abyssinica were macerated and hydro-distilled to explore its biological activities. The seeds of B. abyssinica showed better antioxidant activities; however, they demonstrated good antimicrobial potential against S. aureus, P. aeruginosa, and E. coli and superior antifungal activities against C. albicans and Malassezia spp. Overall, the results of the study suggest that the composition of seeds of B. abyssinica is responsible for its strong antioxidant and antimicrobial properties. Therefore, seeds of B. abyssinica will be a potential source for the discovery of drugs. However, the compounds responsible for the antioxidative and antimicrobial activity are currently unclear. Therefore, further investigation is needed to isolate, identify, and characterize the antioxidant and antimicrobial compounds present in the seeds of the study plant.
Data availability
The data used to support the findings of the present study are available from the corresponding author.
Abbreviations
- ATCC:
-
American type culture collection
- BASE:
-
Bersama abyssinica seed extract
- CFU:
-
Colony forming unity
- DMSO:
-
Dimethyl sulfoxide
- DPPH:
-
Diphenyl picrylhydrazyl
- GC–MS:
-
Gas chromatography–mass spectroscopy
- FRS:
-
Free radical scavenging
- HRSC:
-
Hydroxyl radicals’ scavenging capacities
- IC50 :
-
Half inhibition concentration
- MDA:
-
Malondialdehyde
- NIST:
-
National Institute of Technology and Standards
- TBARS:
-
Thiobarbituric acid reactive species
References
Kaliyaperumal, K., Kaliyaperumal, J., Jegajeevanram, V. & Embialle, M. Traditional medicinal plants: A source of phytotherapeutic modality in resource-constrained health care settings. J. Evid. Based Complement. Altern. Med. 18(1), 67–74. https://doi.org/10.1177/2156587212460241 (2013).
Aragaw, T. J., Afework, D. T. & Getahun, K. A. Assessment of knowledge, attitude, and utilization of traditional medicine among the communities of Debre Tabor Town, Amhara Regional State, North Central Ethiopia: A cross-sectional study. J. Evid. Based Complement. Altern. Med. 20(20), 1–10. https://doi.org/10.1155/2020/6565131 (2020).
Teka, A. et al. Medicinal plant use practice in four ethnic communities (Gurage, Mareqo, Qebena, and Silti), south-central Ethiopia. J. Ethnobiol. Ethnomed. 16(1), 1–12. https://doi.org/10.1186/s13002-020-00377-1 (2020).
Kuadio, I. S. et al. Biopotential of Bersama abyssinica Fresen stem bark extracts: UHPLC profiles, antioxidant, enzyme inhibitory, and antiproliferative propensities. J. Antioxid. 9(163), 1–19. https://doi.org/10.3390/antiox9020163 (2020).
Thabo, M., Dulcie, M. & Geoff, N. Triterpenoids from Bersama swinnyi. J. Phytochem. 49(6), 1819–1820. https://doi.org/10.1016/s0031-9422(98)00306-9 (1998).
Okello, S. V., Nyunja, R. O., Netondo, G. W. & Onyango, J. C. Ethnobotanical study of medicinal plants used by sabaots of Mt. Elgon Kenya. Afr. J. Trad. Complement. Altern. Med. 7(1), 1–10. https://doi.org/10.4314/ajtcam.v7i1.57223 (2010).
Bekele, G. & Ramachandra, R. Ethnobotanical study of medicinal plants used to treat human ailments by Guji Oromo Tribes in Abaya District, Borana, Oromia, Ethiopia. Univ. J. Plant Sci. 3(1), 1–8. https://doi.org/10.13189/ujps.2015.030101 (2015).
Yayesh, L., Shemsu, U. & Messay, W.-M. Ethnobotanical study on traditional medicinal plants in Dega Damot Woreda, Amhara Region, North Ethiopia. IJRPC 5(2), 258–273 (2015).
Solome, M. T., Tiruzer, B. G., Mohammedbrhan, A. & Tezera, J. A. Wound healing activities of hydromethanolic crude extract and solvent fractions of Bersama abyssinica leaves in mice. eCAM 21(21), 1–20. https://doi.org/10.1155/2021/9991146 (2021).
Abebe, W. An overview of Ethiopian traditional medicinal plants used for cancer treatment. EJMP 14(4), 1–16. https://doi.org/10.9734/EJMP/2016/25670 (2016).
Bizuneh, W., Reta, R., Tibebu, A. & Moa, M. Medicinal plants used for treatment of diarrhoeal related diseases in Ethiopia. eCAM 18, 1–21. https://doi.org/10.1155/2018/4630371 (2018).
Kidane, B., van Andel, T., van der Maesen, L. J. G. & Zemede, A. Use and management of traditional medicinal plants by Maale and Ari ethnic communities in southern Ethiopia. J. Ethnobiol. Ethnomed. 10(46), 1–15 (2014).
Nigatu, T., Beyene, P. & Zemede, A. Medicinal plants used by traditional healers to treat malignancies and other human ailments in Dalle District, Sidama Zone. Ethiopia. J. Ethnobiol. Ethnomed. 14(15), 1–21. https://doi.org/10.1186/s13002-018-0213-z (2018).
Teka, A. et al. In vitro antimicrobial activity of plants used in traditional medicine in Gurage and Silti Zones, south-central Ethiopia. BMC Complement. Altern. Med. 15(286), 1–7. https://doi.org/10.1186/s12906-015-0822-1 (2015).
Jelic, M. D. et al. Oxidative stress and its role in cancer. J. Cancer Res. Therap. 17(1), 22–28. https://doi.org/10.4103/jcrt.JCRT_862_16 (2021).
Bilgen, F. et al. The effect of oxidative stress and Raftlin levels on wound healing. Int. Wound J. 16(5), 1178–1184. https://doi.org/10.1111/iwj.13177 (2019).
Yaribeygi, H., Sathyapalan, T. & Atkin, S. L. Molecular mechanisms linking oxidative stress and diabetes mellitus. Oxid. Med. Cell Longev. 2020, 1–13. https://doi.org/10.1155/2020/8609213 (2020).
Ty, M. C. et al. Malaria inflammation by xanthine oxidase-produced reactive oxygen species. EMBO Mol. Med. 11(8), 9903. https://doi.org/10.15252/emmm.201809903 (2019).
Foudah, A. I. et al. Evaluation of the composition and in vitro antimicrobial, antioxidant, and anti-inflammatory activities of Cilantro (Coriandrum sativum L. leaves) cultivated in Saudi Arabia (Al-Kharj). Saudi J. Biol. Sci. 28(6), 3461–3468. https://doi.org/10.1016/j.sjbs.2021.03.011 (2021).
World Health Organization. Fact Sheets Antimicrobial Resistance. https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance (Accessed 17 November 2021) (2022).
Spernovasilis, N., Tsiodras, S. & Poulakou, G. Emerging and re-emerging infectious diseases: Humankind’s companions and competitors. J. Microorg. 10(1), 98. https://doi.org/10.3390/microorganisms10010098 (2022).
Lobo, V., Patil, A., Phatak, A. & Chandra, N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn. Rev. 4(8), 118. https://doi.org/10.4103/0973-7847.70902 (2010).
ElevationMap. Topographic Map of Semar Semarye, Sude, Arsi, Ethiopia. http://elevationmap.net/semar-semarye-sude-arsi-et-1011130067 (Accessed 12 May 2022) (2018).
Tegelberg, R., Virjamo, V. & Julkunen-Tiitto, R. Dry-air drying at room temperature—A practical pre-treatment method of tree leaves for quantitative analyses of phenolics. Phytchem. Anal. https://doi.org/10.1002/pca.2755) (2018).
Parbuntari, H., Prestica, Y., Gunawan, R., Nurman, M. & Adella, F. Preliminary phytochemical screening (qualitative analysis) of cacao leaves (Theobroma cacao L.). EKSAKTA 19(2), 40–48. https://doi.org/10.24036/eksakta/vol19-iss02/142 (2018).
Junaid, R. S. & Patil, M. K. Qualitative tests for preliminary phytochemical screening: An overview. Int. J. Chem. Stud. 8(2), 603–608. https://doi.org/10.22271/chemi.2020.v8.i2i.8834 (2020).
Daisy, S., Ramesh, V. M. & Arunachalam, M. In vitro antidermatophytic activity of bioactive compounds from selected medicinal plants. J. Anal. Sci. Technol. 12(53), 1–13. https://doi.org/10.1186/s40543-021-00304-3 (2021).
Nagaraju, K., Anusha, D., Chitra, K. & Ravi, B. K. Preliminary analysis of phytoconstituents and evaluation of anthelminthic property of Cayratia auriculata (in vitro). J. Clin. Med. 14(4), 350–356. https://doi.org/10.26574/maedica.2019.14.4.350 (2019).
Rohit, K. B. Preliminary test of phytochemical screening of crude ethanolic and aqueous extract of Moringa pterygosperma Gaertn. J. Pharmacogn. Phytochem. 4(1), 7–9 (2015).
Ramya, P., Vasanth, P. M., Prasad, P. V. & Sarath, B. V. Qualitative phytochemical screening tests of Alpinia galanga L.. World J. Pharmac. Res. 8(5), 1064–1077 (2019).
Peter, M. & Maano, V. M. Phytochemical investigation, antioxidant and antimycobacterial activities of Schkuhria pinnata (Lam) thell extracts against Mycobacterium smegmatis. JEBIM 24(19), 1–8. https://doi.org/10.1177/2515690X19866104 (2019).
Mohammadi, M. S., Shahidi-Motlagh, S., Bagherzadeh, H., Azad Forouz, S. & Tafazoli, H. Evaluation of antioxidant activity of Ruta graveolens L. extract on inhibition of lipid peroxidation and DPPH radicals and the effects of some external factors on plant extract’s potency. RJP 1(2014), 45–50 (2014).
Ahmed, D., Fatima, M. & Saeed, S. Phenolic and flavonoid contents and anti-oxidative potential of epicarp and mesocarp of Lagenaria siceraria fruit: A comparative study. Asian Pac. J. Trop. Med. 7(1), 249–255. https://doi.org/10.1016/S1995-7645(14)60241-8 (2014).
Yuan, C. et al. Radical scavenging activities of novel cationic inulin derivatives. MDPI Polym. 10(1295), 1–11. https://doi.org/10.3390/polym10121295 (2018).
Ebani, V. V. et al. Antimicrobial activity of essential oils against Staphylococcus and Malassezia strains isolated from canine dermatitis. MDPI Microorg. 8(252), 252. https://doi.org/10.3390/microorganisms8020252 (2020).
Valgas, C. et al. Screening methods to determine antibacterial activity of natural products. Braz. J. Microbiol. 38, 369–380. https://doi.org/10.1590/S1517-83822007000200034 (2007).
Magaldi, S. et al. Well diffusion for antifungal susceptibility testing. Int. J. Infect. Dis. 8(1), 39–45. https://doi.org/10.1016/j.ijid.2003.03.002 (2004).
Ebrahimabadi, A. H. et al. Essential oil composition and antioxidant and antimicrobial properties of the aerial parts of Salvia eremophila Boiss. from Iran. Food Chem. Toxicol. 48(5), 1371–1376. https://doi.org/10.1016/j.fct.2010.03.003 (2010).
Fitsum, L., Solomon, G., Kebede, S. & Milkyas, E. Antibacterial steroids from roots of Bersama abyssinica. Ethiop. J. Sci. Sustain. Dev. 7(1), 1–8. https://doi.org/10.20372/ejssdastu:v7.i1.2020.156 (2020).
Mathewos, A., Feleke, W., Solomon, L., Fikre, M. & Milkyas, E. Phytochemical screening and antibacterial activity of leaves extract of Bersama abyssinica. J. Adv. Bot. Zool. 3(2), 1–6. https://doi.org/10.15297/JABZ.V3I2.07 (2015).
Watcho, P. et al. Hypoglycemic and hypolipidemic effects of Bersama engleriana leaves in nicotinamide/streptozotocin-induced type 2 diabetic rats. BMC Complement. Altern. Med. 12(264), 1–6 (2012).
Belkheir, A. K. et al. Benzophenone synthase and chalcone synthase accumulate in the mesophyll of Hypericum perforatum leaves at different developmental stages. Front. Plant Sci. 7, 921. https://doi.org/10.3389/fpls.2016.00921 (2016).
Verma, N. & Shukla, S. Impact of various factors responsible for fluctuation in plant secondary metabolites. J. Appl. Res. Med. Aromat. Plants 2(4), 105–113. https://doi.org/10.1016/j.jarmap.2015.09.002 (2015).
Zekeya, N. et al. Potential of natural phenolic antioxidant compounds from Bersama abyssinica (Meliathacea) for treatment of chronic diseases. Saudi J. Biol. Sci. 29(6), 1–7. https://doi.org/10.1016/j.sjbs.2022.03.023 (2022).
Gemechu, A., Aseer, M. & Akbar, I. Phytochemical analysis and antimicrobial activity of Bersama abyssinica Fresen against multidrug-resistant bacterial uropathogens: Picolinyl hydrazide is a major compound. J. Herb. Spices Med. Plants 25(4), 389–400. https://doi.org/10.1080/10496475.2019.1635940 (2019).
Salehi, B. et al. Therapeutic potential of α- and β-pinene: A miracle gift of nature. MDPI Biomol. 9(11), 1–34. https://doi.org/10.3390/biom9110738 (2019).
Zengin, H. & Baysal, A. H. Antibacterial and antioxidant activity of essential oil terpenes against pathogenic and spoilage-forming bacteria and cell structure-activity relationships evaluated by SEM microscopy. J. Mol. 19(11), 17773–17798. https://doi.org/10.3390/molecules191117773 (2014).
de Oliveira, T. M. et al. Evaluation of p-cymene, a natural antioxidant. Pharm. Biol. 53(3), 423–428. https://doi.org/10.3109/13880209.2014.923003 (2015).
Wang, C. Y., Chen, Y. W. & Yao, H. C. Antioxidant and antibacterial activity of seven predominant terpenoids. Int. J. Food Prop. 22(1), 230–238. https://doi.org/10.1080/10942912.2019.1582541 (2019).
Zhang, J. H., Sun, H. L., Chen, S. Y., Zeng, L. & Wang, T. T. Anti-fungal activity, mechanism studies on α-phellandrene and nonanal against Penicillium cyclopium. Bot. Stud. 58(13), 1–19. https://doi.org/10.1186/s40529-017-0168-8 (2017).
Paudel, M. R. & Chand, M. B. Assessment of antioxidant and cytotoxic activities of extracts of Dendrobium crepidatum. MDPI Mol. 9(9), 478–91. https://doi.org/10.3390/biom9090478 (2019).
Raghavendra, H. & Prashith, K. T. R. Antiradical and lipid peroxidation inhibitory activity of ripe and unripe fruit of Rubus steudneri Schweinf. (Rosaceae). Pharmacogn. J. 10(4), 818–822 (2018).
Kouadio, I. S. et al. A comparative study of the HPLC-MS profiles and biological efficiency of different solvent leaf extracts of two African plants: Bersama abyssinica and Scoparia dulcis. Int. J. Environ. Health Res. 31, 1–14. https://doi.org/10.1080/09603123.2019.1652885 (2019).
Tauchen, J. et al. In vitro antioxidant and anti-proliferative activity of Ethiopian medicinal plant extracts. Ind. Crops Prod. 74, 671–679. https://doi.org/10.1016/j.indcrop.2015.05.068 (2015).
Kim, J. S. Evaluation of in vitro antioxidant activity of the water extract obtained from dried pine needle (Pinus densiflora). Prev. Nutr. Food Sci. 23(2), 134–143. https://doi.org/10.3746/pnf.2018.23.2.134 (2018).
Abeyrathne, E. D., Nam, K. & Ahn, D. U. Analytical methods for lipid oxidation and antioxidant capacity in food systems. MDPI Antioxid. 10(1587), 1–19. https://doi.org/10.3390/antiox10101587 (2021).
Niki, E., Yoshida, Y., Saito, Y. & Noguchi, N. Lipid peroxidation: Mechanisms, inhibition, and biological effects. Biochem. Biophys. Res. Commun. 338(1), 668–676. https://doi.org/10.1016/j.bbrc.2005.08.072 (2005).
Saito, M. Polychlorinated biphenyls-induced lipid peroxidation as measured by thiobarbituric acid-reactive substances in liver subcellular fractions of rats. Biochim. Biophys. Acta 1046(3), 301–308. https://doi.org/10.1016/0005-2760(90)90245-S (1990).
Ogunmefun, O. T., Fasola, T. R., Saba, A. B. & Akinyemi, A. J. Inhibitory effect of Phragmanthera incana (Schum.) Harvested from Cocoa (Theobroma cacao) and Kolanut (Cola nitida) trees on Fe(2+) induced lipid oxidative stress in some rat tissues—In vitro. Int. J. Biomed. Sci. 11(1), 16–22 (2015).
Akomolafe, S. F., Oboh, G., Akindahunsi, A., Akinyemi, A. & Tade, O. Inhibitory effect of aqueous extract of stem bark of Cissus populnea on ferrous sulphate- and sodium nitroprusside induced oxidative stress in rat’s testes in vitro. ISRN Pharmacol. 2013, 1–8. https://doi.org/10.1155/2013/130989 (2013).
Nehir, E. S. & Sibel, K. Radical scavenging and iron-chelating activities of some greens used as traditional dishes in Mediterranean diet. Int. J. Food Sci. Nutr. 55(1), 67–74. https://doi.org/10.1080/09637480310001642501 (2004).
Anna, M., Magdalena, J., Dawid, S., Tomasz, C. & Łukasz, M. Anticancer, antioxidant, and antibacterial activities of low molecular weight bioactive subfractions isolated from cultures of wood degrading fungus Cerrena unicolor. PLoS ONE 13(6), 1–14. https://doi.org/10.1371/journal.pone.0197044 (2018).
Moukette, B. M. et al. In vitro antioxidant properties, free radicals scavenging activities of extracts and polyphenol composition of a non-timber forest product used as spice: Monodora myristica. Biol. Res. 48(15), 1–17. https://doi.org/10.1186/s40659-015-0003-1 (2015).
Mathew, S., Emilia, A. T. & Akmar, Z. Z. Reactivity of phenolic compounds towards free radicals under in vitro conditions. J. Food Sci. Technol. 52(9), 5790–5798. https://doi.org/10.1007/s13197-014-1704-0 (2015).
De Zoysa, M. H. N., Rathnayake, H., Hewawasam, R. P. & Wijayaratne, W. M. D. G. B. Determination of in vitro antimicrobial activity of five Sri Lankan medicinal plants against selected human pathogenic bacteria. Int. J. Microbiol. 2019(2019), 1–8. https://doi.org/10.1155/2019/7431439 (2019).
Sader, H. S., Castanheira, M., Flamm, R. K., Farrell, D. J. & Jones, R. N. Antimicrobial activity of ceftazidime-avibactam against Gram-negative organisms collected from US medical centers in 2012. Antimicrob. Agents Chemother. 58(3), 1684–1692. https://doi.org/10.1128/AAC.02429-13 (2014).
Amuka, O., Machocho, A. K., Okemo, P. O. & Mbugua, P. K. Antifungal and antibacterial activity of crude stem bark extracts’ of Bersama abysinicca Verdc. and Faurea saligna Harr. research. J. Med. Plant 9(4), 160–169. https://doi.org/10.3923/rjmp.2015.160.169 (2015).
Mussin, J. E. et al. Antifungal activity of silver nanoparticles in combination with ketoconazole against Malassezia furfur. AMB Expr. 9(131), 1–9. https://doi.org/10.1186/s13568-019-0857-7 (2019).
Anasane, N. et al. Acidophilic actinobacteria synthesized silver nanoparticles showed remarkable activity against fungi-causing superficial mycoses in humans. J. Mycoses 59(3), 157–166. https://doi.org/10.1111/myc.12445 (2016).
Acknowledgements
The authors are grateful to Dilla University, Ethiopia, for sponsoring the study. They acknowledge the University of Gondar for coordination and assistance in laboratory work. They also thank Mr. Getnet Chekol, botanist, for his assistance in identifying the study plant species. They would like to express our gratitude to Dr Worku Negash, Mr. Bogale Damtew, Mr. Kebede Shenkute, Mr. Endalekachew Gugsa, Mr. Wubet Tizazu, Ms. Emebet Getaneh, Mr. Teshome Gezahegn, Mr. Atrsaw Adhanom, and Mr. Dawud Mola for their support during conducting experiments. Our thank goes to Mr. Asegid Ayalew, Mr. Belayhun Ababu, and Mr. Getaw Alemu for their support and assistance in collecting seeds for the study plant.
Author information
Authors and Affiliations
Contributions
Belayhun Alemu performed experiments, analyzed the data, interpreted the data, and wrote the paper. Meseret Derbew, Tadesse Asmamaw, and Hiwot Tezera Aman Dekebo supervise research. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Alemu, B., Molla, M.D., Tezera, H. et al. Phytochemical composition and in vitro antioxidant and antimicrobial activities of Bersama abyssinica F. seed extracts. Sci Rep 14, 6345 (2024). https://doi.org/10.1038/s41598-024-56659-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-56659-1
Keywords
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.