Abstract
Thermal tolerance is a critical factor influencing the survival of living organisms. This study focuses on the thermal resistance of copepod species, Thermocyclops crassus (Fischer, 1853) and T. oithonoides (Sars G.O., 1863), with overlapping distribution ranges in Europe. Short-term heat shock experiments were conducted to assess the thermal resistance of these copepods, considering various temperature increments and exposure durations. Additionally, the study explored the influence of heat shock on egg sac shedding, a vital indicator of population dynamics. Results indicate that widely distributed T. crassus exhibits higher thermal tolerance compared to narrowly distributed T. oithonoides, with survival rates varying under different heat shock conditions. Furthermore, T. crassus demonstrated a quicker response in dropping egg sacs in response to thermal stress, suggesting a potential adaptive mechanism for the survival of adults. However, rapid egg sac droppings pose high risks for eggs facing unfavorable conditions. T. crassus, inhabiting environments with greater temperature fluctuations such as the littoral and pelagial zones, exhibited better survival mechanisms compared to T. oithonoides, which predominantly resides in the pelagic zone. The findings have implications for understanding copepod responses to global warming and thermal pollution. This research contributes insights into the adaptive strategies of thermophilic copepod species and their ecological consequences.
Similar content being viewed by others
Introduction
Thermal tolerance is crucial for survival of any living organism1. Species exhibit varying degrees of tolerance to environmental factors, with death occurring if the tolerance limit of a particular species is exceeded2,3. The ability of a species to resist the new thermal conditions may determine how vulnerable it is to temperature increases brought on by climate change4,5,6,7. Along with long-term increases in temperature, other warming events could affect aquatic animals. The thermoelectric power sector is one of the principal contributors of freshwater thermal pollution8. The ability to withstand heat stress may help animals expand their geographic ranges or adjust to changing climatic circumstances.
Copepod heat tolerance increases as annual temperature of their occurrence increases9. In order to better understand the thermal resistance of aquatic organisms, short-term shock may be used as a predictor of resistance to thermal stresses10,11. Previous work on thermal performance and thermal resistance mainly focused on marine and intertidal copepods, mostly related to Calanoida and Harpacticoida9. Studies on freshwater Cyclopoida in the context of thermal performance are rare. Thermocyclops Kiefer, 1927 (Crustacea, Copepoda, Cyclopoida) is a genus with a large variety of species that inhabit mainly tropics but occur also in temperate regions12,13. This genus is important component of zooplankton communities in many tropical and temperate water bodies14,15,16. Thermocyclops crassus (Fischer, 1853) is considered a cosmopolitan species because its range is dispersed across the globe while the range of T. oithonoides (Sars G.O., 1863) is restricted to Europe. The distribution ranges of T. crassus and T. oithonoides overlap across Europe (Fig. 1). Thermocyclops species found in tropical areas reproduce year-round, while species found in temperate zones reproduce in the warm season only16, therefore all known species in this genus are consider as thermophiles. T. crassus inhabits lakes, rivers, reservoirs, ponds, streams, marshes, typically related to pelagial and litoral zone17. T. oithonoides inhabits mainly pelagial zone of lakes, however is sometimes noted in large rivers and reservoirs17,18.
The aim of this study is to determine whether the current geographical distribution of Thermocyclops crassus and T. oithonoides reflects those species’ tolerance to temperature stress. To test the thermal resistance of copepods, experiments with various heat shock conditions and exposure duration were carried out. The impact of heat shock on egg sac shedding was also studied which is an additional indicator of population characteristics.
Results
Survival analysis
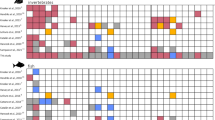
In laboratory conditions, T. oithonoides proved to be a more sensitive to temperature stress than T. crassus (Fig. 2). Despite noticeable differences in survival under control conditions, these distinctions did not reach statistical significance (p > 0.05). Starting from the smallest temperature shock of 5 °C lasting for 30 min, a gradual divergence in the survival curves of the investigated copepods was observed. These differences escalated with the increase in thermal shock intensity and the duration of exposure to this stressor. At a 10 °C thermal shock lasting for 60 min, a substantial decrease in the survival of T. oithonoides was noted. Under these conditions, on the sixth day of the experiment, nearly half of the T. oithonoides population succumbed, whereas only 10% of T. crassus perished. With a 15 °C shock, these differences further amplified, and following a 30-min exposure, 90% of the T. oithonoides population was deceased, while over 90% of the T. crassus population remained viable. A brief 20 °C shock was survivable only by T. crassus individuals, though they did not endure prolonged exposure to this stressor.
Kaplan–Meier survival curves for different exposure durations (a-d – 10 min; e–h – 30 min; i-k – 60 min; l – control) and thermal shock scenarios (a, e, i – 25 °C; b, f, j – 30 °C; c, g, k – 35 °C; d, h – 40 °C). The results of the log-rank test indicate statistical significance at a level of p < 0.05. Circles indicate deceased copepods, and crosses represent censored data.
Egg sacs dropping
In control conditions, T. crassus exhibited a faster egg sac dropping rate compared to T. oithonoides (Fig. 3). On the first day of the experiment under control conditions, none of the T. oithonoides individuals dropped egg sacs, while during the same period, 40% of the T. crassus population released egg sacs. Both investigated species demonstrated a response to thermal stress and its duration by releasing egg sacs. T. crassus exhibited a quicker response to thermal stress than T. oithonoides. On the first day of the experiment, at a + 5 °C shock, 63–67% of the T. crassus population dropped egg sacs, at + 10 °C, 77–90% dropped egg sacs, at + 15 °C, 83–87% dropped egg sacs, and at + 20 °C, 97% dropped egg sacs. Meanwhile, T. oithonoides at + 5 °C dropped egg sacs in 0–3% of the population, at + 10 °C, 3–17% dropped egg sacs, and at + 15 °C, 13–50% dropped egg sacs. Only on the second day of the experiment was a significant increase observed in the egg sac dropping by T. oithonoides. On the fourth day of the experiment, under control conditions, 80% of the T. crassus population dropped egg sacs, at + 5 °C, 87–90% dropped egg sacs, at + 10 °C, 93–97% dropped egg sacs, and at + 15 °C and + 20 °C, 100% dropped egg sacs. During the same period, in control conditions, 50% of the T. oithonoides population dropped egg sacs, at + 5 °C, 63–87% dropped egg sacs, at + 10 °C, 90–100% dropped egg sacs, and at + 15 °C, 97–100% dropped egg sacs. In cases where survival on the initial day of the experiment was 0, indicating that copepods did not survive beyond the first day, egg sacs were not dropped before their death.
The ANOVA results indicated that temperature was a decisive factor in relation to egg sacs dropping, while exposure time did not play a significant role in this case (Table 1). A stronger impact of temperature was observed for T. crassus (p = 0.0003) than for T. oithonoides (p = 0.0430).
Discussion
Species may be able to withstand temperature extremes higher than those to which they are typically exposed19. Despite both Thermocyclops species being acclimated to the same thermal conditions and developing their populations in the same central European region, their responses to thermal stress differed. The limited studies on intraspecific variability in copepod temperature tolerance indicate that some copepods exhibit diverse responses to thermal stress, depending on the local conditions to which they have adapted20,21,22. Pereira et al.21, studying the mechanisms driving thermal adaptation in the intertidal copepod Tigriopus californicus (Baker, 1912), revealed significant variations in both genetic adaptation and developmental phenotypic plasticity among populations. Hence, the increased resilience observed in T. crassus may indicate genetic differences between the studied species, potentially affecting both acclimation capacity and/or developmental temperature. The results of our study suggest that the geographic occurrence range of Thermocyclops species may be impacted by their resistance to thermal stress; species like T. crassus that possess traits supporting its survival under conditions of thermal stress may be able to survive across a wide range of habitats.
In only a few taxonomic groups has the association between heat tolerance and latitudinal ranges of species been assessed19. In general, a species’ latitudinal range reflects its thermal tolerance, where heat tolerance corresponds to the peak summer temperature of its range1. However, temperature differences between regions at the same latitude are substantial. Latitude is a proxy indicator of temperature; hence, it does not always accurately reflect the thermal characterization of a region where a species occurs. The maximum temperature in the warmest months of the year served as our reference point. Maximum temperatures of locations where T. crassus has occur (according to species distribution presented in Fig. 1) are reaching 32 °C. Verbitsky et al.23, studying T. crassus populations in East Europe, concluded that the optimum thermal conditions for T. crassus are 25–30 °C, and a temperature of 34 °C is lethal for this species. We demonstrated that a high percentage of the population could survive temporary exposure to a temperature of 35 °C. Our results confirm the ability of T. crassus to withstand strong thermal stress, induced suddenly without acclimatization to conditions with elevated temperatures. This characteristic enables T. crassus expansion; therefore, this species is observed in many areas with a tropical climate. Despite T. oithonoides thermal preferences (this species occurs in Central Europe during the summer) suggesting a spread towards the equator, its range is limited to a maximum air temperature of 24.8 °C in Central Europe and 27 °C in South Europe. The limited ability of this species to survive thermal stress, on the one hand, and its narrow, restricted range associated with temperature, on the other hand, suggests that it is a thermophilic but also stenothermic species.
To better comprehend the enhanced thermotolerance of T. crassus, consideration should be given not only to the current distribution of species but also to migrations over the past few thousand years. It is speculated that during the last glaciation, T. oithonoides was present on the southeastern edge of the retreating ice sheet. After the glacier’s retreat, those niches were occupied by this species. In contrast, warmer refugia in Asia might have been inhabited by T. crassus (which suggests the current distribution of the species). Therefore, the colonization of Central European areas by T. crassus after the glacial retreat could have been carried out by populations of eastern origin that were previously adapted to higher temperatures.
T. crassus exhibits better-developed survival mechanisms under thermal stress. The genus Thermocyclops, most likely of tropical origin24, probably possesses mechanisms to mitigate excessive thermal stress. However, T. oithonoides, whose range is associated with temperate climates, has seemingly lost some of its heat stress mitigation potential. We hypothesize that this divergence is due to different habitat preferences. The littoral environment is more thermally variable due to the wind-driven water masses and the faster heating and cooling of shallower water layers25. T. crassus, which resides both pelagial and littoral habitats, is exposed to greater temperature amplitudes than T. oithonoides, which predominantly inhabits pelagial of lakes. The potential for T. crassus to competitively replace T. oithonoides is substantial, owing to the former’s broader range of thermotolerance. This observation aligns with research conducted on closely related marine cyclopoids, including the indigenous Oithona nana Giesbrecht, 1893 and the invasive O. davisae Ferrari F.D. & Orsi, 1984, as documented by Isinibilir et al.26.
Rahlff27, in a study of the molecular effects of heat shock on the species Acartia tonsa Dana, 1849 and Eurytemora affinis (Poppe, 1880), demonstrated that exposure to high temperature induces molecular changes, specifically an increase in the production of heat shock proteins, even in the absence of a visible reaction in copepods. The production of thermal proteins rises with increasing temperature, playing a role in maximizing the chances of surviving heat shock by repairing damage at the molecular level28. Low et al.2, investigating thermal stress in the widely distributed tropical copepod Pseudodiaptomus annandalei Sewell, 1919, revealed that maximal expressions of hsp70 and hsp90 protein genes occur at 32–33 °C. They found that maximum protein expression occurred 3 °C-3.5 °C above the mean water temperature (29.32 °C) of the copepod in the field. Individuals producing more thermal proteins can withstand greater thermal shock. Therefore, it can be inferred that in T. crassus, the production of these proteins may be higher than in T. oithonoide, resulting in greater thermal shock tolerance. Alternatively, T. crassus may have higher thermal limits and thus experience less stress than T. oithonoides at any given temperature, potentially resulting in lower expression of heat shock proteins. Further research on this variability at the molecular level is necessary to demonstrate whether T. crassus exhibits greater activity of proteins associated with the organism’s response to thermal stress compared to T. oithonoides.
The release of egg sacs in T. crassus was faster than in T. oithonoides, although both species were sensitive to thermal stress, leading to a more frequent release of egg sacs as the temperature increases. The ability for a quicker release of egg sacs may be an adaptation that enhances survival in unfavorable thermal conditions. Examples are known among other animals (eg. king penguin Aptenodytes patagonicus J. F. Miller, 1778) where females, subjected to strong stress, abandon eggs29. Moreover, in some crustaceans (crabs: Lithodes santolla (Molina, 1782) and Paralomis granulosa (Hombron & Jacquinot, 1846)) egg loss were observed in stress conditions30. There are examples in copepods where unattended eggs successfully hatched, especially in Calanoida11,31. Recent studies on copepod survival capabilities in challenging environmental conditions suggest that the release of eggs subjected to thermal shock could be a mechanism increasing not only the female’s chances of survival but also enabling the survival of the offspring. Bartholmeé et al.32, conducted an experiment to assess if the egg sacs of calanoids (Eudiaptomus gracilis (Sars G.O., 1863), E. graciloides (Lilljeborg, 1888)) and cyclopoids (Cyclops abyssorum Sars G.O., 1863, Macrocyclops albidus (Jurine, 1820)) passing through the digestive system of a fish can survive. They found that a total of 50–70% of the calanoid eggs and 11–29% of the cyclopoid eggs survived ingestion and gut passage. However, in our experiment, we did not monitor the hatching of young individuals from the released egg sacs. The shedding of egg sacs may serve as an adaptation for adult females to survive stressful conditions, as it enables quicker escape and reduces energy expenditure in case of increased effort33. Shedding egg sacs can present both opportunities and risks for the released eggs. It serves as an opportunity when the egg sacs fall onto favorable environmental conditions (e.g., well-oxygenated surfaces devoid of predators), while posing a risk if the eggs land in an unfavorable zone for their survival (e.g., oxygen-deprived zone or with limiting biological factors). However, this matter necessitates further research.
The obtained results also have implications for zooplankton assemblages affected by thermal pollution. Changes in abundance and zooplankton communities were investigated in cooling canals at the Konin and Pątnów power plants, Poland34. Tunowski34 demonstrated that a change as small as 7–8 °C leads to a decrease in the abundance of crustacean zooplankton ranging from 44 to 60%. In the case of the studied species, T. oithonoides, being a more sensitive species, has lower chances of surviving thermal shock compared to T. crassus. As each species has specific resilience to stressful conditions, communities under thermal pressure likely shift towards species characterized by greater resistance to thermal stress.
Richardson35 conducted research on the potential impact of global warming on zooplankton. The results obtained indicated that even a slight increase in temperature will causes a decrease in zooplankton abundance. Consequently, if climate change disrupts the zooplankton population, the induction of a short-term thermal shock associated with thermal pollution will lead to synergy of harmful conditions and reduction in copepod survival. As an organism capable of entering diapause, Thermocyclops possesses excellent abilities to survive adverse thermal conditions during the winter period (low temperature). In T. oithonoides, diapause occurs in copepodid stages IV–V during winter, either in sediments or in the pelagic zone14. However, in the case of climate changes leading to an increase in maximum temperatures, these thermal changes during the summer, may impact Thermocyclops to some extent. It is conceivable that an extension of the growing season due to global warming could lead to an elongation of the period where Thermocyclops reproduce. Nilssen and Wærvågen14 demonstrated, based on studies of T. oithonoides populations, that in the cold lakes in Norway, this species exhibited only one generation per year, while in warmer lakes, there could be two or three generations. Therefore it is likely that Thermocyclops will be a more constant component of Central European waters during the year. In addition to temperature, trophic status may also influence the rate of copepods development, accelerating it under adequate nutritional conditions. It seems that both thermal changes and increased eutrophication will favor an increase in the number of generations per year in T. oithonoides. With higher temperatures in the summer, both species may descend into deeper and cooler lake layers14. However, one of the threats to them could be local oxygen deficiencies preventing their migration36. Therefore, populations of T. oithonoides in shallow lakes appear to be the most vulnerable in the case of significant global thermal changes. Current changes seem to facilitate the further spread of T. crassus and other thermophilic copepod species. Our findings could be important in understanding the impact of global warming on thermophilic organisms.
Methods
We selected two copepod species whose ranges overlap in some regions but have distinct climatic zone distributions12,14. This enables us to assess the thermal resistance of both species, assuming that both are adapted to the local thermal conditions. To assess the effect of short-term heat shock, we chose planktonic micro-crustaceans, T. crassus (Fischer, 1853), and T. oithonoides (G.O. Sars, 1863), which are closely related taxonomically and morphologically. The following four variants of thermal shock has been chosen: 25 °C (+ 5 °C), 30 °C (+ 10 °C), 35 °C (+ 15 °C) and 40 °C (+ 20 °C) in relation to the reference conditions (20 °C). Temperature change for copepods was instantaneous; individuals were not acclimated to the increased temperature. For all temperature variants, three exposure times were established: 10, 30, and 60 min. Individuals were subjected to heat shock only once at the beginning of the experiment. Both species were obtained from waters within the city of Szczecin, Poland. T. crassus was obtained (June 04, 2021) from the Oder river (53.427757N, 14.573041E) and T. oithonoides was obtained (June 04, 2021) from Głębokie lake (53.474399N, 14.478487E). The mentioned water bodies do not form thermal layers, and their temperatures are similar from the surface to the bottom, and both are eutrophic.
The water was heated in laboratory water bath (Microm, SB 80) with an accuracy of 0.1 °C. The vessels with water were placed in the bath, and after attaining a certain temperature, copepod individuals were introduced into the heated container for a defined period of time. Additional details about the experimental procedures are provided in the following paragraph. To monitor the accuracy of the temperature values, we used a multifunctional field laboratory device (Elmetron CX-401). The oxygen concentration in the heated containers was also controlled, but no significant decrease in oxygenation was observed. Thermal shock conducted on copepods has shown that the acclimation temperature has a large effect on the tolerance of individuals of a given species10,27. Therefore, in the experiment, individuals under similar thermal conditions were used by carrying out their acclimation. Twenty four hours before the experiment, all specimens were acclimated to the laboratory condition.
For each variant and exposure time, 30 individuals were gently selected and placed in three separate 120 ml containers (10 individuals in each container). For the experiment, we used only females: 360 specimens per taxon. We examined only adult females with egg sacs. This was because determining the sex of juvenile copepod (without stressing the animal) is difficult. After thermal shock was induced, crustaceans were placed under stable laboratory conditions at approximately 20 °C. To provide the crustaceans with optimal conditions during the experiment, all individuals were placed under the same lighting conditions (LED light, white color 6 W;16 h light/8 h dark). During the experiment T. crassus individuals were held in filtered water (mesh size: 100 µm) from the Oder river (at the time of copepod collection conductivity was 749 µS/cm, oxygen concentration was 6.9 mg/L, pH was 7.61, and the temperature was 19.5 °C). In T. oithonoides experiment, we used filtered water (mesh size: 100 µm) from Głębokie lake (at the time of copepod collection conductivity was 560 µS/cm, oxygen concentration was 7.43 mg/L, pH 7.31, and the temperature was 20.5 °C). Additionally, the individuals were fed regularly (1 µg of powder Chlorella sp. per container every alternate day). Before classifying the specimen as dead, its vital functions were checked twice under a microscope. Egg sac dropping was checked along with the assessment of adult survival. However, the morphology or development of the eggs in the egg sacs was not assessed. Adult T. crassus females had an average body size (mean and standard deviation) of 0.755 ± 30 mm and T. oithonoides of 0.708 ± 14 mm, calculated as the sum of the lengths of the cephalothorax, abdomen, and caudal furca.
The map of the distribution ranges was created based on the literature for T. crassus13,15,37,38,39,40,41,42,43,44,45,46,47,48 and T. oithonoides14,49,50,51,52,53,54. The map for surface temperature refers to maximum temperature (°C) data for 1970–2000 for globally the warmest month55(www.worldclim.org). Averaged spatial data for July were utilized to generate the map. A color gradient was applied to the resulting spatial data. The map was constructed using the World Geodetic System 1984 ensemble (EPSG:6326). The map illustrating the distribution of species in relation to thermal conditions across space was created using QGIS version 3.20.3. (https://www.qgis.org)56.
Survival analysis was conducted using the log-rank test, a non-parametric method for comparing survival distributions between groups11,57. Survival analysis was chosen for its ability to handle censored data and time-to-event outcomes. Chi-square and p-values (significance level set at α = 0.05) were computed to assess the significance of differences in survival curves. Additionally, we tested for differences in egg sacs dropped among species regarding the main factors in our experiment (temperature and exposure time) using a two-way analysis of variance (ANOVA). Before applying the statistical test, we checked the data distribution using descriptive statistics and histograms. We also assessed the homogeneity of variances with Levene’s test. All analyses were performed using Statistica 13.1 (StatSoft).
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Stevens, G. C. The latitudinal gradient in geographical range: how so many species coexist in the tropics. Am. Nat. 133(2), 240–256 (1989).
Low, J. S. et al. Heat shock response and metabolic stress in the tropical estuarine copepod Pseudodiaptomus annandalei converge at its upper thermal optimum. J. Therm. Biol. 74, 14–22. https://doi.org/10.1016/j.jtherbio.2018.02.012 (2018).
Sasaki, M. C. & Dam, H. G. Integrating patterns of thermal tolerance and phenotypic plasticity with population genetics to improve understanding of vulnerability to warming in a widespread copepod. Glob. Chang Biol. 25(12), 4147–4164 (2019).
Stillman, J. H. Acclimation capacity underlies susceptibility to climate change. Science 301(5629), 65–65. https://doi.org/10.1126/science.1083073 (2003).
Buckley, L. B. & Kingsolver, J. G. Functional and phylogenetic approaches to forecasting species’ responses to climate change. Annu. Rev. Ecol. Evol. Syst. 43, 205–226 (2012).
Birrell, J. H., Frakes, J. I., Shah, A. A. & Woods, H. A. Mechanisms underlying thermal breadth differ by species in insects from adjacent but thermally distinct streams–A test of the climate variability hypothesis. J. Therm. Biol. 112, 103435. https://doi.org/10.1016/j.jtherbio.2022.103435 (2023).
Uttieri, M. & Svetlichny, L. Escape performance in the cyclopoid copepod Oithona davisae. Sci. Rep. 14(1), 1078. https://doi.org/10.1038/s41598-024-51288-0 (2024).
Raptis, C. E., van Vliet, M. T. & Pfister, S. Global thermal pollution of rivers from thermoelectric power plants. Environ. Res. Lett. 11(10), 104011 (2016).
Sasaki, M. & Dam, H. G. Global patterns in copepod thermal tolerance. J. Plankton Res. 43(4), 598–609 (2021).
Jiang, Z. et al. Tolerance of copepods to short-term thermal stress caused by coastal power stations. J. Therm. Biol. 33(7), 419–423 (2008).
Nowakowski, K. & Sługocki, Ł. Short-term heat shock perturbation affects populations of Daphnia magna and Eurytemora carolleeae: A warning to the water thermal pollution. Sci. Rep. 11(1), 16909. https://doi.org/10.1038/s41598-021-96464-8 (2021).
Mirabdullayev, I. M., Reid, J. W. & Ueda, H. Genus Thermocyclops Kiefer, 1927. In Copepoda: Cyclopoida. Genera Mesocyclops and Thermocyclops (eds Ueda, H. & Reid, J. W.) 213–302 (Backhuys Publishers, Leiden, 2003).
Hołyńska, M. On species of the genus Thermocyclops (Copepoda: Cyclopidae) occurring in northern Queensland Australia. Ann. Zool. 56(2), 335–367 (2006).
Nilssen, J. P. & Wærvågen, S. B. Superficial ecosystem similarities vs autecological stripping: The “twin species” Mesocyclops leuckarti (Claus) and Thermocyclops oithonoides (Sars)-seasonal habitat utilisation and life history traits. J. Limnol. 59(2), 79–102 (2000).
Burgis, M. J. The effect of temperature on the development time of eggs of Thermocyclops sp., a tropical cyclopoid copepod from Lake George Uganda. Limnol. Oceanogr. 15(5), 742–747 (1970).
Maier, G. The seasonal cycle of Thermocyclops crassus (Fischer, 1853)(Copepoda: Cyclopoida) in a shallow, eutrophic lake. Hydrobiologia 178, 43–58 (1989).
Błędzki, L. A. & Rybak, J. I. Freshwater Crustacean Zooplankton of Europe: Cladocera & Copepoda (Calanoida, Cyclopoida) Key to species identification, with notes on ecology, distribution, methods and introduction to data analysis (Springer, 2016).
Sługocki, Ł & Czerniawski, R. Trophic state (TSISD) and mixing type significantly influence pelagic zooplankton biodiversity in temperate lakes (NW Poland). PeerJ 6, e5731. https://doi.org/10.7717/peerj.5731 (2018).
Sunday, J. M., Bates, A. E. & Dulvy, N. K. Thermal tolerance and the global redistribution of animals. Nat. Clim. Change 2(9), 686–690 (2012).
Schoville, S. D., Barreto, F. S., Moy, G. W., Wolff, A. & Burton, R. S. Investigating the molecular basis of local adaptation to thermal stress: Population differences in gene expression across the transcriptome of the copepod Tigriopus californicus. BMC Evol. Biol. 12, 1–17 (2012).
Pereira, R. J., Sasaki, M. C. & Burton, R. S. Adaptation to a latitudinal thermal gradient within a widespread copepod species: The contributions of genetic divergence and phenotypic plasticity. Proc. R. Soc. B. 284(1853), 20170236 (2017).
Kelly, M. W., Pankey, M. S., DeBiasse, M. B. & Plachetzki, D. C. Adaptation to heat stress reduces phenotypic and transcriptional plasticity in a marine copepod. Funct. Ecol. 31(2), 398–406 (2017).
Verbitsky, V. B. et al. The preferred and avoidance temperatures of Thermocyclops crassus (Fischer, 1853) and their relation to the temperature of optimal, pessimal and normal performance of the species. J. Therm. Biol. 78, 106–113. https://doi.org/10.1016/j.jtherbio.2018.09.009 (2018).
Hołyńska, M. & Sługocki, Ł. Freshwater microcrustaceans (Copepoda: Cyclopidae) on islands: A review. Hydrobiologia 850(1), 183–201. https://doi.org/10.1007/s10750-022-05053-x (2023).
Finlay, K. P., Cyr, H. & Shuter, B. J. Spatial and temporal variability in water temperatures in the littoral zone of a multibasin lake. Can. J. Fish. Aquat. Sci. 58(3), 609–619. https://doi.org/10.1139/f01-017 (2001).
Isinibilir, M., Svetlichny, L. & Hubareva, E. Competitive advantage of the invasive copepod Oithona davisae over the indigenous copepod Oithona nana in the Marmara Sea and Golden Horn Estuary. Mar. Freshw. Behav. Physiol. 49(6), 391–405 (2016).
Rahlff, J. et al. Short-term molecular and physiological responses to heat stress in neritic copepods Acartia tonsa and Eurytemora affinis. Comp. Biochem. Physiol. Part A Mol. Integr. 203, 348–358 (2017).
Lindquist, S. & Craig, E. A. The heat-shock proteins. Annu. Rev. Genet. 22(1), 631–677 (1988).
Groscolas, R., Lacroix, A. & Robin, J. P. Spontaneous egg or chick abandonment in energy-depleted king penguins: A role for corticosterone and prolactin?. Horm. Behav. 53(1), 51–60. https://doi.org/10.1016/j.yhbeh.2007.08.010 (2008).
Gowland-Sainz, M., Tapella, F. & Lovrich, G. A. Egg loss in females of two lithodid species following different return-to-the-water protocols. Fish. Res. 161, 77–85 (2015).
Belmonte, G. The suspected contradictory role of parental care in the adaption of planktonic Calanoida to temporary freshwater. Water 13(1), 100. https://doi.org/10.3390/w13010100 (2021).
Bartholmeé, S., Samchyshyna, L., Santer, B. & Lampert, W. Subitaneous eggs of freshwater copepods pass through fish guts: Survival, hatchability, and potential ecological implications. Limnol. Oceanogr. 50(3), 923–929 (2005).
Svetlichny, L., Rudi Strickler, J. & Obertegger, U. Swimming and respiration in cyclopoid copepods Thermocyclops oithonoides and Oithona davisae and calanoid copepod Paracalanus parvus. J. Exp. Zool. A. 337(8), 835–851 (2022).
Tunowski, J. Changes in zooplankton abundance and community structure in the cooling channel system of the Konin and Pątnów power plants. Fish. Aquat. Life. 17(4), 279–289. https://doi.org/10.2478/v10086-009-0020-1 (2009).
Richardson, A. J. In hot water: Zooplankton and climate change. ICES J. Mar. Sci. 65(3), 279–295 (2008).
Karpowicz, M., Ejsmont-Karabin, J., Kozłowska, J., Feniova, I. & Dzialowski, A. R. Zooplankton community responses to oxygen stress. Water 12(3), 706. https://doi.org/10.3390/w12030706 (2020).
Collado, C., Defaye, D., Dussart, B. H. & Fernando, C. H. The freshwater Copepoda (Crustacea) of Costa Rica with notes on some species. Hydrobiologia 119, 89–99 (1984).
Mirabdullayev, I. M. & Kuzmetov, A. R. The genus Thermocyclops (Crustacea: Copepoda) in Uzbekistan (Central Asia). Int. Rev. Gesamten Hydrobiol. Hydrogr. 82(2), 201–212 (1997).
Kobari, T. & Ban, S. Life cycles of two limnetic cyclopoid copepods, Cyclops vicinus and Thermocyclops crassus, in two different habitats. J. Plankton Res. 20(6), 1073–1086 (1998).
Guo, X. The genus Thermocyclops Kiefer, 1927 (Copepoda: Cyclopidae) in China. Hydrobiologia 403, 87–95 (1999).
Gutiérrez-Aguirre, M. & Suárez-Morales, E. The Eurasian Thermocyclops crassus (Fischer, 1853)(Copepoda, Cyclopoida) found in southeastern Mexico. Crustaceana 73(6), 705–713 (2000).
Viaroli, P., Ferrari, I. & Rossetti, G. Long-term limnological research in a quarry lake of the Po River. Italy. Verh. Int. Ver. Theor. Angew. Limnol. 28(2), 576–581 (2002).
Aquino, M. R. Y., Cho, C. D., Cruz, M. A. S., Saguiguit, M. A. G. & Papa, R. D. S. Zooplankton composition and diversity in Paoay Lake Luzon Is., Philippines. Philipp. J. Sci. 137(2), 169–177 (2008).
Chaicharoen, R., Sanoamuang, L. O. & Hołyńska, M. A review of the genus Thermocyclops (Crustacea: Copepoda: Cyclopoida) in Cambodia. Zool. Stud. 50(6), 780–803 (2011).
Alekseev, V. R., Haffner, D. G., Vaillant, J. J. & Yusoff, F. M. Cyclopoid and calanoid copepod biodiversity in Indonesia. J. Limnol. 72(s2), 245–274. https://doi.org/10.4081/jlimnol.2013.s2.e12 (2013).
Bozkurt, A. & Can, M. F. Seasonal variations in body length and fecundity of 2 copepod species: Thermocyclops crassus (Fischer, 1853) and Eudiaptomus drieschi (Poppe & Mrázek, 1895). Turk. J. Zool. 38(2), 222–228 (2014).
Connolly, J. K., Watkins, J. M., Hinchey, E. K., Rudstam, L. G. & Reid, J. W. New cyclopoid copepod (Thermocyclops crassus) reported in the Laurentian Great Lakes. J. Great Lakes Res. 43(3), 198–203 (2017).
Stamou, G., Kourkoutmani, P. & Michaloudi, E. The Inland Cladocera and Copepoda Fauna in Greece. Diversity 14(11), 997 (2022).
Reid, J. W. The distribution of species of the genus Thermocyclops (Copepoda, Cyclopoida) in the western hemisphere, with description of T. parvus, new species. Hydrobiologia 175, 149–174 (1989).
Anufriieva, E., Hołyńska, M. & Shadrin, N. Current invasions of Asian Cyclopid species (Copepoda: Cyclopidae) in Crimea, with taxonomical and zoogeographical remarks on the hypersaline and freshwater fauna. Ann. Zool. 64(1), 109–130. https://doi.org/10.3161/000345414X680636 (2014).
Kolozin, V. A. Interannual changes in the structure of summer zooplankton community in the Iriklinsky Reservoir (Ural River, Russia). Inland Water Biol. 15(5), 580–592 (2022).
Rybka, T. S. & Yuryshynets, V. I. Symbiont fauna of freshwater zooplankton in several water bodies of the Dnipro river basin. Vestn. Zool. 52(6), 439–450 (2018).
Lazareva, V. I. Long-term changes in the composition and abundance of the zooplankton community in Kama River reservoirs. Inland Water Biol. 13, 214–229 (2020).
Lazareva, V. I., Zhdanova, S. M. & Sabitova, R. Z. The spread of east asian copepod Thermocyclops taihokuensis (Crustacea, Copepoda) in the Volga River basin. Inland Water Biol. 15(2), 139–148 (2022).
Fick, S. E. & Hijmans, R. J. WorldClim 2: New 1 km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 37(12), 4302–4315 (2017).
QGIS.Org, QGIS geographic information system version 3.20.3. https://www.qgis.org. Accessed 29 June 2023.
Kaplan, E. L. & Meier, P. Nonparametric estimation from incomplete observations. J. Am. Stat. Assoc. 53(282), 457–481 (1958).
Acknowledgements
Co-financed by the Minister of Science under the “Regional Excellence Initiative” program for 2024-2027.
Author information
Authors and Affiliations
Contributions
Ł.S. and K.N. research conceptualization: Ł.S. and K.N. sample design and methodology: K.N. and Ł.S. investigation and data collection: K.N. and Ł.S. data analysis and interpretation: K.N. and Ł.S. ethics approval: Ł.S. and K.N. funding provision: K.N. and Ł.S. roles/writing - original draft: Ł.S. and K.N. writing - review & editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nowakowski, K., Sługocki, Ł. Contrasting responses of Thermocyclops crassus and T. oithonoides (Crustacea, Copepoda) to thermal stress. Sci Rep 14, 7660 (2024). https://doi.org/10.1038/s41598-024-58230-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-58230-4
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.