Abstract
Colorectal cancer is the third most commonly diagnosed cancer and the second leading cause of cancer-related death, thus a novel chemotherapeutic agent for colon cancer therapy is needed. In this study, analogues of echinomycin, a cyclic peptide natural product with potent toxicity to several human cancer cell lines, were synthesized, and their biological activities against human colon cancer cells were investigated. Analogue 3 as well as 1 inhibit HIF-1α-mediated transcription. Notably, transcriptome analysis indicated that the cell cycle and its regulation were involved in the effects on cells treated with 3. Analogue 3 exhibited superior in vivo efficacy to echinomycin without significant toxicity in mouse xenograft model. The low dose of 3 needed to be efficacious in vivo is also noteworthy and our data suggest that 3 is an attractive and potentially novel agent for the treatment of colon cancer.
Similar content being viewed by others
Introduction
Cancer is a leading cause of death from disease in many countries, and 9.9 million people died of cancer in 2020 worldwide1. Colorectal cancer is the third most commonly diagnosed cancer and the second leading cause of cancer-related death among men and women. The distant metastasis of cancer cells is also an important issue, a colorectal cancer tends to metastasize to the liver and lungs. In particularly, the 5-year survival rate for stage IV colorectal cancer patients with distant metastases to the liver and lungs is still extremely low. Current treatments for colorectal cancer include surgical resection and chemotherapy. However, even after successful surgical resection followed by chemotherapy, the 5-year recurrence rate is still high. Therefore, a novel chemotherapeutic agent for colon cancer therapy is urgently needed.
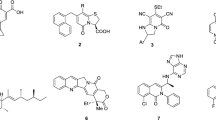
Echinomycin2 (1, Fig. 1), a bicyclic octadepsipeptide possessing two quinoxaline chromophores, is in a class of natural products that can bind to double-stranded DNA due to bisintercalation by the chromophores3,4,5,6. Because of its potent toxicity against human cancer cells, several phase I and II clinical trials with this compound were conducted in the 1990s3,4,5,6,7,8,9,10,11,12,13,14. However, drug development was halted because inimal therapeutic effects were observed for terminal patients with solid tumors. Migration, angiogenesis, and invasion are important for tumor progression and metastasis and are regulated by the transcription factor, hypoxia-inducible factor-1 (HIF-1)15,16. In 2015, it was reported that echinomycin suppresses the HIF-1 pathway by inhibiting the binding of HIF-1α to the hypoxia-responsive element (HRE), which is the cis-element of HIF-1α17. In addition, it has been suggested that 1 suppresses the Notch signaling pathway, resulting in potent inhibitory activity against pancreatic cancer by targeting stem cells18. It has also been reported that 1 can selectively kill leukemia-initiating cells in patients with relapsed acute myeloid leukemia (AML) without causing normal stem cell toxicity19. In these studies, the dose of 1 demonstrating efficacy was quite low compared to those used in previous clinical trials. It has been suggested that 1 is still a promising anticancer drug lead compound because of its ability to alter the clinical protocol. We accomplished the first total synthesis of 1 by constructing the characteristic thioacetal moiety via a Pummerer rearrangement of 2 in the late stage of the synthesis20. A brief structure–activity relationship study revealed that 2 reduces but still retains the toxicity of 1 to human pancreatic cancer MIA PaCa-2 cells. We further found that the chromophore analogue 3, which possesses 3-hydroxyquinoline-2-carbonyl groups, exhibited a similar cytotoxic potency to that of 1. The fact that sulfide analogues 2 and 3 exhibit potencies similar that of 1 is advantageous because these analogues are more synthetically accessible than 1. The toxicity of these analogues to human colorectal cells has not yet been tested, and the structural requirements of the chromophores remain to be investigated from our previous study. In this study, the synthesis of chromophore analogues of 2 and an in vitro and in vivo evaluation of their antiproliferative activity against SW620 cell lines, which are human colon cancer cells derived from highly invasive lymph node metastases, are described.
Results
Synthesis of echinomycin analogues
First, the impact of other pendant chromophores was investigated to expand our current knowledge of the structure–activity relationships for 2 (Fig. 2). Analogues 4–7 were designed to determine the impact of the number and position of each nitrogen in the quinoxalines of 2 on the cytotoxic activity. Quinoxaline is a fused aromatic group composed of two six-membered rings. Similarly, to determine the effect of chromophore shape, analogues 8–17, which have five-membered aromatic rings fused to benzene as the chromophores, 18 and 19, which have extended chromophores, and 20, where the phenyl rings of 2 were truncated, were designed. One of the characteristics of our synthetic strategy toward 2 and 3 is the installation of chromophores at the late stage of synthesis, which allows us to access a range of chromophore analogues efficiently. Analogues 4–20 were synthesized as shown in Fig. 3. Analogues 4–7 were synthesized by deprotection of the Cbz groups on the amines of the D-Ser residues of 21 using TFA in the presence of thioanisole followed by acylation of the resulting amines with the corresponding carboxylic acids using EDCI, HOAt, and Et3N in DMF. As for the synthesis of 8–20, the liberated diamine obtained from 21 was once protected with the Boc group to provide 22 in 39% yield over two steps. There are two advantages for the changing the protecting group from Cbz to Boc. The first is to reduce the time for the synthesis of multiple compounds, because the Boc group is more quickly removed by under an acidic conditions than the Cbz group. The second is to simplify the purification of the products. Specifically, the benzylmethylphenylsulfonium trifluoroacetate was generated as a byproduct during Cbz deprotection of 21 using TFA and thioanisole. The sulfonium salt is difficult to separate from the liberated amine, whereas the removal of the Boc group does not generate such problematic byproducts. This change largely improved the synthetic access to a range of analogues. Deprotection of the Boc groups of 22 by HCl in dioxane followed by condensation with the corresponding carboxylic acids afforded 8–20.
Toxicity of echinomycin analogues to human colon cancer cells
With a range of chromophore analogues in hand, their toxicity to SW620 cells was investigated by WST assays. Additionally, the 50% inhibitory concentration (IC50) values were determined and compared to those of 2, which has 2-quinoxaline chromophores, as a positive control for 3–20 (Table 1). To compare the toxicities of the new analogues to pancreatic cancer cells with the toxicity of 1 side by side, their activity against SW620 cells and MIA PaCa-2 cells is also shown in Table 1. Similar to a previous investigation with MIA PaCa-2 cells20, 2 retained its potent toxicity to SW620 cells with an IC50 value of 26 nM although a tenfold reduction in the activity was observed. Analogue 3, which has 3-hydroxyquinoline chromophores, exhibited cytotoxicity equipotent to that of 1 (2.5 nM for 1 vs. 0.85 nM for 3). Under these conditions, the 2-quinoline analogue 4 showed slightly better cytotoxicity than 2 (IC50 26 nM), indicating that the nitrogens at the 4-positions of the quinoxaline chromophores in 2 are not necessary for cytotoxicity. In contrast to 4, analogues 5 and 6, where the nitrogens at the 1-positions of the chromophores in 4 are transposed, displayed largely reduced activity, with IC50 values of 1210 and 1193 nM, respectively. Naphthalene analogue 7, which lacks all the nitrogens in 2, showed completely diminished activity. Analogues 8–17 possess 5-membered heterocycles fused to the benzene ring. This structural alteration has a great impact on cytotoxicity, and all of these analogues completely lost their activity (IC50 > 10,000 nM). Extended chromophore analogues 18 and 19 as well as truncated analogue 20 also lost their cytotoxicity.
Induction of apoptosis by 3
Compound 3 exhibits very potent toxicity to SW620, as expected. Therefore, the expression of cleaved caspase-3 in SW620 cells was subsequently evaluated to clarify the effects of 3 on cancer cell apoptosis. Western blotting analysis of SW620 cells treated with 3 revealed that the expression of cleaved caspase-3 was significantly greater than that in untreated cells (Fig. 4). TUNEL staining was then performed. The number of apoptotic SW620 cells increased after treatment with 3 compared to the number of apoptotic control cells. These data indicated that 3 exhibited a tumor-suppressive effect by inducing colon cancer cell apoptosis. In both cases, 3 exhibited better activity than 1.
Inhibition of the HIF-1 pathway
Echinomycin is known to inhibit the HIF-1 pathway by inhibiting the binding of HIF-1α to HRE15,21,22. The ability of 3 to inhibit HIF-1α-mediated transcription was investigated by using an HRE reporter gene assay in SW620 cells transiently overexpressing HIF-1α. As shown in Figs. 3 and 5 markedly decreased HIF-1α-induced HRE reporter activity to a similar extent as echinomycin, indicating that 3 also has an inhibitory effect on HIF-1α-mediated transcription (Fig. 4).
Effect of echinomycin (1), 2 and 3 on HIF-1α-dependent transcription in SW620 cells. SW620 cells were transiently transfected with empty vector pCI-neo-3 × FLAG or HIF-1α expression vector pCI-neo-3 × FLAG-HIF-1α, together with pGL3-5 × HRE-Luc and pGL4.75 [hRluc/CMV]. One day after transfection, echinomycin (1), 2, or 3 was added to the cell culture media and incubated for an additional 16 h. The cells were lysed, and a dual luciferase assay was performed. Firefly luciferase activity was normalized to Renilla luciferase activity, and the data are presented as fold induction relative to the values obtained in cells DMSO-treated cells transfected with empty vector. The error bars represent standard deviations (n = 3). *P < 0.05 and **P < 0.01 compared to DMSO-treated cells transfected with HIF-1α (Student’s test).
Transcriptome analysis
Although modulation of the HIF-1 pathway has been reported to be one of the modes of action of 1, it has also been suggested that 1 inhibits other signaling pathways including the Notch pathway19. Therefore, transcriptome analysis was then performed to gain insight into the mode of action of 3. Namely, to determine the changes in mRNA expression in SW620 cells treated with 3, a high-throughput sequencing analysis was conducted in a manner similar to our previous study23.
Seven thousand and three hundred seventy mRNAs exhibited more than twofold changes in expression levels that were significant (p < 0.05) in comparison to their expression levels in control cells (Supplementary Table 1). Pathway analysis was performed using the software program MetaCore, and the results are shown in Table 2. These results indicated that the cell cycle and its regulation were involved in the changes in the expression of these mRNAs in the cells treated with 3. Notably the HIF-1 pathway was not indicated by this analysis, suggesting that the HIF-1 pathway may not be the primary target of 3.
To identify potential target molecules of echinomycin, two types of probe compounds were designed (Fig. 6). The first one was biotinylated probe 27 with an ethylene glycol linker. Target identification using this type of probe is one of the most frequently used techniques. The other one was photochemical probe 29 with a diazirine photo cross-linker containing an alkyne handle, which was developed by Yao et al.24 The diazirine moiety forms a covalent bond with the target molecule by the generation of a carbene upon UV irradiation, while the alkyne is used for subsequent immobilization on the beads via a click reaction. The most important feature of this linker is that it can minimize structural changes due to the introduction of the linker, and it has been reported to be used even in living cells. These compounds were synthesized from sulfoxide 23 in a manner similar to the total synthesis of 1, via a scheme that was developed by our group20. Namely, Pummerer rearrangement of 23 by AcCl in CH2Cl2 gave the corresponding chloride, which was sequentially treated with 24 as a nucleophile and ZnCl2 as a promoter in THF at room temperature for 15 h to provide 25 in 26% yield over two steps from 23.
After removal of the t-Bu group of 25 by aq. TFA, the resulting carboxylic acid was condensed with biotinylated amine 26 to afford the desired biotinylated probe 27. Chemical probe 29 was synthesized using the minimalist linker 28. Identification of the target molecule(s) of echinomycin using 27 and 29 is currently underway.
Metabolic stability of 3
Before investigating the in vivo efficacy of 3, its metabolic stability was evaluated by treatment with human or mouse liver microsomes at 37 °C for 30 min, and the remaining analogues were analyzed by LC–MS/MS (Table 3). The clearance vale of 3 were 83.7 and 386 mL/min/kg for human and mouse liver microsomes, respectively, in the presence of NADPH. This means that approximately 67% of 3 was unaffected by human liver microsomes. Thus, it was revealed that these analogues were metabolically stable, especially in human liver microsomes. Furthermore, the metabolic clearance values in the absence of NADPH were 45.1 and 118 mL/min/kg in human and mouse liver microsomes, respectively, suggesting that in liver microsomes in both species, approximately half of the metabolic reactions are oxidative reactions and the other half are hydrolytic reactions involving the peptide and/or ester bonds in 3.
Investigation of the maximum tolerated dose of 3
Toxicity Body weight change is an indicator of systemic toxicity. Accordingly, the body weights of mice intraperitoneally treated with different doses (0.04 and 0.4 µg/mouse) of either 1 or 3 were measured (Fig. S1). After treatment with dosages of 0.04 and 0.4 µg/mouse, the body weights did not differ greatly from those of the control, indicating that 1 and 3 did not exhibit severe systemic toxicity at these dosages administered over 14 days. A blood hematological examination (including nineteen parameters) was also conducted on these mice on Day 14 (Fig. S2). There were no significant changes in almost any of the parameters in the mice treated with either 1 or 3 at doses of 0.04 or 0.4 µg/mouse. At a dose of 0.4 µg/mouse, a decrease in total bilirubin (T-BIL) and an increase in aspartate aminotransferase (AST), alanine aminotransferase (ALT), and lactate dehydrogenase (LDH) were observed in both groups treated with 1 and 3 (indicated by arrows). These results indicate that 1 and 3 do not show significant toxicity at least up to a dose of 0.04 µg/mouse, which is expected to be the maximum tolerated dose of 3 for the subsequent in vivo evaluations.
Tumor growth suppression by 3 in vivo
Since the metabolic stability and identification of the maximum tolerated dose of 3 were confirmed, the effect of 3 on colon cancer progression in vivo was then investigated (Fig. 7). A xenograft model was generated by injecting a suspension of 2 × 106 SW620 cells into the backs of nude mice. The doses of the compounds were 0.04 and 0.4 µg/mouse, which correspond to approximately 1.6 and 16 μg/kg, respectively, according to the results obtained in Fig. S1. A solution of 1, 3, or PBS was injected intraperitoneally into each mouse, and tumor sizes were measured each day. Tumor growth in the groups treated with 1 and 3 was significantly suppressed compared to that in the PBS-injected group. Of note is the efficacy of tumor growth inhibition with 3. The efficacy of 3 at a dose of 0.04 µg/mouse is equivalent to that of 1 at a dose of 0.4 µg/mouse, and it is revealed that 3 possesses superior tumor growth inhibition properties to 1.
In vivo anticancer activity of echinomycin and 3 on the SW620 xenograft mice model. Male BALB/C nude mice were administered by intraperitoneal injection with the vehicle, echinomycin and 3, at dosages of 0.04 and 0.4 µg/mice (n = 10 each group), every day for 10 days (a, b). Average tumor volume (c) was measured. Data are expressed as the mean ± standard error (n = 8). *Indicates P < 0.05 by Student's t test. The error bars show the s.d. (n = 10).
Discussion
Echinomycin analogues were synthesized and biologically evaluated in this study. Evaluating the impact of other pendant chromophores was considered the first goal of this study, with which we aimed to significantly expand our current knowledge on the structure–activity relationship of 2. Analogue 3 was designed inspired by the fact that congener natural products of 1, such as SW-163C25, thiocoraline26, sandramycin27, and quinaldopeptin28, share 3-hydroxyquinoline-2-carbonyl groups as chromophores and display strong toxicity to a range of human cancer cell lines. An investigation of the cytotoxicity of these analogues to SW620 cells revealed the following structure–activity relationship for the chromophore. (1) The nitrogens at the 4-positions of the quinoxaline chromophores in 2 are not necessary. (2) The nitrogens at the 1-position contribute significantly to the cytotoxicity. (3) Naphthalene analogue 7, which lacks all the nitrogens of 2, displayed completely diminished activity. (4) The shape of the 6-membered heterocycles fused to the benzene ring is important for cytotoxicity. The SARs of echinomycin elucidated in this study in conjunction with those previously reported20 are summarized in Fig. 8.
Analogue 3 as well as 1 inhibited HIF-1α-mediated transcription (Fig. 4). It has also been suggested that 1 inhibits other signaling pathways including the Notch pathway19. Our transcriptome analysis indicated that treatment with 3 altered the expression of 7370 mRNAs in SW620 cells. Pathway analysis revealed that the cell cycle and its regulation were involved in these changes in mRNA expression in the cells treated with 3 (Table 2). Interestingly, the HIF-1 and NOTCH signaling pathways were not identified as the main pathways in this analysis. Further studies will be necessary to elucidate the detailed modes of action of 3 as well as 1 by using chemical probes 27 and 29.
Analogue 3 showed lower clearance values with mouse and human liver microsomes. Although the observed difference in metabolic stability could arise from differences in the chemical stability of a thioacetal and thioether moieties or from conformational changes throughout the entire cyclic peptide caused by changes in the thioacetal linkage, more detailed studies are needed to determine which contribution is greater.
Echinomycin analogue 3 exhibited superior in vivo efficacy to echinomycin without significant toxicity in mouse xenograft model. The low dose of 3 needed in vivo efficacy is also noteworthy. The chemical structures of echinomycin and 3 are not very different and their in vitro cytotoxic activities are similar. However, the in vivo activity of 3 was better than that of echinomycin, even though the clearance of 3 was worse. One possible reason is the difference in the mechanism of 1 and 3, which was suggested by our transcriptome analysis. Although the details are not apparent, the HIF-1 pathway likely influences the difference between the in vivo efficacy of 1 and 3. Another reason for this difference is the difference in protein binding rates. Namely, in vivo bioactivity tends to be worse for protein binding rates that are higher at the same plasma concentration. On the other hand, the higher the protein binding rate is, the slower the drug is lost from the body and the longer it stays in the body, so its contribution to the effect of the drug is greater. It is thought that the difference in in vivo activity may be due to a difference in the balance between the two. A dose of 0.04 µg/mouse corresponds to 1.6 μg/kg for mice, and a calculated human equivalent dose of 0.13 μg/kg (4.8 μg/m2)29. No obvious toxicity was observed at this dose in the mice judging from body weight loss and the blood hematological examination, while there was significant efficacy in the xenograft model. As previously noted, human clinical trials of 1 have suggested that echinomycin at doses ranging from 1200 to 2128 μg/m2 given intravenously is toxic. However, there was no observed toxicity at the lower doses of 60 and 120 μg/m230. Sulfide 3 retains the cytotoxic activity of 1 both in vitro and in vivo and is more accessible and scalable than 1. Our data suggest that echinomycin is an attractive and potentially novel agent for the treatment of colon cancer in addition to pancreatic cancer and AML.
Experimental section
General experimental methods
Compounds 4–29 were prepared using methods previously reported by our group20. Namely, all reactions except those carried out in the aqueous phase were performed under an argon atmosphere unless otherwise noted. Materials were purchased from commercial suppliers and used without further purification unless otherwise noted. Solvents were distilled according to the standard protocol. Isolated yields were calculated by weighing products. The weight of the starting materials and the products were not calibrated. Analytical thin layer chromatography (TLC) was performed on Merck silica gel 60F254 plates. Normal-phase column chromatography was performed on Merck silica gel 5715 or Wakogel 60N. Flash column chromatography was performed on Kanto Chemical Silica Gel 60N (spherical, neutral, 40–50 µm). Hi-flash column chromatography was performed on YAMAZEN Hi-Flash™ column silica gel (40 µm) or Fuji Silysia Chromatorex MB/PSQ (50–200 µm). 1H NMR spectra were measured in CDCl3, DMSO-d6 or CD3OD solution and reported in parts per million (δ) relative to tetramethylsilane (0.00 ppm) as an internal standard or referenced to residual solvent peak of DMSO-d6 (2.49 ppm) or CD3OD (3.31 ppm) using JEOL ECS400 (400 MHz) and ECX400P (400 MHz) spectrophotometers, unless otherwise noted. 13C NMR spectra were measured in CDCl3, DMSO-d6 or CD3OD solution and referenced to residual solvent peaks of CDCl3 (77.0 ppm), DMSO-d6 (39.52 ppm) or CD3OD (49.00 ppm) using JEOL ECS400 (100 MHz) and ECX400P (100 MHz) spectrophotometers. The coupling constant (J) was reported in hertz (Hz). Abbreviations of multiplicity were as follows; s: singlet, d; doublet, t: triplet, q: quartet, m: multiplet, br: broad. Data were presented as follows; chemical shift (multiplicity, integration, coupling constant). The assignment was based on 1H–1H COSY, HMBC and HMQC NMR spectra. Mass spectra were obtained on Waters MICRO MASS LCT–premier or Advion expression CMS and the mass analyzer type used for the HRMS measurements was TOF. Optical rotation was measured on a Rudolph Research Analytical Autopol IV automatic polarimeter. Liquid chromatography-mass spectrometry (LC–MS) was performed on the systems as follows: Shimadzu Prominence-i LC-2030C Plus as an HPLC system; FCV-20AH2 as a flow-line selection valve; LCMS-8040 as a Liquid chromatograph mass spectrometer, and LabSolutions as a system controller.
General procedure for the synthesis of 4–7
Compounds were prepared using methods previously reported by our group20. Namely, compound 22 (25.3 mg, 25.0 µmol) in TFA (2.5 mL) was treated with thioanisole (117.2 µL, 1.00 mmol) at room temperature for 24 h. The reaction mixture was concentrated in vacuo, and then washed with Et2O (5 mL × 3) to afford a pale yellow solid. The residue, iPr2NEt (25.5 µL, 125 µmol), carboxylic acid (100 µmol) and HOAt (17.0 mg, 125 µmol) in DMF (1.0 mL) was treated with EDCI (24.0 mg, 100 µmol) at 0 °C, and the whole mixture was stirred at room temperature for 5 h. The reaction mixture was diluted with EtOAc (5 mL), and washed with H2O (5 mL), 1 M aq. HCl (5 mL), sat. aq. NaHCO3 (5 mL) and brine (5 mL). The organic layer was dried over Na2SO4, filtered, and concentrated in vacuo. The residue was purified to afford 4–7.
{N-(Quinoline-2-carbonyl)-D-Ser-L-Ala-N-Boc-N-Me-L-Cys-N-Me-L-Val}2 sulfide (4)
Compound 4 (5.3 mg, 5.0 µmol, 20%) was synthesized using methods previously reported by our group20, from quinaldic acid (100 µmol), and purified with flash silica gel column chromatography (\(\Phi\) 0.7 × 5.2 cm, CHCl3/MeOH: 100/0 → 99.5/0.5 → 99/1 → 98.5/1.5) as a white amorphous solid. 1H NMR (CDCl3, 400 MHz) δ 8.95 (d, 2H, Ser-NH, JSer-NH, Ser-α-CH = 7.2 Hz), 8.30 (d, 2H, Ar–H, J = 8.2 Hz), 8.24 (d, 2H, Ar–H, J = 8.2 Hz), 7.89–7.84 (m, 4H, Ar–H), 7.70 (ddd, 2H, Ar–H, J = 1.3, 6.9, 8.2 Hz), 7.58 (ddd, 2H, Ar–H, J = 1.1, 6.9, 8.0 Hz), 6.84 (d, 2H, Ala-NH, JAla-NH, Ala-α-CH = 7.4 Hz), 6.26 (dd, 2H, Cys-α-CH, JCys-α-CH, Cys-β-CH = JCys-α-CH, Cys-β-CH = 6.6 Hz), 5.13 (d, 2H, Val-α-CH, JVal-α-CH, Val-β-CH = 10.0 Hz), 4.89–4.78 (m, 6H, Ala-α-CH, Ser-α-CH, Ser-β-CH), 4.67 (dd, 2H, Ser-β-CH, JSer-β-CH, Ser-α-CH = 1.4, JSer-β-CH, Ser-β-CH = 11.4 Hz), 3.38 (dd, 2H, Cys-β-CH, JCys-β-CH, Cys-α-CH = 6.6, JCys-β-CH, Cys-β-CH = 14.8 Hz), 3.24 (s, 6H, NCH), 2.95 (s, 6H, NCH), 2.52–2.45 (m, 2H, Cys-β-CH), 2.36 (qqd, 2H, Val-β-CH, JVal-β-CH, Val-γ-CH = JVal-β-CH, Val-γ-CH = 6.9, JVal-β-CH, Val-α-CH = 10.0 Hz), 1.34 (d, 6H, Ala-β-CH, JAla-β-CH, Ala-α-CH = 6.9 Hz), 1.10 (d, 6H, Val-γ-CH, JVal-γ-CH, Val-β-CH = 6.9 Hz), 0.92 (d, 6H, Val-γ-CH, JVal-γ-CH, Val-β-CH = 6.9 Hz); 13C NMR (CDCl3, 100 MHz) δ 173.2, 171.3, 170.3, 168.0, 165.6, 148.5, 146.6, 138.0, 130.5, 129.8, 129.5, 128.5, 128.1, 118.9, 64.2, 62.3, 55.0, 53.5, 46.3, 36.1, 31.7, 30.3, 28.1, 20.5, 19.4, 17.6; ESIMS-LR m/z 1075.2 [(M + Na)+]; ESIMS-HR calcd for C52H65N10O12S 1053.4499, found 1053.4515; [α]22D −172.8 (c 0.14, CHCl3).
{N-(Quinoline-3-carbonyl)-D-Ser-L-Ala-N-Boc-N-Me-L-Cys-N-Me-L-Val}2 sulfide (5)
Compound 5 (4.6 mg, 4.4 µmol, 17%) was synthesized using methods previously reported by our group20, from 3-quinolinecarboxylic acid (17.3 mg, 100 µmol), and purified with flash silica gel column chromatography (\(\Phi\) 0.7 × 5.2 cm, CHCl3/MeOH: 100/0 → 99/1 → 98.5/1.5 → 98/2 → 97.5/2.5 → 97/3) as a white amorphous solid. 1H NMR (DMSO-d6, 400 MHz) δ 9.26 (d, 2H, Ar–H, J = 2.2 Hz), 8.83 (d, 2H, Ar–H, J = 2.2 Hz), 8.80 (d, 2H, Ser-NH, JSer-NH, Ser-α-CH = 7.9 Hz), 8.14 (d, 2H, Ar–H, J = 7.6 Hz), 8.10 (d, 2H, Ar–H, J = 8.8 Hz), 7.91–7.86 (m, 4H, Ala-NH, Ar–H), 7.72 (td, 2H, Ar–H, J = 1.4, 7.7 Hz), 6.29 (dd, 2H, Cys-α-CH, JCys-α-CH, Cys-β-CH = JCys-α-CH, Cys-β-CH = 6.6 Hz), 4.84 (d, 2H, Val-α-CH, JVal-α-CH, Val-β-CH = 10.8 Hz), 4.71 (ddd, 2H, Ser-α-CH, JSer-α-CH, Ser-β-CH = 2.9, JSer-α-CH, Ser-β-CH = 7.7, JSer-α-CH, Ser-NH = 7.9 Hz), 4.58 (dd, 2H, Ala-α-CH, JAla-α-CH, Ala-β-CH = JAla-α-CH, Ala-NH = 6.6 Hz), 4.50–4.45 (m, 2H, Ser-β-CH), 4.37 (dd, 2H, Ser-β-CH, JSer-β-CH, Ser-α-CH = 2.9, JSer-β-CH, Ser-β-CH = 13.0 Hz), 3.17–3.07 (m, 2H, Cys-β-CH), 3.03 (s, 6H, NCH), 2.81 (s, 6H, NCH), 2.53–2.49 (m, 2H, Cys-β-CH), 2.23 (qqd, 2H, Val-β-CH, JVal-β-CH, Val-γ-CH = 6.4, JVal-β-CH, Val-γ-CH = 6.8, JVal-β-CH, Val-α-CH = 10.8 Hz), 1.27 (d, 6H, Ala-β-CH, JAla-β-CH, Ala-α-CH = 6.6 Hz), 1.00 (d, 6H, Val-γ-CH, JVal-γ-CH, Val-β-CH = 6.4 Hz), 0.76 (d, 6H, Val-γ-CH, JVal-γ-CH, Val-β-CH = 6.8 Hz); 13C NMR (DMSO-d6, 100 MHz) δ172.3, 170.3, 169.8, 167.7, 165.3, 149.0, 148.5, 136.1, 131.4, 129.2, 128.8, 127.5, 126.7, 126.4, 64.7, 61.5, 54.1, 53.1, 46.1, 35.2, 30.5, 29.7, 26.5, 20.2, 18.8, 16.6; ESIMS-LR m/z 1075.4 [(M + Na)+]; ESIMS-HR calcd for C52H65N10O12S 1053.4499, found 1053.4506; [α]23D −175.3 (c 0.20, DMSO).
{N-(Quinoline-6-carbonyl)-D-Ser-L-Ala-N-Boc-N-Me-L-Cys-N-Me-L-Val}2 sulfide (6)
Compound 6 (5.0 mg, 4.8 µmol, 19%) was synthesized using methods previously reported by our group20, from 6-quinolinecarboxylic acid (17.3 mg, 100 µmol), and purified with flash silica gel column chromatography (\(\Phi\) 0.7 × 6.3 cm, CHCl3/MeOH: 100/0 → 99/1 → 98.5/1.5 → 98/1 → 97.5/2.5 → 97/3 → 95/5) as a white amorphous solid. 1H NMR (DMSO-d6, 400 MHz) δ 9.00 (dd, 2H, Ar–H, J = 1.8, 4.2 Hz), 8.70 (d, 2H, Ser-NH, JSer-NH, Ser-α-CH = 7.8 Hz), 8.53–8.51 (m, 4H, Ar–H), 8.17–8.11 (m, 4H, Ar–H), 7.85 (d, 2H, Ala-NH, JAla-NH, Ala-α-CH = 5.2 Hz), 7.63 (dd, 2H, Ar–H, J = 4.2, 8.6 Hz), 6.28 (dd, 2H, Cys-α-CH, JCys-α-CH, Cys-β-CH = JCys-α-CH, Cys-β-CH = 6.8 Hz), 4.86 (d, 2H, Val-α-CH, JVal-α-CH, Val-β-CH = 10.4 Hz), 4.71 (ddd, 2H, Ser-α-CH, JSer-α-CH, Ser-β-CH = 3.0, JSer-α-CH, Ser-NH = 7.8, JSer-α-CH, Ser-β-CH = 8.0 Hz), 4.59–4.47 (m, 4H, Ala-α-CH, Ser-β-CH), 4.33 (dd, 2H, Ser-β-CH, JSer-β-CH, Ser-α-CH = 3.0, JSer-β-CH, Ser-β-CH = 11.4 Hz), 3.09 (dd, 2H, Cys-β-CH, JCys-β-CH, Cys-α-CH = 6.8, JCys-β-CH, Cys-β-CH = 14.8 Hz), 3.01 (s, 6H, NCH), 2.81 (s, 6H, NCH), 2.54–2.49 (m, 2H, Cys-β-CH), 2.22 (qqd, 2H, Val-β-CH, JVal-β-CH, Val-γ-CH = 6.4, JVal-β-CH, Val-γ-CH = 7.2, JVal-β-CH, Val-α-CH = 10.4 Hz), 1.27 (d, 6H, Ala-β-CH, JAla-β-CH, Ala-α-CH = 7.2 Hz), 0.99 (d, 6H, Val-γ-CH, JVal-γ-CH, Val-β-CH = 6.4 Hz), 0.76 (d, 6H, Val-γ-CH, JVal-γ-CH, Val-β-CH = 7.2 Hz); 13C NMR (DMSO-d6, 100 MHz) δ172.3, 170.2, 169.7, 167.8, 166.0, 152.3, 148.8, 137.1, 131.6, 129.1, 128.4, 127.8, 127.0, 122.3, 64.8, 61.4, 54.1, 53.2, 46.0, 35.3, 30.4, 29.8, 26.5, 20.2, 18.6, 16.6; ESIMS-LR m/z 1075.4 [(M + Na)+]; ESIMS-HR calcd for C52H65N10O12S 1053.4499, found 1053.4536; [a]23D −208.5 (c 0.20, DMSO).
{N-(Naphthalene-2-carbonyl)-D-Ser-L-Ala-N-Boc-N-Me-L-Cys-N-Me-L-Val}2 sulfide (7)
Compound 7 (8.8 mg, 8.4 µmol, 33%) was synthesized using methods previously reported by our group20, from 2-naphthoic acid (17.2 mg, 100 µmol) with flash silica gel column chromatography (\(\Phi\) 0.7 × 8.4 cm, CHCl3/MeOH: 100/0 → 99/1 → 98.5/1.5) as a white amorphous solid. 1H NMR (DMSO-d6, 400 MHz) δ 8.60 (d, 2H, Ser-NH, JSer-NH, Ser-α-CH = 7.8 Hz), 8.44 (s, 2H, Ar–H), 8.07–7.98 (m, 6H, Ar–H), 7.91 (dd, 2H, Ar–H, J = 1.6, 8.6 Hz), 7.82 (d, 2H, Ala-NH, JAla-NH, Ala-α-CH = 5.6 Hz), 7.65–7.59 (m, 4H, Ar–H), 6.29 (dd, 2H, Cys-α-CH, JCys-α-CH, Cys-β-CH = 6.6, JCys-α-CH, Cys-β-CH = 6.8 Hz), 4.86 (d, 2H, Val-α-CH, JVal-α-CH, Val-β-CH = 10.4 Hz), 4.71 (ddd, 2H, Ser-α-CH, JSer-α-CH, Ser-β-CH = 2.8, JSer-α-CH, Ser-NH = 7.8, JSer-α-CH, Ser-β-CH = 8.0 Hz), 4.57 (dd, 2H, Ala-α-CH, JAla-α-CH, Ala-β-CH = JAla-α-CH, Ala-NH = 6.4 Hz), 4.52–4.47 (m, 2H, Ser-β-CH), 4.32 (dd, 2H, Ser-β-CH, JSer-β-CH, Ser-α-CH = 2.8, JSer-β-CH, Ser-β-CH = 11.0 Hz), 3.10 (dd, 2H, Cys-β-CH, JCys-β-CH, Cys-α-CH = 6.6, JCys-β-CH, Cys-β-CH = 14.8 Hz), 3.01 (s, 6H, NCH), 2.81 (s, 6H, NCH), 2.53–2.49 (m, 2H, Cys-β-CH), 2.22 (qqd, 2H, Val-β-CH, JVal-β-CH, Val-γ-CH = 6.4, JVal-β-CH, Val-γ-CH = 6.8, JVal-β-CH, Val-α-CH = 10.4 Hz), 1.27 (d, 6H, Ala-β-CH, JAla-β-CH, Ala-α-CH = 7.2 Hz), 0.99 (d, 6H, Val-γ-CH, JVal-γ-CH, Val-β-CH = 6.8 Hz), 0.76 (d, 6H, Val-γ-CH, JVal-γ-CH, Val-β-CH = 6.4 Hz); 13C NMR (CDCl3, 100 MHz) δ173.2, 173.1, 169.7, 168.3, 167.9, 135.2, 132.7, 129.3, 129.0, 128.5, 128.4, 128.3, 127.9, 127.1, 123.3, 65.7, 61.9, 55.1, 54.9, 46.6, 36.5, 30.7, 30.3, 27.0, 20.5, 18.3, 17.6; ESIMS-LR m/z 1073.2 [(M + Na)+]; ESIMS-HR calcd for C54H67N8O12S 1051.4594, found 1051.4565; [α]24D −235.0 (c 0.20, CHCl3).
(N-Boc-D-Ser-L-Ala-N-Boc-N-Me-L-Cys-N-Me-L-Val)2 sulfide (22)
Compounds were prepared using methods previously reported by our group20. Namely, compound 21 (20.0 mg, 19.8 µmol) was treated with thioanisole (185 µL, 1.58 mmol) and TFA (3.9 mL) at room temperature, and the whole mixture was stirred at 40 °C for 15 h. The resulting mixture was concentrated in vacuo, and washed with Et2O (15 mL × 3) to afford a pale yellow solid. A solution of the residue and Boc2O (18.2 µL, 79.2 µmol) in THF (0.4 mL) was treated with Et3N (21.9 µL, 158 µmol) at room temperature, and the whole mixture was stirred at 40 °C for 5 h. The resulting mixture was diluted with AcOEt (15 mL), and the solution was washed with 1 M aq. HCl (5 mL × 2), brine (5 mL), dried (Na2SO4), filtered, and concentrated in vacuo. The residue was purified by silica gel column chromatography (\(\Phi\) 1 × 10 cm, CHCl3/MeOH: 100/0 → 99/1 → 98/2 → 97/3) to afford 22 (7.3 mg, 7.7 µmol, 39% over 2 steps) as a colorless solid. 1H NMR (CDCl3, 400 MHz) δ 6.81 (s, 2H, Ala-NH), 6.22 (br s, 2H, Cys-α-CH), 5.28 (d, 2H, Ser-NH, JSer-NH, Ser-α-CH = 6.4 Hz), 5.05 (d, 2H, Val-α-CH, JVal-α-CH, Val-β-CH = 10.6 Hz), 4.81 (br s, 2H, Ala-α-CH), 4.65 (dd, 2H, Ser-β-CH, Jgem = 11.9, JSer-β-CH, Ser-α-CH = 4.1 Hz), 4.53 (d, 2H, Ser-β-CH, Jgem = 11.9 Hz), 4.38 (br s, 2H, Ser-α-CH), 3.30 (m, 2H, Cys-β-CH), 3.07 (s, 6H, N–CH), 2.92 (s, 6H, N–CH) 2.48 (br s, 2H, Cys-β-CH), 2.23 (m, 2H, Val-β-CH), 1.47 (s, 18H, Boc), 1.37 (d, 6H, Ala-β-CH, JAla-β-CH, Ala-α-CH = 7.3 Hz), 1.080 (d, 6H, Val-γ-CH, JVal-γ-CH, Val-β-CH = 6.4 Hz), 0.82 (d, 6H, Val-γ-CH, JVal-γ-CH, Val-β-CH = 6.9 Hz); 13C NMR (CDCl3, 100 MHz) δ 173.2, 171.6, 169.9, 168.4, 155.6, 81.5, 64.3, 62.1, 55.0, 54.8, 46.1, 36.2, 31.4, 30.3, 28.3, 27.5, 20.4, 18.9, 17.7; ESIMS-LR m/z 943.87 [(M + H)+]; ESIMS-HR calcd for C42H71N8O14S 943.4805; found 943.4833; [α]17D − 187.33 (C 0.73, CHCl3).
General procedure for the synthesis of 8–20
Compounds were prepared using methods previously reported by our group20. Namely, compound 22 (100 mg, 106 µmol) was treated with 4 M HCl/1,4-dioxane (8 mL) at room temperature for 1 h. The resulting mixture was concentrated in vacuo, and the residue was washed with Et2O (15 mL × 3) to afford a white solid. The residue was divided into 5 portions. A suspension of each portion, carboxylic acid (63.6 µmol), HOAt (8.7 mg, 63.6 µmol) and Et3N (20.6 µL, 148 µmol) in DMF (0.3 mL) was treated with EDCI (12.2 mg, 63.6 µmol) at 0 °C, and the whole mixture was stirred at room temperature for several hours. The resulting mixture was diluted with AcOEt, and the solution was washed with H2O, 1 M aq. HCl, sat. aq. NaHCO3 and, brine, dried over Na2SO4, filtered, and concentrated in vacuo. The residue was purified to afford 8–20.
{N-(Indol-2-carbonyl)-D-Ser-L-Ala-N-Boc-N-Me-L-Cys-N-Me-L-Val}2 sulfide (8)
Compound 8 (6.5 mg, 6.3 µmol, 30% over 2 steps) was synthesized using methods previously reported by our group20, from indole-2-carboxylic acid (10.2 mg, 63.6 µmol), and purified with high flash silica gel column chromatography (\(\Phi\) 2 × 6 cm, CHCl3/MeOH: 100/0 → 94/6) as a white solid. 1H NMR (DMSO-d6, 400 MHz) δ 11.7 (d, 2H, Ar–NH), 8.40 (d, 2H, Ser-NH, JSer-NH, Ser-α-CH = 7.3 Hz), 7.84 (d, 2H, Ala-NH, JAla-NH, Ala-α-CH = 4.1 Hz), 7.65 (d, 2H, Ar, J = 7.8 Hz), 7.44 (d, 2H, Ar, J = 7.8 Hz), 7.21–7.04 (m, 6H, Ar), 6.28 (t, 2H, Cys-α-CH, JCys-α-CH, Cys-β-CH = 6.0 Hz), 4.83 (d, 2H, Val-α-CH, JVal-α-CH, Val-β-CH = 8.7 Hz), 4.65 (m, 2H, Ser-α-CH), 4.55 (qd, 2H, Ala-α-CH, JAla-α-CH, Ala-NH = 5.0, JAla-α-CH, Ala-β-CH = 4.1 Hz), 4.45 (m, 2H, Ser-β-CH), 4.31 (d, 2H, Ser-β-CH, Jgem = 11.0 Hz), 3.08 (dd, 2H, Cys-β-CH, Jgem = 14.6, JCys-β-CH, Cys-α-CH = 5.5 Hz), 3.03 (s, 6H, N–CH), 2.80 (s, 6H, N–CH), 2.50–2.45 (overlap, 2H, Cys-β-CH), 2.23 (m, 2H, Val-β-CH), 1.27 (d, 6H, Ala-β-CH, JAla-β-CH, Ala-α-CH = 5.0 Hz), 0.99 (d, 6H, Val-γ-CH, JVal-γ-CH, Val-β-CH = 4.6 Hz), 0.76 (d, 6H, Val-γ-CH, JVal-γ-CH, Val-β-CH = 5.0 Hz); 13C NMR (DMSO-d6, 100 MHz) δ 172.3, 170.3, 169.8, 167.8, 161.2, 136.6, 130.8, 126.9, 123.8, 121.7, 119.9, 112.4, 103.7, 64.8, 61.5, 54.2, 52.7, 46.0, 30.5, 29.8, 29.0, 26.6, 20.3, 18.8, 16.6; ESIMS-LR m/z 1029.53 [(M + H)+]; ESIMS-HR calcd for C50H65N10O12S 1029.4499, found 1029.4497; [α]22D − 177.67 (C 1.08, DMSO).
{N-(Indol-3-carbonyl)-D-Ser-L-Ala-N-Boc-N-Me-L-Cys-N-Me-L-Val}2 sulfide (9)
Compound 9 (4.2 mg, 4.1 µmol, 19% over 3 steps) was synthesized using methods previously reported by our group20, from N-Boc-indole-3-carboxylic acid (16.6 mg, 63.6 µmol). After amide coupling, the residue was treated with 50% TFA/CH2Cl2 (1 mL) at room temperature, and the whole mixture was stirred at 40 °C for 5 h. The resulting mixture was concentrated in vacuo, and the residue was purified by high flash silica gel column chromatography (\(\Phi\) 2 × 6 cm, CHCl3/MeOH: 100/0 → 94/6) to afford compound 9 as a white solid. 1H NMR (DMSO-d6, 400 MHz) δ 11.7 (d, 2H, Ar–NH), 8.06 (d, 2H, Ar, J = 8.2 Hz), 8.05 (s, 2H, Ar-C2H), 7.82 (d, 2H, Ser-NH, JSer-NH, Ser-α-CH = 7.3 Hz), 7.78 (d, 2H, Ala-NH, JAla-NH, Ala-α-CH = 5.0 Hz), 7.18–7.11 (m, 4H, Ar), 6.28 (dd, 2H, Cys-α-CH, JCys-α-CH, Cys-β-CH = 6.9, 5.9 Hz), 4.81 (d, 2H, Val-α-CH, JVal-α-CH, Val-β-CH = 10.5 Hz), 4.69 (m, 2H, Ser-α-CH), 4.57 (qd, 2H, Ala-α-CH, JAla-α-CH, Ala-β-CH = 6.0, JAla-α-CH, Ala-NH = 5.0 Hz), 4.43 (m, 2H, Ser-β-CH), 4.30 (d, 2H, Ser-β-CH, Jgem = 10.5 Hz), 3.08 (dd, 2H, Cys-β-CH, Jgem = 14.6, JCys-β-CH, Cys-α-CH = 5.9 Hz), 3.02 (s, 6H, N–CH), 2.80 (s, 6H, N–CH), 2.50–2.45 (dd, 2H, Cys-β-CH, Jgem = 14.6, JCys-β-CH, Cys-α-CH = 6.9 Hz), 2.21 (m, 2H, Val-β-CH), 1.26 (d, 6H, Ala-β-CH, JAla-β-CH, Ala-α-CH = 6.4 Hz), 0.99 (d, 6H, Val-γ-CH, JVal-γ-CH, Val-β-CH = 5.5 Hz), 0.76 (d, 6H, Val-γ-CH, JVal-γ-CH, Val-β-CH = 6.0 Hz); 13C NMR (DMSO-d6, 100 MHz) δ 172.3, 170.3, 169.9, 168.5, 164.5, 136.2, 129.0, 125.8, 122.1, 120.6, 112.1, 109.8, 65.1, 61.6, 54.2, 52.4, 46.0, 35.3, 30.5, 29.7, 26.6, 20.2, 18.9, 16.7; ESIMS-LR m/z 1029.88 [(M + H)+]; ESIMS-HR calcd for C50H65N10O12S1 1029.4499, found 1029.4497; [α]23D − 113.26 (C 0.19, DMSO).
{N-(Indol-5-carbonyl)-D-Ser-L-Ala-N-Boc-N-Me-L-Cys-N-Me-L-Val}2 sulfide (10)
Compound 10 (12.4 mg, 12.1 µmol, 57% over 2 steps) was synthesized using methods previously reported by our group20, from indole-5-carboxylic acid (10.2 mg, 63.6 µmol), and purified with high flash silica gel column chromatography (\(\Phi\) 2 × 6 cm, CHCl3/MeOH: 100/0 → 94/6) as a white solid. 1H NMR (DMSO-d6, 400 MHz) δ 11.4 (d, 2H, Ar–NH), 8.29 (d, 2H, Ser-NH, JSer-NH, Ser-α-CH = 7.3 Hz), 8.12 (s, 2H, Ar), 7.79 (d, 2H, Ala-NH, JAla-NH, Ala-α-CH = 5.0 Hz), 7.61 (d, 2H, Ar, J = 8.7 Hz), 7.46 (d, 2H, Ar, J = 8.7 Hz), 7.45 (s, 2H, Ar), 6.56 (s, 2H, Ar), 6.27 (dd, 2H, Cys-α-CH, JCys-α-CH, Cys-β-CH = 6.6, 6.2 Hz), 4.83 (d, 2H, Val-α-CH, JVal-α-CH, Val-β-CH = 10.5 Hz), 4.66 (m, 2H, Ser-α-CH), 4.56 (qd, 2H, Ala-α-CH, JAla-α-CH, Ala-β-CH = 6.9, JAla-α-CH, Ala-NH = 5.0 Hz), 4.44 (m, 2H, Ser-β-CH), 4.28 (d, 2H, Ser-β-CH, Jgem = 10.6 Hz), 3.09 (dd, 2H, Cys-β-CH, Jgem = 15.1, JCys-β-CH, Cys-α-CH = 6.2 Hz), 3.01 (s, 6H, N–CH), 2.80 (s, 6H, N–CH), 2.50 (overlap, 2H, Cys-β-CH), 2.24–2.18 (m, 2H, Val-β-CH), 1.26 (d, 6H, Ala-β-CH, JAla-β-CH, Ala-α-CH = 6.9 Hz), 0.98 (d, 6H, Val-γ-CH, JVal-γ-CH, Val-β-CH = 6.4 Hz), 0.76 (d, 6H, Val-γ-CH, JVal-γ-CH, Val-β-CH = 6.4 Hz); 13C NMR (DMSO-d6, 100 MHz) δ 172.3, 170.3, 169.8, 168.3, 167.5, 136.7, 127.0, 124.6, 120.7, 120.3, 111.1, 102.2, 65.0, 61.5, 54.2, 53.1, 45.9, 35.3, 30.5, 29.8, 26.6, 20.2, 18.8, 16.7; ESIMS-LR m/z 1051.95 [(M + Na)+]; ESIMS-HR calcd for C50H65N10O12S 1029.4499, found 1029.4497; [α]24D − 234.83 (C 0.81, DMSO).
{N-(Indol-6-carbonyl)-D-Ser-L-Ala-N-Boc-N-Me-L-Cys-N-Me-L-Val}2 sulfide (11)
Compound 11 (6.1 mg, 5.9 µmol, 28% over 2 steps) was synthesized using methods previously reported by our group20, from indole-6-carboxylic acid (10.2 mg, 63.6 µmol), and purified with high flash silica gel column chromatography (\(\Phi\) 2 × 6 cm, CHCl3/MeOH: 100/0 → 94/6) as a white solid. 1H NMR (DMSO-d6, 400 MHz) δ 11.5 (d, 2H, Ar–NH), 8.30 (d, 2H, Ser-NH, JSer-NH, Ser-α-CH = 7.4 Hz), 7.92 (s, 2H, Ar), 7.78 (d, 2H, Ala-NH, JAla-NH, Ala-α-CH = 5.5 Hz), 7.62 (d, 2H, Ar J = 8.5 Hz), 7.54 (s, 2H, Ar), 7.49 (d, 2H, J = 8.5 Hz), 6.50 (s, 2H, Ar), 6.28 (t, 2H, Cys-α-CH, JCys-α-CH, Cys-β-CH = 6.4 Hz), 4.83 (d, 2H, Val-α-CH, JVal-α-CH, Val-β-CH = 10.1 Hz), 4.66 (m, 2H, Ser-α-CH), 4.57 (qd, 2H, Ala-α-CH, JAla-α-CH, Ala-β-CH = 7.3, JAla-α-CH, Ala-NH = 5.5 Hz), 4.45 (m, 2H, Ser-β-CH), 4.28 (d, 2H, Ser-β-CH, Jgem = 10.1 Hz), 3.08 (dd, 2H, Cys-β-CH, Jgem = 15.1, JCys-β-CH, Cys-α-CH = 6.4 Hz), 3.01 (s, 6H, N–CH), 2.79 (s, 6H, N–CH), 2.50 (overlap, 2H, Cys-β-CH), 2.20 (m, 2H, Val-β-CH), 1.25 (d, 6H, Ala-β-CH, JAla-β-CH, Ala-α-CH = 7.3 Hz), 0.98 (d, 6H, Val-γ-CH, JVal-γ-CH, Val-β-CH = 6.0 Hz), 0.75 (d, 6H, Val-γ-CH, JVal-γ-CH, Val-β-CH = 6.4 Hz); 13C NMR (DMSO-d6, 100 MHz) δ 172.3, 170.3, 169.8, 168.2, 167.4, 135.1, 130.2, 128.4, 126.3, 119.6, 118.2, 111.6, 101.4, 64.9, 61.5, 54.2, 53.2, 45.9, 35.4, 30.5, 29.8, 26.6, 20.3, 18.8, 16.7; ESIMS-LR m/z 1051.91 [(M + Na)+]; ESIMS-HR calcd for C50H65N10O12S 1029.4499, found 1029.4497; [α]24D − 216.52 (C 0.26, DMSO).
{N-(Indol-7-carbonyl)-D-Ser-L-Ala-N-Boc-N-Me-L-Cys-N-Me-L-Val}2 sulfide (12)
Compound 12 (6.1 mg, 5.9 µmol, 28% over 2 steps) was synthesized using methods previously reported by our group20, from indole-7-carboxylic acid (10.2 mg, 63.6 µmol), and purified with high flash silica gel column chromatography (\(\Phi\) 2 × 6 cm, CHCl3/MeOH: 100/0 → 94/6) as a white solid. 1H NMR (DMSO-d6, 400 MHz) δ 11.2 (d, 2H, Ar–NH), 8.45 (d, 2H, Ser-NH, JSer-NH, Ser-α-CH = 7.3 Hz), 7.84 (d, 2H, Ala-NH, JAla-NH, Ala-α-CH = 5.9 Hz), 7.78 (d, 2H, Ar J = 8.2 Hz), 7.62 (d, 2H, Ar, J = 7.3 Hz), 7.36 (t, 2H, J = 2.7 Hz), 7.12 (t, 2H, J = 7.6 Hz), 6.51 (dd, 2H, Ar, J = 3.2, 2.3 Hz), 6.27 (dd, 2H, Cys-α-CH, JCys-α-CH, Cys-β-CH = 6.9, 6.4 Hz), 4.84 (d, 2H, Val-α-CH, JVal-α-CH, Val-β-CH = 10.5 Hz), 4.72 (ddd, 2H, Ser-α-CH, JSer-α-CH, Ser-β-CH = 8.2, JSer-α-CH, Ser-NH = 7.3, JSer-α-CH, Ser-β-CH = 2.6 Hz), 4.56 (qd, 2H, Ala-α-CH, JAla-α-CH, Ala-β-CH = 6.8, JAla-α-CH, Ala-NH = 5.9 Hz), 4.47 (dd, 2H, Ser-β-CH, Jgem = 11.2, JSer-β-CH, Ser-α-CH = 8.2 Hz), 4.34 (dd, 2H, Ser-β-CH, Jgem = 11.2, JSer-β-CH, Ser-α-CH = 2.6 Hz), 3.09 (dd, 2H, Cys-β-CH, Jgem = 14.6, JCys-β-CH, Cys-α-CH = 6.4 Hz), 3.01 (s, 6H, N–CH), 2.79 (s, 6H, N–CH), 2.50 (overlap, 2H, Cys-β-CH), 2.21 (m, 2H, Val-β-CH), 1.25 (d, 6H, Ala-β-CH, JAla-β-CH, Ala-α-CH = 6.8 Hz), 0.98 (d, 6H, Val-γ-CH, JVal-γ-CH, Val-β-CH = 6.4 Hz), 0.75 (d, 6H, Val-γ-CH, JVal-γ-CH, Val-β-CH = 6.9 Hz); 13C NMR (DMSO-d6, 100 MHz) δ 172.3, 170.4, 169.8, 168.1, 167.0, 134.1, 129.3, 127.0, 124.4, 120.4, 118.1, 116.2, 101.2, 64.9, 61.5, 54.2, 52.9, 46.0, 35.4, 30.5, 29.8, 26.6, 20.3, 18.7, 16.7; ESIMS-LR m/z 1051.71 [(M + Na)+]; ESIMS-HR calcd for C50H65N10O12S 1029.4499, found 1029.4497; [α]25D − 207.55 (C 0.61, DMSO).
{N-(Benzofurane-2-carbonyl)-D-Ser-L-Ala-N-Boc-N-Me-L-Cys-N-Me-L-Val}2 sulfide (13)
Compound 13 (18.5 mg, 18.0 µmol, 85% over 2 steps) was synthesized using methods previously reported by our group20, from benzofuran-2-carboxylic acid (10.3 mg, 63.6 µmol), and purified with silica gel column chromatography (\(\Phi\) 1 × 10 cm, CHCl3/MeOH: 100/0 → 99/1 → 98/2 → 97/3) as a white solid. 1H NMR (DMSO-d6, 400 MHz) δ 8.38 (d, 2H, Ser-NH, JSer-NH, Ser-α-CH = 8.7 Hz), 7.98 (d, 2H, Ala-NH, JAla-NH, Ala-α-CH = 6.0 Hz), 7.80 (d, 2H, Ar, J = 8.2 Hz), 7.67 (d, 2H, Ar, J = 8.2 Hz), 7.64 (s, 2H, Ar), 7.49 (m, 2H,r Ar), 7.35 (m, 2H, Ar), 6.28 (dd, 2H, Cys-α-CH, JCys-α-CH, Cys-β-CH = 6.9, 6.4 Hz), 4.84 (d, 2H, Val-α-CH, JVal-α-CH, Val-β-CH = 10.5 Hz), 4.69 (m, 2H, Ser-α-CH), 4.57 (qd, 2H, Ala-α-CH, JAla-α-CH, Ala-β-CH = 6.9, JAla-α-CH, Ala-NH = 6.0 Hz), 4.46 (dd, 2H, Ser-β-CH, Jgem = 11.4, JCys-β-CH, Cys-α-CH = 7.8 Hz), 4.31 (d, 2H, Ser-β-CH, Jgem = 11.4 Hz), 3.09 (dd, 2H, Cys-β-CH, Jgem = 15.1, JCys-β-CH, Cys-α-CH = 6.4 Hz), 3.03 (s, 6H, N–CH), 2.80 (s, 6H, N–CH), 2.46 (overlap, 2H, Cys-β-CH), 2.20 (m, 2H, Val-β-CH), 1.28 (d, 6H, Ala-β-CH, JAla-β-CH, Ala-α-CH = 6.9 Hz), 0.98 (d, 6H, Val-γ-CH, JVal-γ-CH, Val-β-CH = 6.9 Hz), 0.77 (d, 6H, Val-γ-CH, JVal-γ-CH, Val-β-CH = 6.9 Hz); 13C NMR (DMSO-d6, 100 MHz) δ 172.3, 170.3, 169.8, 168.1, 167.0, 134.1, 129.3, 126.9, 124.4, 120.4, 118.1, 116.2, 101.2, 64.9, 61.5, 54.2, 52.9, 46.0, 35.4, 30.5, 29.8, 26.6, 20.2, 18.7, 16.7; ESIMS-LR m/z 1053.41 [(M + Na)+]; ESIMS-HR calcd for C50H63N8O14S 1031.4179, found 1031.4124; [α]25D − 238.12 (C 0.97, DMSO).
{N-(Benzothiophene-2-carbonyl)-D-Ser-L-Ala-N-Boc-N-Me-L-Cys-N-Me-L-Val}2 sulfide (14)
Compound 14 (19.6 mg, 18.5 µmol, 87% over 2 steps) was synthesized using methods previously reported by our group20, from benzothiophene-2-carboxylic acid (11.3 mg, 63.6 µmol), and purified with silica gel column chromatography (\(\Phi\) 1 × 10 cm, CHCl3/MeOH: 100/0 → 99/1 → 98/2 → 97/3) as a white solid. 1H NMR (DMSO-d6, 400 MHz) δ 8.71 (d, 2H, Ser-NH, JSer-NH, Ser-α-CH = 7.8 Hz), 8.14 (s, 2H, Ar), 8.06–8.00 (m, 4H, Ar), 7.85 (d, 2H, Ala-NH, JAla-NH, Ala-α-CH = 5.5 Hz), 7.51–7.44 (m, 4H, Ar), 6.29 (t, 2H, Cys-α-CH, JCys-α-CH, Cys-β-CH = 6.4 Hz), 4.84 (d, 2H, Val-α-CH, JVal-α-CH, Val-β-CH = 10.5 Hz), 4.64 (m, 2H, Ser-α-CH), 4.56 (dq, 2H, Ala-α-CH, JAla-α-CH, Ala-β-CH = 6.9, JAla-α-CH, Ala-NH = 5.5 Hz), 4.50 (m, 2H, Ser-β-CH), 4.31 (m, 2H, Ser-β-CH), 3.08 (dd, 2H, Cys-β-CH, Jgem = 15.1, JCys-β-CH, Cys-α-CH = 6.4 Hz), 3.02 (s, 6H, N–CH), 2.80 (s, 6H, N–CH), 2.50 (overlap, 2H, Cys-β-CH), 2.22 (m, 2H, Val-β-CH), 1.28 (d, 6H, Ala-β-CH, JAla-β-CH, Ala-α-CH = 6.9 Hz), 0.99 (d, 6H, Val-γ-CH, JVal-γ-CH, Val-β-CH = 6.4 Hz), 0.75 (d, 6H, Val-γ-CH, JVal-γ-CH, Val-β-CH = 6.4 Hz); 13C NMR (DMSO-d6, 100 MHz) δ 172.3, 170.2, 169.8, 161.7, 140.3, 138.9, 138.6, 128.4, 126.5, 126.0, 125.4, 125.1, 122.9, 64.6, 61.5, 54.2, 53.1, 46.1, 35.4, 30.5, 29.8, 26.6, 20.3, 18.8, 16.6; ESIMS-LR m/z 1085.44 [(M + Na)+]; ESIMS-HR calcd for C50H63N8O12S3 1063.3722, found 1063.3690; [α]26D − 246.77 (C 0.58, DMSO).
{N-(Benzoxazole-5-carbonyl)-D-Ser-L-Ala-N-Boc-N-Me-L-Cys-N-Me-L-Val}2 sulfide (15)
Compound 15 (7.2 mg, 7.0 µmol, 33% over 2 steps) was synthesized using methods previously reported by our group20, from benzoxazole-5-carboxylic acid (10.4 mg, 63.6 µmol), and purified with high flash silica gel column chromatography (\(\Phi\) 2 × 6 cm, CHCl3/MeOH: 100/0 → 99/1 → 98/2 → 97/3) as a white solid. 1H NMR (DMSO-d6, 400 MHz) δ 8.88 (d, 2H, Ar, J = 2.1 Hz), 8.57 (d, 2H, Ser-NH, JSer-NH, Ser-α-CH = 6.9 Hz), 8.33 (s, 2H, Ar), 7.96 (d, 2H, Ar, J = 8.7 Hz), 7.92 (d, 2H, Ar, J = 8.7, 2.1 Hz), 7.81 (d, 2H, Ala-NH, JAla-NH, Ala-α-CH = 4.6 Hz), 6.27 (dd, 2H, Cys-α-CH, JCys-α-CH, Cys-β’-CH = 6.0, JCys-α-CH, Cys-β-CH = 5.7 Hz), 4.82 (d, 2H, Val-α-CH, JVal-α-CH, Val-β-CH = 9.2 Hz), 4.65 (m, 2H, Ser-α-CH), 4.56 (qd, 2H, Ala-α-CH, JAla-α-CH, Ala-β-CH = 5.5, JAla-α-CH, Ala-NH = 4.6 Hz), 4.44 (m, 2H, Ser-β-CH), 4.32 (d, 2H, Ser-β-CH, Jgem = 11.0 Hz), 3.08 (dd, 2H, Cys-β-CH, Jgem = 15.1, JCys-β-CH, Cys-α-CH = 5.7 Hz), 3.02 (s, 6H, N–CH), 2.80 (s, 6H, N–CH), 2.50 (overlap, 2H, Cys-β-CH), 2.19 (m, 2H, Val-β-CH), 1.26 (d, 6H, Ala-β-CH, JAla-β-CH, Ala-α-CH = 5.5 Hz), 0.99 (d, 6H, Val-γ-CH, JVal-γ-CH, Val-β-CH = 5.0 Hz), 0.75 (d, 6H, Val-γ-CH, JVal-γ-CH, Val-β-CH = 5.5 Hz); 13C NMR (DMSO-d6, 100 MHz) δ 172.3, 170.3, 169.8, 167.9, 166.1, 155.6, 151.3, 139.5, 130.8, 125.8, 119.7, 111.2, 64.8, 61.5, 54.1, 53.3, 46.0, 35.3, 30.5, 29.8, 26.5, 20.3, 18.7, 16.6; ESIMS-LR m/z 1033.57 [(M + H)+]; ESIMS-HR calcd for C48H61N10O14S 1033.4084, found 1033.4098; [α]26D − 412.43 (C 0.21, DMSO).
{N-(Benzoxazole-6-carbonyl)-D-Ser-L-Ala-N-Boc-N-Me-L-Cys-N-Me-L-Val}2 sulfide (16)
Compound 16 (11.9 mg, 11.5 µmol, 54% over 2 steps) was synthesized using methods previously reported by our group20, from benzoxazole-6-carboxylic acid (10.4 mg, 63.6 µmol), and purified with high flash silica gel column chromatography (\(\Phi\) 2 × 6 cm, CHCl3/MeOH: 100/0 → 99/1 → 98/2 → 97/3) as a white solid. 1H NMR (CD3OD, 400 MHz) δ 8.63 (s, 2H, Ar), 8.25 (s, 2H, Ar), 7.98 (dd, 2H, Ar, J = 8.2, 1.8 Hz), 7.87 (d, 2H, Ar, J = 8.2 Hz), 6.24 (dd, 2H, Cys-α-CH, JCys-α-CH, Cys-β-CH = 7.3, 6.4 Hz), 4.93–4.89 (overlap, 4H, Val-α-CH, Ser-α-CH), 4.70 (q, 2H, Ala-α-CH, JAla-α-CH, Ala-β-CH = 7.3 Hz), 4.63 (dd, 2H, Ser-β-CH, Jgem = 11.9, JSer-β-CH, Ser-α-CH = 5.4 Hz), 4.54 (dd, 2H, Ser-β-CH, Jgem = 11.9, JSer-β-CH, Ser-α-CH = 2.3 Hz), 3.34 (dd, 2H, Cys-β-CH, Jgem = 15.1, JCys-β-CH, Cys-α-CH = 6.4 Hz), 3.16 (s, 6H, N–CH), 3.01 (s, 6H, N–CH), 2.65 (dd, 2H, Cys-β-CH, Jgem = 15.1, JCys-β-CH, Cys-α-CH = 7.3 Hz), 2.25 (m, 2H, Val-β-CH), 1.39 (d, 6H, Ala-β-CH, JAla-β-CH, Ala-α-CH = 7.3 Hz), 1.04 (d, 6H, Val-γ-CH, JVal-γ-CH, Val-β-CH = 6.4 Hz), 0.84 (d, 6H, Val-γ-CH, JVal-γ-CH, Val-β-CH = 6.9 Hz); 13C NMR (CD3OD, 100 MHz) δ 175.3, 172.2, 171.9, 170.2, 169.5, 157.4, 151.1, 143.9, 133.1, 125.7, 121.0, 112.0, 65.4, 63.9, 56.1, 54.8, 35.6, 32.2, 30.8, 28.4, 20.5, 19.6, 16.6, 7.9; ESIMS-LR m/z 1033.63 [(M + H)+]; ESIMS-HR calcd for C48H61N10O14S 1033.4084, found 1033.4098; [α]24D − 257.59 (C 0.45, DMSO).
{N-(Benzthiazole-6-carbonyl)-D-Ser-L-Ala-N-Boc-N-Me-L-Cys-N-Me-L-Val}2 sulfide (17)
Compound 17 (11.5 mg, 10.8 µmol, 51% over 2 steps) was synthesized using methods previously reported by our group20, from benzothiazole-6-carboxylic acid (11.4 mg, 63.6 µmol), and purified with high flash silica gel column chromatography (\(\Phi\) 2 × 6 cm, CHCl3/MeOH: 100/0 → 99/1 → 98/2 → 97/3) as a white solid. 1H NMR (DMSO-d6, 400 MHz) δ 9.56 (s, 2H, Ar), 8.69 (d, 2H, Ar, J = 1.4 Hz), 8.62 (d, 2H, Ser-NH, JSer-NH, Ser-α-CH = 8.2 Hz), 8.20 (d, 2H, Ar, J = 8.7 Hz), 8.00 (d, 2H, Ar, J = 8.7, 1.4 Hz), 7.84 (d, 2H, Ala-NH, JAla-NH, Ala-α-CH = 5.5 Hz), 6.27 (dd, 2H, Cys-α-CH, JCys-α-CH, Cys-β-CH = 6.9, 6.4 Hz), 4.84 (d, 2H, Val-α-CH, JVal-α-CH, Val-β-CH = 10.6 Hz), 4.66 (ddd, 2H, Ser-α-CH, JSer-α-CH, Ser-β-CH = 9.2, JSer-α-CH, Ser-NH = 8.2, JSer-α-CH, Ser-β-CH = 2.8 Hz), 4.55 (qd, 2H, Ala-α-CH, JAla-α-CH, Ala-β-CH = 6.9, JAla-α-CH, Ala-NH = 5.5 Hz), 4.45 (dd, 2H, Ser-β-CH, Jgem = 11.4, JSer-β-CH, Ser-α-CH, = 9.2 Hz), 4.31 (dd, 2H, Ser-β-CH, Jgem = 11.4, JSer-β-CH, Ser-α-CH, = 2.8 Hz), 3.08 (dd, 2H, Cys-β-CH, Jgem = 15.1, JCys-β-CH, Cys-α-CH = 6.4 Hz), 3.00 (s, 6H, N–CH), 2.80 (s, 6H, N–CH), 2.54–48(overlap, 2H, Cys-β-CH), 2.24–2.17 (m, 2H, Val-β-CH), 1.26 (d, 6H, Ala-β-CH, JAla-β-CH, Ala-α-CH = 6.9 Hz), 0.99 (d, 6H, Val-γ-CH, JVal-γ-CH, Val-β-CH = 6.4 Hz), 0.75 (d, 6H, Val-γ-CH, JVal-γ-CH, Val-β-CH = 6.4 Hz); 13C NMR (DMSO-d6, 100 MHz) δ 172.3, 170.3, 169.8, 167.9, 166.1, 159.3, 154.9, 133.7, 131.0, 125.6, 122.8, 122.6, 64.8, 61.4, 54.1, 53.2, 46.1, 35.3, 30.5, 29.8, 26.5, 20.3, 18.7, 16.6; ESIMS-LR m/z 1065.53 [(M + H)+]; ESIMS-HR calcd for C48H61N10O12S3 1065.3627, found 1065.3638; [α]25D − 224.92 (C 0.95, DMSO).
{N-(4-Benzoylphenylcarbonyl)-D-Ser-L-Ala-N-Boc-N-Me-L-Cys-N-Me-L-Val}2 sulfide (18)
Compound 18 (17.4 mg, 15.0 µmol, 71% over 2 steps) was synthesized using methods previously reported by our group20, from p-benzoylbenzoic acid (14.4 mg, 63.6 µmol), and purified with high flash silica gel column chromatography (\(\Phi\) 2 × 6 cm, CHCl3/MeOH: 100/0 → 99/1 → 98/2) as a white solid. 1H NMR (DMSO-d6, 400 MHz) δ 8.62 (d, 2H, Ser-NH, JSer-NH, Ser-α-CH = 7.8 Hz), 7.99 (d, 4H, Ar, J = 8.2 Hz), 7.84 (d, 4H, Ar, J = 8.2 Hz), 7.82 (m, 2H, Ala-NH), 7.77–7.69 (m, 6H, Ar), 7.60–7.57 (m, 4H, Ar), 6.29 (t, 2H, Cys-α-CH, JCys-α-CH, Cys-β-CH = 6.4 Hz), 4.83 (d, 2H, Val-α-CH, JVal-α-CH, Val-β-CH = 10.6 Hz), 4.66 (m, 2H, Ser-α-CH), 4.56 (m, 2H, Ala-α-CH), 4.45 (m, 2H, Ser-β-CH), 4.31 (d, 2H, Ser-β-CH, Jgem = 11.0 Hz), 3.08 (dd, 2H, Cys-β-CH, Jgem = 14.6, JCys-β-CH, Cys-α-CH = 6.4 Hz), 3.00 (s, 6H, N–CH), 2.79 (s, 6H, N–CH), 2.50 (overlap, 2H, Cys-β-CH), 2.22 (m, 2H, Val-β-CH), 1.25 (d, 6H, Ala-β-CH, JAla-β-CH, Ala-α-CH = 6.9 Hz), 0.99 (d, 6H, Val-γ-CH, JVal-γ-CH, Val-β-CH = 6.4 Hz), 0.75 (d, 6H, Val-γ-CH, JVal-γ-CH, Val-β-CH = 6.4 Hz); 13C NMR (DMSO-d6, 100 MHz) δ 195.4, 172.3, 170.3, 169.8, 167.7, 165.9, 139.6, 137.1, 136.6, 133.1, 129.7, 129.6, 128.7, 127.8, 64.7, 61.4, 54.1, 53.2, 46.0, 35.3, 30.5, 29.8, 26.5, 20.3, 18.7, 16.6; ESIMS-LR m/z 1181.50 [(M + Na)+]; ESIMS-HR calcd for C60H71N8O14S 1159.4805, found 1159.4802; [α]24D − 189.17 (C 0.23, DMSO).
{N-(Anthraquinone-2carbonyl)-D-Ser-L-Ala-N-Boc-N-Me-L-Cys-N-Me-L-Val}2 sulfide (19)
Compound 19 (16.0 mg, 13.2 µmol, 62% over 2 steps) was synthesized using methods previously reported by our group20, from anthoraquinone-2-carboxylic acid (16.0 mg, 63.6 µmol), and purified with high flash silica gel column chromatography (\(\Phi\) 2 × 6 cm, CHCl3/MeOH: 100/0 → 99/1 → 98/2) as a pale yellow solid. 1H NMR (DMSO-d6, 400 MHz) δ 8.87 (d, 2H, Ser-NH, JSer-NH, Ser-α-CH = 7.8 Hz), 8.60 (s, 2H, Ar), 8.32 (s, 4H, Ar), 8.23 (dd, 4H, Ar, J = 4.6, 3.2 Hz), 7.96–7.94 (m, 4H, Ar), 7.91 (d, 2H, Ala-NH, JAla-NH, Ala-α-CH = 5.5 Hz), 6.30 (t, 2H, Cys-α-CH, JCys-α-CH, Cys-β-CH = 6.9 Hz), 4.85 (d, 2H, Val-α-CH, JVal-α-CH, Val-β-CH = 10.5 Hz), 4.68 (m, 2H, Ser-α-CH), 4.59 (m, 2H, Ala-α-CH), 4.49 (m, 2H, Ser-β-CH), 4.35 (d, 2H, Ser-β-CH, Jgem = 9.6 Hz), 3.10 (dd, 2H, Cys-β-CH, Jgem = 14.2, JCys-β-CH, Cys-α-CH = 6.9 Hz), 3.05 (s, 6H, N–CH), 2.81 (s, 6H, N–CH), 2.54 (overlap, 2H, Cys-β-CH), 2.26 (m, 2H, Val-β-CH), 1.29 (d, 6H, Ala-β-CH, JAla-β-CH, Ala-α-CH = 6.9 Hz), 0.99 (d, 6H, Val-γ-CH, JVal-γ-CH, Val-β-CH = 6.4 Hz), 0.77 (d, 6H, Val-γ-CH, JVal-γ-CH, Val-β-CH = 6.9 Hz); 13C NMR (DMSO-d6, 100 MHz) δ 182.1, 172.3, 170.3, 169.8, 167.6, 165.2, 138.7, 134.9, 134.8, 133.3, 133.1, 133.0, 127.2, 126.9, 125.8, 64.6, 61.5, 54.2, 53.3, 46.1, 35.3, 30.5, 29.8, 26.5, 20.3, 18.8, 16.6; ESIMS-LR m/z 1233.60 [(M + Na)+]; ESIMS-HR calcd for C62H67N8O16S 1211.4390, found 1211.4396; [α]24D − 192.98 (C 0.79, DMSO).
{N-(Pyradine-2carbonyl)-D-Ser-L-Ala-N-Boc-N-Me-L-Cys-N-Me-L-Val}2 sulfide (20)
Compound 20 (8.3 mg, 8.7 µmol, 49% over 2 steps) was synthesized using methods previously reported by our group20, from pyrazine-2-carboxylic acid (7.4 mg, 63.6 µmol), and purified with high flash silica gel column chromatography (\(\Phi\) 1 × 10 cm, CHCl3/MeOH: 100/0 → 98/2 → 96/4 → 94/6) as a white solid. 1H NMR (CDCl3, 400 MHz) δ 9.41 (d, 2H, Ar, J = 1.4 Hz), 8.80 (d, 2H, Ar, J = 2.3 Hz), 8.50 (dd, 2H, Ar, J = 2.3, 1.4 Hz), 8.45 (d, 2H, Ser-NH, JSer-NH, Ser-α-CH = 7.3 Hz), 6.72 (d, 2H, Ala-NH, JAla-NH, Ala-α-CH = 7.3 Hz), 6.23 (dd, 2H, Cys-α-CH, JCys-α-CH, Cys-β-CH = 6.9, 6.4 Hz), 5.10 (d, 2H, Val-α-CH, JVal-α-CH, Val-β-CH = 10.1 Hz), 4.88–4.85 (m, 2H, Ser-α-CH), 4.80 (dq, 2H, Ala-α-CH, JAla-NH, Ala-α-CH = 7.3, JAla-α-CH, Ala-β-CH = 7.3 Hz), 4.75 (dd, 2H, Ser-β-CH, Jgem = 11.4, JSer-β-CH, Ser-α-CH = 4.6 Hz), 4.63 (dd, 2H, Ser-β-CH, Jgem = 11.4, JSer-β-CH, Ser-α-CH = 1.4 Hz), 3.36 (dd, 2H, Cys-β-CH, Jgem = 15.1, JCys-β-CH, Cys-α-CH = 6.4 Hz), 3.11 (s, 6H, N–CH), 2.93 (s, 6H, N–CH), 2.47 (dd, 2H, Cys-β-CH, Jgem = 15.1, JCys-β-CH, Cys-α-CH = 6.9 Hz), 2.24 (m, 2H, Val-β-CH), 1.35 (d, 6H, Ala-β-CH, JAla-β-CH, Ala-α-CH = 7.3 Hz), 1.06 (d, 6H, Val-γ-CH, JVal-γ-CH, Val-β-CH = 6.9 Hz), 0.84 (d, 6H, Val-γ-CH, JVal-γ-CH, Val-β-CH = 6.9 Hz); 13C NMR (CDCl3, 100 MHz) δ 173.2, 171.4, 170.0, 167.5, 164.1, 148.2, 144.8, 143.4, 142.9, 64.0, 32.0, 54.9, 53.3, 46.4, 36.1, 31.4, 30.3, 27.7, 20.5, 19.0, 17.5; ESIMS-LR m/z 955.48 [(M + H)+]; ESIMS-HR not obtained; [α]19D − 201.55 (C 0.83, CHCl3).
Echinomycin probe 27
Compound 27 was prepared using methods previously reported by our group20. Namely, 25 (5.9 mg, 4.4 µmol) was treated with triisopropylsilane (0.96 µL, 4.96 µmol) and 80% aq. TFA (400 µL) at room temperature for 2.5 h. The reaction mixture was concentrated in vacuo, and then azeotroped with toluene to afford a crude carboxylic acid. A mixture of the residue and biotin-PEG2-amine 26 (2.2 mg, 5.9 µmol) in 50% CHCl3/MeOH (100 µL) was treated with DMT-MM (1.8 mg, 6.5 µmol) at room temperature for 3.5 h. The reaction mixture was concentrated in vacuo, and the crude was purified by preparative TLC (20 × 20 cm, 10% MeOH in CHCl3) to afford an inseparable mixture containing unreacted carboxylic acid (3.6 mg). Then the crude carboxylic acid (3.6 mg), iPr2NEt (1.90 µL, 11.2 µmol), EDCI (0.8 mg, 4.2 µmol) and HOAt (0.6 mg, 4.2 µmol) in DMF (100 µL) was treated with biotin-PEG2-amine (26) (2.1 mg, 5.6 µmol) at room temperature for 14 h. The reaction mixture was concentrated in vacuo, and then azeotroped with toluene to afford a white solid. The crude was purified by preparative TLC (20 × 20 cm, 10% MeOH in CHCl3) to afford compound 27 (1.94 mg, 1.18 µmol, 27% over 3 steps) as a white amorphous solid. 1H NMR (CDCl3, 400 MHz) δ 9.64 (s, 1H, Ar–H), 9.64 (s, 1H, Ar–H), 8.83 (d, 1H, Ser-NH, JSer-NH, Ser-α-CH = 6.4 Hz), 8.69 (d, 1H, Ser-NH, JSer-NH, Ser-α-CH = 7.6 Hz), 8.20 (d, 1H, Ar–H, J = 4.8 Hz), 8.18 (d, 1H, Ar–H, J = 5.2 Hz), 8.00 (d, 1H, Ar–H, J = 7.6 Hz), 7.96 (d, 1H, Ar–H, J = 8.0 Hz), 7.90–7.80 (m, 4H, Ar–H), 7.01–6.97 (m, 3H, Ala-NH, biotin-NH), 6.56 (s, 1H, biotin-NH), 6.43 (d, 1H, Cys-α-CH, JCys-α-CH, Cys-β-CH = 8.4 Hz), 6.19 (d, 1H, Cys-α-CH, JCys-α-CH, Cys-β-CH = 10.4 Hz), 5.59 (s, 1H, CONH), 5.59 (s, 1H, CONH), 5.16–5.11 (m, 3H, Val-α-CH, Cys-β-CH), 4.98–4.91 (m, 2H, Ser-α-CH, Ala-α-CH), 4.85–4.79 (m, 2H, Ser-α-CH, Ala-α-CH), 4.72–4.59 (m, 4H, Ser-β-CH), 4.51–4.48 (m, 1H, biotin-NHCH), 4.31–4.29 (m, 1H, biotin-NHCH), 3.78–3.40 (m, 25H, Cys-β-CH, Hb, Hc, Hd, He, Hf, Hg, Hi, Hj, Hk, Hl, Hm, Hn), 3.16–3.11 (m, 7H, NCH, biotin-SCH), 3.00 (s, 3H, NCH), 2.99 (s, 3H, NCH), 2.94–2.80 (m, 3H, Cys-β-CH, Hh), 2.74–2.68 (m, 2H, biotin-SCH2), 2.51 (t, 2H, Ha, JHa, Hb = 6.0 Hz), 2.38–2.28 (m, 2H, Val-β-CH), 2.23 (t, 2H, Ho, JHo, Hp = 7.2 Hz), 1.79–1.63 (m, 2H, Hp), 1.49–1.43 (m, 2H, Hr), 1.40 (d, 3H, Ala-β-CH, JAla-β-CH, Ala-α-CH = 6.4 Hz), 1.39 (d, 3H, Ala-β-CH, JAla-β-CH, Ala-α-CH = 6.0 Hz), 1.32–1.25 (m, 2H, Hq), 1.09 (d, 3H, Val-γ-CH, JVal-γ-CH, Val-β-CH = 6.4 Hz), 1.07 (d, 3H, Val-γ-CH, JVal-γ-CH, Val-β-CH = 6.0 Hz), 0.92 (d, 3H, Val-γ-CH, JVal-γ-CH, Val-β-CH = 6.8 Hz), 0.87 (d, 3H, Val-γ-CH, JVal-γ-CH, Val-β-CH = 6.4 Hz); 13C NMR (CDCl3, 100 MHz) δ173.8, 173.5, 173.3, 172.0, 171.2, 170.9, 170.4, 169.0, 167.8, 167.5, 164.3, 164.1, 163.2, 144.4, 144.3, 143.8, 143.8, 142.5, 142.5, 140.3, 140.3, 132.3, 132.2, 131.3, 131.2, 129.9, 129.8, 129.6, 129.5, 77.4, 70.6, 70.5, 70.4, 70.3, 70.0, 67.5, 65.2, 65.1, 62.9, 62.1, 61.9, 60.2, 60.0, 55.4, 53.7, 53.5, 52.4, 46.8, 46.4, 40.7, 39.3, 37.1, 35.9, 32.5, 31.5, 31.0, 30.4, 30.0, 29.8, 28.2, 27.9, 27.8, 25.5, 20.6, 20.5, 19.3, 18.9, 18.3, 17.4; ESIMS-LR m/z 1669.6 [(M + Na)+]; ESIMS-HR calcd for C75H106N16NaO20S3 1669.6824, found 1669.6798; [α]23D − 184.42 (c 0.19, CHCl3).
Echinomycin probe 29
Compound 29 was prepared using methods previously reported by our group20. Namely, 23 (27.8 mg, 26.0 µmol) in CH2Cl2 (519 µL) was treated with acetyl chloride (5.6 µL, 77.9 µmol) at room temperature for 1.5 h. Acetyl chloride (5.6 µL, 77.9 µmol) was added to the mixture, and the whole mixture was further stirred for 1 h. The reaction mixture was concentrated in vauo, and then azeotroped with toluene to afford a pale yellow solid. A mixture of the residue, 2-(3-but-3-ynyl-3H-diazirin-3-yl)-ethanol (28) (35.9 mg, 259.0 µmol) in THF (200 µL) was treated with zinc bromide (29.2 mg, 130 µmol) at room temperature for 20 h. The reaction mixture was diluted with EtOAc (50 mL) and CHCl3 (10 mL), and washed with H2O (20 mL × 2) and brine (20 mL). The organic layer was dried over Na2SO4, filtered, concentrated in vacuo and washed with Et2O. The crude was purified by preparative TLC (20 × 20 cm, 5% MeOH in CHCl3) to afford compound 29 (0.9 mg, 0.76 µmol, 3%) as a white amorphous solid. 1H NMR (CDCl3, 400 MHz) δ 9.64 (s, 1H, Ar–H), 9.64 (s, 1H, Ar–H), 8.82 (d, 1H, Ser-NH, JSer-NH, Ser-α-CH = 6.0 Hz), 8.74 (d, 1H, Ser-NH, JSer-NH, Ser-α-CH = 6.9 Hz), 8.20 (dd, 1H, Ar–H, J = 3.2, 1.6 Hz), 8.18 (dd, 1H, Ar–H, J = 3.4, 1.1 Hz), 8.04 (d, 1H, Ar–H, J = 6.9 Hz), 8.02 (d, 1H, Ar–H, J = 7.8 Hz), 7.90–7.81 (m, 4H, Ar–H), 7.07–7.04 (m, 2H, Ala-NH), 6.46 (d, 2H, Cys-α-CH, JCys-α-CH, Cys-β-CH = 7.3 Hz), 5.18–5.13 (m, 3H, Val-α-CH, Cys-β-CH), 4.96–4.82 (m, 4H, Ser-α-CH, Ala-α-CH), 4.71–4.56 (m, 4H, Ser-β-CH), 3.76 (td, 1H, H-1, Jgem = 9.7, JH-1, H-2 = 6.6 Hz), 3.24–3.19 (m, 1H, H-1), 3.12 (s, 3H, NCH), 3.08 (s, 3H, NCH), 3.04–3.02 (m, 1H, Cys-β-CH), 2.97 (s, 3H, NCH), 2.96 (s, 3H, NCH), 2.78–2.71 (m, 1H, Cys-β-CH), 2.40–2.27 (m, 2H, Val-β-CH), 2.03–2.00 (m, 2H, H-3, H-5), 1.93–1.86 (1H, m, H-2), 1.78–1.71 (m, 1H, H-2), 1.66–1.62 (m, 1H, H-3), 1.44–1.41 (m, 1H, H-4), 1.43 (d, 3H, Ala-β-CH, JAla-β-CH, Ala-α-CH = 6.9 Hz), 1.42 (d, 3H, Ala-β-CH, JAla-β-CH, Ala-α-CH = 6.9 Hz), 1.36–1.25 (m, 1H, H-4), 1.08 (d, 6H, Val-γ-CH, JVal-γ-CH, Val-β-CH = 6.0 Hz), 0.90 (d, 3H, Val-γ-CH, JVal-γ-CH, Val-β-CH = 6.9 Hz), 0.86 (d, 3H, Val-γ-CH, JVal-γ-CH, Val-β-CH = 6.9 Hz); 13C NMR (CDCl3, 100 MHz) δ174.3, 173.6, 171.2, 170.2, 168.8, 164.3, 164.1, 144.3, 143.8, 143.8, 142.6, 140.4, 140.3, 132.2, 131.2, 131.2, 129.8, 129.7, 129.7, 83.0, 77.4, 69.7, 65.3, 65.1, 64.8, 62.4, 62.0, 59.5, 53.6, 53.3, 52.5, 46.9, 46.7, 32.8, 32.1, 32.0, 31.2, 30.8, 29.9, 29.8, 27.6, 27.5, 26.9, 20.5, 20.5, 18.9, 18.6, 18.2, 18.0, 13.3; ESIMS-LR m/z 1213.6 [(M + Na)+]; ESIMS-HR calcd. for C57H70N14NaO13S 1213.4860, found 1213.4882; [α]24D − 239.91 (c 0.42, CHCl3).
Evaluation of cytotoxicity
Basically, the evaluation was performed using methods previously reported by our group20,23. Cytotoxic activities of compounds against MIA PaCa-2 (American Type Culture Collection), SW620 (European Collection of Authenticated Cell Cultures) were measured by WST-8 assay according to manufacturer’s protocol. Briefly, cells (1 × 104 cells/well) in a 96-well plate were cultured in medium (for MIA PaCa-2, D-MEM (Wako, Japan) containing 10% fetal bovine serum (Funakoshi, Japan), 100 units/mL penicillin G and 100 µg/mL streptomycin (Wako, Japan), for SW620, RPMI-1640 (Wako, Japan) containing 10% fetal bovine serum, 100 units/mL penicillin G and 100 µg/mL streptomycin) were cultured at 37 °C for 24 h under 5% CO2 atmosphere. Then, cells were treated with test compounds (final DMSO concentration was 1%) at 37 °C for 72 h under 5% CO2 atmosphere. Then, cells were treated with a solution of Cell Counting Kit-8® (Dojindo, Japan) for 3 h. After that, cell viabilities were determined based on the measurement of absorption at 450 nm of the wells by using TECAN Infinite® M200 microplate reader. The results were expressed as a percentage of the negative control (cells treated with DMSO only), and that value was fixed at 100%. IC50 values were calculated by using the Microsoft Excel 15.24 software and the GraphPad Prism 8.3.0/9.0 software and each data is shown as mean ± SE (n = 3) in Table 3.
Western blotting
Basically, the analysis was performed using methods previously reported by our group23. Proteins were extracted from samples using NP-40 Cell Lysis Buffer (Thermo Fisher Scientific, MA, USA) supplemented with Complete protease inhibitor (Sigma Aldrich) and Halt Phosphatase Inhibitor Cocktail (Thermo Fisher Scientific). Equal amounts of protein were separated by SDS-PAGE (12.5%), transferred onto a nitrocellulose membrane, and blocked with SuperBlock T-20 (PBS; ThermoFisher Scientific). Primary antibody of cleaved caspase-3 (Cell Signaling Technology, Inc, MA, USA, #9661) were diluted to 1:1000 in SuperBlock T-20 (PBS) and incubated with blots overnight at 4 ℃. After washing in T-PBS, blots were probed with HRP-conjugated secondary antibodies (R&D Systems, Inc., MN, USA), washed in T-PBS, and then developed using the Super-Signal West Pico enhanced chemiluminescence system (ThermoFisher Scientific). Protein expression levels were normalized to the actin expression (BD Transduction Laboratories, KY, USA).
Terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL) staining
Basically, the analysis was performed using methods previously reported by our group23. SW-620 cells were plated on a Lab-Tek® Chamber Slide™ (ThermoFisher Scientific) 24 h prior to stimulation. After fixing the slides in 4% paraformaldehyde and through PBS washing, an In Situ Cell Death Detection Kit and TMR red (Roche Diagnostic, Indianapolis, IN, USA) were used for staining according to the manufacturer’s instructions. The cells were mounted with an anti-fade mounting medium, and fluorescence microscopy (KEYENCE Corporation, Osaka, Japan) was employed for visualizing TUNEL-positive cells.
Reporter gene assay
Basically, the assay was performed using methods previously reported by our group21. SW620 cells were transfected with either HIF-1α expression vector pCI-neo-3 × FLAG-HIF-1α or empty vector pCI-neo-3 × FLAG, a derivative of pCI-neo (Promega, Madison, WI, USA), together with pGL3-5xHRE-Luc and pGL4.75 [hRluc/CMV] (Promega), by using PEI MAX (Polysciences Inc., Warrington, PA, USA). At one day after transfection, the cells were exposed to the test compounds for 16 h, and then cell lysates were prepared. A dual-luciferase assay was performed with a dual-luciferase reporter assay system (Promega) and GloMax20/20 luminometer (Promega).
Transcriptome analysis
Basically, the analysis was performed using methods previously reported by our group23. RNA libraries were generated using an Ion Total RNA-Seq Kit v2 (Thermo Fisher Scientific) according to the manufacturer’s instructions. Emulsion PCR was carried out with an Ion OneTouchTM system and an Ion OneTouch 200 Template Kit v3 (Thermo Fisher Scientific). Template-positive Ion Sphere™ particles were enriched and purified for the sequencing reaction with an Ion OneTouch™ ES system (Thermo Fisher Scientific). The template-positive Ion Sphere™ Particles were loaded onto Ion PI™ Chips (Thermo Fisher Scientific) and for high throughput sequencing with an Ion Proton™ Semiconductor sequencer (Thermo Fisher Scientific). Sequencing data were mapped on a human reference genome sequence (GRCh38/hg38) using the Torrent Suite software program (Life Technologies). The expression analysis was performed in the CLC Genomics Workbench software program (CLC bio, Aarhus, Denmark), and differences among the samples were determined using an unpaired Student’s t-test. The gene list describing fold change and p-value was uploaded to the MetaCore software (Clarivate Analytics, PA, USA, URL; https://portal.genego.com/, version 6.33.69110.), and then pathway analysis was performed.
Hepatic microsomal stability assay
Basically, the analysis was performed using methods previously reported31. Disappearance of the parent compound over time was measured by using the amount of drug at time zero as a reference. After 5 min of preincubation, 1 mM NADPH (final concentration, the same applies to the following, NADPH( +)) or 0.1 M phosphate buffer (pH 7.4, NADPH(−))) was added to a mixture containing 1 μM of the test compound, 0.2 mg/mL of human or mouse liver microsomes (Sekisui XenoTech LLC (Kansas City, KS)), 1 mM EDTA and 0.1 M phosphate buffer (pH 7.4) and incubated at 37 °C for 30 min by rotation at 60 rpm. An aliquot of 50 μL of the incubation mixture was sampled and added to 250 μL of chilled acetonitrile/internal standard (IS). After centrifuging for 15 min at 3150×g (4 °C), the supernatants were analyzed by LC–MS/MS. Hepatic microsomal stability (mL/min/kg, CLint) was calculated according to the previous report32, using 48.8 and 45.4 mg MS protein/g liver and 25.7 and 87.5 g liver/kg body weight as scaling factors for human and mouse, respectively.
LC–MS/MS quantification method
Basically, the analysis was performed using methods previously reported31. An LC-MS8060 instrument equipped with a Shimadzu Nexera series LC system (Shimadzu, Kyoto, Japan) was used. All compounds were analyzed in multi-reaction monitoring mode under electron spray ionization conditions. The analytical column used was a CAPCELLPAK C18 MGIII (3 μm × 2.0 mmID × 35 mm; OSAKA SODA, Osaka, Japan) at 50 °C. The gradient mobile phase consisted of 0.1% formic acid in water (mobile phase A,) and 0.1% formic acid in acetonitrile (mobile phase B) at a total flow rate of 1 mL/min. The initial mobile phase composition was 10% B, which was held constant for 0.2 min, increased in a linear fashion to 90% B over 1 min, then held constant for 0.8 min, and finally brought back to the initial condition of 10% B over 0.01 min and re-equilibrated for 1 min. The transitions (precursor ion > product ion) of echinomycin, 3 and IS (methyl testosterone) are 1101.45 > 1053.5 (positive), 1083.5 > 523.95 (negative), and 303.1 > 109.1 (positive), respectively.
Xenografts
Basically, the experiments were performed using methods previously reported33. Male BALB/c nude mice (6 weeks old) were purchased from Charles River Laboratories Japan, Inc. SW-620 cells (2 × 106 cells/35 μL PBS/tumor) were mixed with matrigel (15 μL/tumor) (BD Transduction Laboratories). Subsequently, 50 μL of cell suspension was subcutaneously injected into the back of BALB/c nude mice. Daily intraperitoneal injections of each compound began on the day following the SW-620 cell injection. The tumor size was calculated by the following formula: Tumor size (mm2) = (major diameter) × (minor diameter).
Serum biochemistry
Male BALB/c mice (7 weeks old) were purchased from Charles River Laboratories Japan, Inc. Each compound was intraperitoneally injected daily for 14 days. The whole blood of mice was obtained from the inferior vena cava under anesthesia with isoflurane by inhalation. Serum was collected by centrifuging blood at 5000 rpm for 10 min. Biochemistry was performed in Oriental Yeast Co., Ltd, Japan.
Ethics declaration
The study received ethical approval for the use of an opt-out methodology from the Medical Ethics Committee of Asahikawa Medical University (Approval No. 16069, 20057). All methods were carried out in accordance with relevant guidelines and regulations, and they are reported in compliance with the ARRIVE guidelines.
Data availability
Data supporting the findings of this manuscript are available from the corresponding author upon request.
References
WHO, Global Cancer Observation. https://gco.iarc.fr
Corbaz, R. et al. Stoffwechselprodukte von actinomyceten. 7. Mitteilung. Echinomycin. Helv. Chim. Acta. 40, 199 (1957).
Zolova, O. E., Mady, A. S. A. & Tsodikova, S. G. Recent developments in bisintercalator natural products. Biopolymers 93, 777 (2010).
Dawson, S., Malkinson, J. P., Paumier, D. & Searcey, M. Bisintercalator natural products with potential therapeutic applications: Isolation, structure determination, synthetic and biological studies. Nat. Prod. Rep. 24, 109 (2007).
Waring, M. J. & Wakelin, L. P. G. Echinomycin: A bifunctional intercalating antibiotic. Nature 252, 653 (1974).
Ughetto, G. et al. A comparison of the structure of echinomycin and triostin A complexed to a DNA fragment. Nucleic Acid Res. 13, 2305 (1985).
Chang, A. Y. et al. A randomized phase II trial of echinomycin, trimetrexate, and cisplatin plus etoposide in patients with metastatic non-small cell lung carcinoma: An Eastern Cooperative Oncology Group Study (E1587). Cancer 82, 292–300 (1998).
Gradishar, W. J. et al. A phase II clinical trial of echinomycin in metastatic soft tissue sarcoma. An Illinois Cancer Center Study. Invest. New Drugs 13, 171–174 (1995).
Wadler, S. et al. Phase II trial of echinomycin in patients with advanced or recurrent colorectal cancer. Cancer Chemother. Pharmacol. 34, 266–269 (1994).
Shevrin, D. H. et al. Phase II trial of echinomycin in advanced hormone-resistant prostate cancer. An Illinois Cancer Council study. Invest. New Drugs 12, 65–66 (1994).
Chang, A. et al. Phase II study of echinomycin in the treatment of renal cell carcinoma ECOG study E2885. Invest. New Drugs 12, 151–153 (1994).
Muss, H. B., Blessing, J. A. & DuBeshter, B. Echinomycin in recurrent and metastatic endometrial carcinoma.A phase II trial of the Gynecologic Oncology Group. J. Clin. Oncol. 16, 492–493 (1993).
Marshall, M. E. et al. Phase II trial of echinomycin for the treatment of advanced renal cell carcinoma. A Southwest Oncology Group study. Invest. New Drugs 11, 207–209 (1993).
Taylor, S. A. et al. Phase II evaluation of echinomycin (NSC-526417) in patients with central nervous system malignancies. A southwest Oncology Group study. J. Neurooncol. 15, 181–184 (1993).
Smenza, G. L. & Wang, G. L. A nuclear factor induced by hypoxia via de novo protein synthesis binds to the human erythropoietin gene enhancer at a site required for transcriptional activation. Mol. Cell. Biol. 12, 5447 (1992).
Masoud, G. N. & Li, W. HIF-1α pathway: Role, regulation and intervention for cancer therapy. Acta Pharm. Sin. B 5, 378 (2015).
Kong, D. et al. Echinomycin, a small-molecule inhibitor of hypoxia-inducible factor-1 DNA-binding activity. Cancer Res. 65, 9047 (2005).
Ponnurangam, S. et al. Quinomycin A targets Notch signaling pathway in pancreatic cancer stem cells. Oncotarget 7, 3217 (2016).
Wang, Y. et al. Echinomycin protects mice against relapsed acute myeloid leukemia without adverse effect on hematopoietic stem cells. Blood 14, 1127 (2014).
Kojima, K., Yakushiji, F., Katsuyama, A. & Ichikawa, S. Total synthesis of echinomycin and its analogues. Org. Lett. 22, 4217–4221 (2020).
Katayama, K., Nakagawa, K., Takeda, H., Matsuda, A. & Ichikawa, S. Total synthesis of sandramycin and its analogues via a multi-component assemblage. Org. Lett. 16, 428–431 (2014).
Katayama, K. et al. Synthesis and biological evaluation of quinaldopeptin. J. Org. Chem. 79, 2580–2590 (2014).
Konishi, H. et al. Probiotic-derived ferrichrome inhibits colon cancer progression via JNK-mediated apoptosis. Nat. Commun. 7, 12365 (2016).
Li, Z. et al. Design and synthesis of minimalist terminal alkyne-containing diazirine photo-crosslinkers and their incorporation into kinase inhibitors for cell- and tissue-based proteome profiling. Angew. Chem. Int. Ed. 52, 8551–8556 (2013).
Takahashi, K., Koshino, H., Esumi, Y., Tsuda, E. & Kurosawa, K. SW-163C and E, novel antitumor depsipeptides produced by Streptomyces sp II. Structure elucidation. J. Antibiot. 54, 622 (2001).
Romero, F. et al. Thiocoraline, a new depsipeptide with antitumor activity produced by a marine Micromonospora I taxonomy, fermentation, isolation, and biological activities. J. Antibiot. 50, 734 (1997).
Matson, J. A. & Bush, J. A. Sandramycin, a novel antitumor antibiotic produced by a Nocardioides sp. production, isolation, characterization and biological properties. J. Antibiot. 42, 1763 (1989).
Toda, S. et al. Quinaldopeptin, a novel antibiotic of the quinomycin family. J. Antibiot. 43, 796 (1990).
Nair, A. B. & Jacob, S. A simple practice guide for dose conversion between animals and human. J. Basic Clin. Pharma. 7, 27–31 (2016).
Kuhn, J. G. et al. Phase I trial of echinomycin (NSC 526417), a bifunctional intercalating agent, administered by 24-hour continuous infusion. Eur. J. Cancer Clinic. Oncol. 25, 797–803 (1989).
Kitakami, R. et al. Inhibitory activities of anthraquinone and xanthone derivatives against transthyretin amyloidogenesis. Bioorg. Med. Chem. 44, 116292 (2021).
Shah, P. et al. An Automated high-throughput metabolic stability assay using an integrated high-resolution accurate mass method and automated data analysis software. Drug Metab. Dispos. 10, 1653–1661 (2016).
Konishi, H. et al. Probiotic Aspergillus oryzae produces anti-tumor mediator and exerts anti-tumor effects in pancreatic cancer through the p38 MAPK signaling pathway. Sci. Rep. 11, 11070 (2021).
Acknowledgements
This research was supported in part by JSPS KAKENHI Grant-in-Aid for Scientific Research (B) (Grant Number JP16H05097, JP19H03345 and JP22H02738 to S.I.), JSPS KAKENHI Grant-in-Aid for Scientific Research (C) (JP21K07929 to M.F.), Grant-in-Aid for Scientific Research on Innovative Areas “Frontier Research on Chemical Communications” (No 18H04599 and 20H04757 to S.I.), and was partly supported by Hokkaido University, Global Facility Center (GFC), Pharma Science Open Unit (PSOU), funded by MEXT under "Support Program for Implementation of New Equipment Sharing System", Platform Project for Supporting Drug Discovery and Life Science Research (Basis for Supporting Innovative Drug Discovery and Life Science Research (BINDS)) from AMED (JP18am0101093j0002, JP19ak0101118h0001, and JP22ama121039 to S.I. and A.K., JP21am0101087 (support number 1559) to K.K.).
Author information
Authors and Affiliations
Contributions
K.K., H.K., M.F., and. S.I. designed the research, and K.K., H.K., K.N., M.F., and S.I. designed the experiments. K.K., K.M., and Y.K. synthesized compounds. K.M. and F.Y. performed a cytotoxicity assay. H.K. performed a TUNEL assay, transcriptome analysis, and in vivo evaluation. K.N. performed assays associated with HIF inhibition. K.K. performed a hepatic microsomal stability assay. S.I. wrote the paper. All authors discussed the results and commented on the paper and have given approval to the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kojima, K., Konishi, H., Momosaki, K. et al. Synthesis and biological evaluation of echinomycin analogues as potential colon cancer agent. Sci Rep 14, 7628 (2024). https://doi.org/10.1038/s41598-024-58196-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-58196-3
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.