Abstract
Background
The repurposing of FDA-approved drugs for anti-cancer therapies is appealing due to their established safety profiles and pharmacokinetic properties and can be quickly moved into clinical trials. Cancer progression and resistance to conventional chemotherapy remain the key hurdles in improving the clinical management of colon cancer patients and associated mortality.
Methods
High-throughput screening (HTS) was performed using an annotated library of 1,600 FDA-approved drugs to identify drugs with strong anti-CRC properties. The candidate drug exhibiting most promising inhibitory effects in in-vitro studies was tested for its efficacy using in-vivo models of CRC progression and chemoresistance and patient derived organoids (PTDOs).
Results
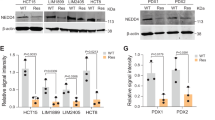
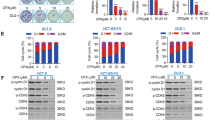
Albendazole, an anti-helminth drug, demonstrated the strongest inhibitory effects on the tumorigenic potentials of CRC cells, xenograft tumor growth and organoids from mice. Also, albendazole sensitized the chemoresistant CRC cells to 5-fluorouracil (5-FU) and oxaliplatin suggesting potential to treat chemoresistant CRC. Mechanistically, Albendazole treatment modulated the expression of RNF20, to promote apoptosis in CRC cells by delaying the G2/M phase and suppressing anti-apoptotic-Bcl2 family transcription.
Conclusions
Albendazole, an FDA approved drug, carries strong therapeutic potential to treat colon cancers which are aggressive and potentially resistant to conventional chemotherapeutic agents. Our findings also lay the groundwork for further clinical testing.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 24 print issues and online access
$259.00 per year
only $10.79 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The datasets generated and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209–49.
Fatima I, Uppada JP, Chhonker YS, Gowrikumar S, Barman S, Roy S, et al. Identification and characterization of a first-generation inhibitor of claudin-1 in colon cancer progression and metastasis. Biomed Pharmacother. 2023;159:114255.
Miller KD, Nogueira L, Devasia T, Mariotto AB, Yabroff KR, Jemal A, et al. Cancer treatment and survivorship statistics, 2022. CA Cancer J Clin. 2022;72:409–36.
Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin. 2023;73:17–48.
Ciardiello F, Ciardiello D, Martini G, Napolitano S, Tabernero J, Cervantes A. Clinical management of metastatic colorectal cancer in the era of precision medicine. CA Cancer J Clin. 2022;72:372–401.
Xie YH, Chen YX, Fang JY. Comprehensive review of targeted therapy for colorectal cancer. Signal Transduct Target Ther. 2020;5:22.
Modest DP, Rivera F, Bachet JB, de Braud F, Pietrantonio F, Koukakis R, et al. Panitumumab-based maintenance after oxaliplatin discontinuation in metastatic colorectal cancer: A retrospective analysis of two randomised trials. Int J Cancer. 2019;145:576–85.
Hu T, Li Z, Gao CY, Cho CH. Mechanisms of drug resistance in colon cancer and its therapeutic strategies. World J Gastroenterol. 2016;22:6876–89.
El Zarif T, Yibirin M, De Oliveira-Gomes D, Machaalani M, Nawfal R, Bittar G, et al. Overcoming Therapy Resistance in Colon Cancer by Drug Repurposing. Cancers (Basel). 2022;14:2105.
Yeu Y, Yoon Y, Park S. Protein localization vector propagation: a method for improving the accuracy of drug repositioning. Mol Biosyst. 2015;11:2096–102.
Scannell JW, Blanckley A, Boldon H, Warrington B. Diagnosing the decline in pharmaceutical R&D efficiency. Nat Rev Drug Discov. 2012;11:191–200.
Horton J. Albendazole: a review of anthelmintic efficacy and safety in humans. Parasitology. 2000;121:S113–S132.
Conterno LO, Turchi MD, Correa I, Monteiro, de Barros Almeida RA. Anthelmintic drugs for treating ascariasis. Cochrane Database Syst Rev. 2020;4:CD010599.
Zhou F, Du J, Wang J. Albendazole inhibits HIF-1alpha-dependent glycolysis and VEGF expression in non-small cell lung cancer cells. Mol Cell Biochem. 2017;428:171–8.
Pourgholami MH, Cai ZY, Wang L, Badar S, Links M, Morris DL. Inhibition of cell proliferation, vascular endothelial growth factor and tumor growth by albendazole. Cancer Invest. 2009;27:171–7.
Pourgholami MH, Cai ZY, Badar S, Wangoo K, Poruchynsky MS, Morris DL. Potent inhibition of tumoral hypoxia-inducible factor 1alpha by albendazole. BMC Cancer. 2010;10:143.
Pourgholami MH, Khachigian LM, Fahmy RG, Badar S, Wang L, Chu SW, et al. Albendazole inhibits endothelial cell migration, tube formation, vasopermeability, VEGF receptor-2 expression and suppresses retinal neovascularization in ROP model of angiogenesis. Biochem Biophys Res Commun. 2010;397:729–34.
Wang LJ, Lee YC, Huang CH, Shi YJ, Chen YJ, Pei SN, et al. Non-mitotic effect of albendazole triggers apoptosis of human leukemia cells via SIRT3/ROS/p38 MAPK/TTP axis-mediated TNF-alpha upregulation. Biochem Pharm. 2019;162:154–68.
Patel K, Doudican NA, Schiff PB, Orlow SJ. Albendazole sensitizes cancer cells to ionizing radiation. Radiat Oncol. 2011;6:160.
Jung YY, Baek SH, Ha IJ, Ahn KS. Regulation of apoptosis and autophagy by albendazole in human colon adenocarcinoma cells. Biochimie. 2022;198:155–66.
Pourgholami MH, Akhter J, Wang L, Lu Y, Morris DL. Antitumor activity of albendazole against the human colorectal cancer cell line HT-29: in vitro and in a xenograft model of peritoneal carcinomatosis. Cancer Chemother Pharm. 2005;55:425–32.
Gowrikumar S, Primeaux M, Pravoverov K, Wu C, Szeglin BC, Sauve CG, et al. A claudin-based molecular signature identifies high-risk, chemoresistant colorectal cancer patients. Cells. 2021;10:2211.
Sato T, Stange DE, Ferrante M, Vries RG, Van Es JH, Van den Brink S, et al. Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett’s epithelium. Gastroenterology. 2011;141:1762–72.
Fatima I, Barman S, Uppada J, Chauhan S, Rauth S, Rachagani S, et al. MASTL regulates EGFR signaling to impact pancreatic cancer progression. Oncogene. 2021;40:5691–704.
New-Aaron M, Koganti SS, Ganesan M, Kanika S, Kumar V, Wang W, et al. Hepatocyte-specific triggering of hepatic stellate cell profibrotic activation by apoptotic bodies: the role of hepatoma-derived growth factor, HIV, and ethanol. Int J Mol Sci. 2023;24:5346.
Fatima I, Saxena R, Kharkwal G, Hussain MK, Yadav N, Hajela K, et al. The anti-proliferative effect of 2-[piperidinoethoxyphenyl]-3-[4-hydroxyphenyl]-2H-benzo(b) pyran is potentiated via induction of estrogen receptor beta and p21 in human endometrial adenocarcinoma cells. J Steroid Biochem Mol Biol. 2013;138:123–31.
Fatima I, El-Ayachi I, Taotao L, Lillo MA, Krutilina RI, Seagroves TN, et al. The natural compound Jatrophone interferes with Wnt/beta-catenin signaling and inhibits proliferation and EMT in human triple-negative breast cancer. PLoS One. 2017;12:e0189864.
Kumar B, Ahmad R, Sharma S, Gowrikumar S, Primeaux M, Rana S, et al. PIK3C3 inhibition promotes sensitivity to colon cancer therapy by inhibiting cancer stem cells. Cancers (Basel). 2021;13:2168.
Luzak B, Siarkiewicz P, Boncler M. An evaluation of a new high-sensitivity PrestoBlue assay for measuring cell viability and drug cytotoxicity using EA.hy926 endothelial cells. Toxicol Vitr. 2022;83:105407.
Kaemmerer E, Klaus C, Jeon MK, Gassler N. Molecular classification of colorectal carcinomas: the genotype-to-phenotype relation. World J Gastroenterol. 2013;19:8163–7.
Zhu L, Yang Q, Hu R, Li Y, Peng Y, Liu H, et al. Novel therapeutic strategy for melanoma based on albendazole and the CDK4/6 inhibitor palbociclib. Sci Rep. 2022;12:5706.
Petersen J, Baird SK. Treatment of breast and colon cancer cell lines with anti-helmintic benzimidazoles mebendazole or albendazole results in selective apoptotic cell death. J Cancer Res Clin Oncol. 2021;147:2945–53.
Fatima I, El-Ayachi I, Taotao L, Angeles Lillo M, Krutilina RI, Seagroves TN, et al. Correction: The natural compound Jatrophone interferes with Wnt/beta-catenin signaling and inhibits proliferation and EMT in human triple-negative breast cancer. PLoS One. 2018;13:e0197796.
Xia W, Spector S, Hardy L, Zhao S, Saluk A, Alemane L, et al. Tumor selective G2/M cell cycle arrest and apoptosis of epithelial and hematological malignancies by BBL22, a benzazepine. Proc Natl Acad Sci USA. 2000;97:7494–9.
Hulkower KI, Herber RL. Cell migration and invasion assays as tools for drug discovery. Pharmaceutics. 2011;3:107–24.
Kowtharapu BS, Murin R, Junemann AGM, Stachs O. Role of corneal stromal cells on epithelial cell function during wound healing. Int J Mol Sci. 2018;19:464.
Fatima I, El-Ayachi I, Playa HC, Alva-Ornelas JA, Khalid AB, Kuenzinger WL, et al. Simultaneous multi-organ metastases from chemo-resistant triple-negative breast cancer are prevented by interfering with WNT-signaling. Cancers (Basel). 2019;11:2039.
Ehteda A, Galettis P, Pillai K, Morris DL. Combination of albendazole and 2-methoxyestradiol significantly improves the survival of HCT-116 tumor-bearing nude mice. BMC Cancer. 2013;13:86.
Will Castro L, Pieters W, Alemdehy MF, Aslam MA, Buoninfante OA, Raaijmakers JA, et al. The widely used antihelmintic drug albendazole is a potent inducer of loss of heterozygosity. Front Pharm. 2021;12:596535.
Duan Y, Huo D, Gao J, Wu H, Ye Z, Liu Z, et al. Corrigendum: Ubiquitin ligase RNF20/40 facilitates spindle assembly and promotes breast carcinogenesis through stabilizing motor protein Eg5. Nat Commun. 2016;7:13462.
Pavri R, Zhu B, Li G, Trojer P, Mandal S, Shilatifard A, et al. Histone H2B monoubiquitination functions cooperatively with FACT to regulate elongation by RNA polymerase II. Cell. 2006;125:703–17.
Schulze JM, Jackson J, Nakanishi S, Gardner JM, Hentrich T, Haug J, et al. Linking cell cycle to histone modifications: SBF and H2B monoubiquitination machinery and cell-cycle regulation of H3K79 dimethylation. Mol Cell. 2009;35:626–41.
Chandrashekar DS, Bashel B, Balasubramanya SAH, Creighton CJ, Ponce-Rodriguez I, Chakravarthi B, et al. UALCAN: a portal for facilitating tumor subgroup gene expression and survival analyses. Neoplasia. 2017;19:649–58.
Parang B, Barrett CW, Williams CS. AOM/DSS model of colitis-associated cancer. Methods Mol Biol. 2016;1422:297–307.
Moser AR, Pitot HC, Dove WF. A dominant mutation that predisposes to multiple intestinal neoplasia in the mouse. Science. 1990;247:322–4.
Schneider D, Chua RL, Molitor N, Hamdan FH, Rettenmeier EM, Prokakis E, et al. The E3 ubiquitin ligase RNF40 suppresses apoptosis in colorectal cancer cells. Clin Epigenetics. 2019;11:98.
Zhu B, Zheng Y, Pham AD, Mandal SS, Erdjument-Bromage H, Tempst P, et al. Monoubiquitination of human histone H2B: the factors involved and their roles in HOX gene regulation. Mol Cell. 2005;20:601–11.
Saijo T, Ishii G, Ochiai A, Yoh K, Goto K, Nagai K, et al. Eg5 expression is closely correlated with the response of advanced non-small cell lung cancer to antimitotic agents combined with platinum chemotherapy. Lung Cancer. 2006;54:217–25.
Yan GR, Zou FY, Dang BL, Zhang Y, Yu G, Liu X, et al. Genistein-induced mitotic arrest of gastric cancer cells by downregulating KIF20A, a proteomics study. Proteomics. 2012;12:2391–9.
Jin Q, Dai Y, Wang Y, Zhang S, Liu G. High kinesin family member 11 expression predicts poor prognosis in patients with clear cell renal cell carcinoma. J Clin Pathol. 2019;72:354–62.
Kim M, Jeong HJ, Ju HM, Song JY, Jang SJ, Choi J. Overexpression of the NEK9-EG5 axis is a novel metastatic marker in pathologic stage T3 colon cancer. Sci Rep. 2023;13:342.
Sun L, Lu J, Niu Z, Ding K, Bi D, Liu S, et al. A potent chemotherapeutic strategy with Eg5 inhibitor against gemcitabine resistant bladder cancer. PLoS One. 2015;10:e0144484.
Hicke L. Protein regulation by monoubiquitin. Nat Rev Mol Cell Biol. 2001;2:195–201.
Hammond WA, Swaika A, Mody K. Pharmacologic resistance in colorectal cancer: a review. Ther Adv Med Oncol. 2016;8:57–84.
de Gramont A, Figer A, Seymour M, Homerin M, Hmissi A, Cassidy J, et al. Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol. 2000;18:2938–47.
Pardini B, Kumar R, Naccarati A, Novotny J, Prasad RB, Forsti A, et al. 5-Fluorouracil-based chemotherapy for colorectal cancer and MTHFR/MTRR genotypes. Br J Clin Pharm. 2011;72:162–3.
Denise C, Paoli P, Calvani M, Taddei ML, Giannoni E, Kopetz S, et al. 5-fluorouracil resistant colon cancer cells are addicted to OXPHOS to survive and enhance stem-like traits. Oncotarget. 2015;6:41706–21.
Sanchez-Diez M, Alegria-Aravena N, Lopez-Montes M, Quiroz-Troncoso J, Gonzalez-Martos R, Menendez-Rey A, et al. Implication of different tumor biomarkers in drug resistance and invasiveness in primary and metastatic colorectal cancer cell lines. Biomedicines. 2022;10:1083.
Hirschmann-Jax C, Foster AE, Wulf GG, Nuchtern JG, Jax TW, Gobel U, et al. A distinct “side population” of cells with high drug efflux capacity in human tumor cells. Proc Natl Acad Sci USA. 2004;101:14228–33.
Longley DB, Johnston PG. Molecular mechanisms of drug resistance. J Pathol. 2005;205:275–92.
Pantziarka P, Verbaanderd C, Sukhatme V, Rica Capistrano I, Crispino S, Gyawali B, et al. ReDO_DB: the repurposing drugs in oncology database. Ecancermedicalscience. 2018;12:886.
Ashburn TT, Thor KB. Drug repositioning: identifying and developing new uses for existing drugs. Nat Rev Drug Discov. 2004;3:673–83.
Lacey E. Mode of action of benzimidazoles. Parasitol Today. 1990;6:112–5.
Pourgholami MH, Woon L, Almajd R, Akhter J, Bowery P, Morris DL. In vitro and in vivo suppression of growth of hepatocellular carcinoma cells by albendazole. Cancer Lett. 2001;165:43–49.
Pourgholami MH, Yan Cai Z, Lu Y, Wang L, Morris DL. Albendazole: a potent inhibitor of vascular endothelial growth factor and malignant ascites formation in OVCAR-3 tumor-bearing nude mice. Clin Cancer Res. 2006;12:1928–35.
Laudisi F, Maronek M, Di Grazia A, Monteleone G, Stolfi C. Repositioning of anthelmintic drugs for the treatment of cancers of the digestive system. Int J Mol Sci. 2020;21:4957.
Zhang P, Zhang Y, Liu K, Liu B, Xu W, Gao J, et al. Ivermectin induces cell cycle arrest and apoptosis of HeLa cells via mitochondrial pathway. Cell Prolif. 2019;52:e12543.
Shangguan F, Liu Y, Ma L, Qu G, Lv Q, An J, et al. Niclosamide inhibits ovarian carcinoma growth by interrupting cellular bioenergetics. J Cancer. 2020;11:3454–66.
Chen D, Sun X, Zhang X, Cao J. Targeting mitochondria by anthelmintic drug atovaquone sensitizes renal cell carcinoma to chemotherapy and immunotherapy. J Biochem Mol Toxicol. 2018;32:e22195.
Furst R, Vollmar AM. A new perspective on old drugs: non-mitotic actions of tubulin-binding drugs play a major role in cancer treatment. Pharmazie. 2013;68:478–83.
Risinger AL, Dybdal-Hargreaves NF, Mooberry SL. Breast cancer cell lines exhibit differential sensitivities to microtubule-targeting drugs independent of doubling time. Anticancer Res. 2015;35:5845–50.
Bates DJ, Danilov AV, Lowrey CH, Eastman A. Vinblastine rapidly induces NOXA and acutely sensitizes primary chronic lymphocytic leukemia cells to ABT-737. Mol Cancer Ther. 2013;12:1504–14.
Chu B, Liu F, Li L, Ding C, Chen K, Sun Q, et al. A benzimidazole derivative exhibiting antitumor activity blocks EGFR and HER2 activity and upregulates DR5 in breast cancer cells. Cell Death Dis. 2015;6:e1686.
Haschka MD, Soratroi C, Kirschnek S, Hacker G, Hilbe R, Geley S, et al. The NOXA-MCL1-BIM axis defines lifespan on extended mitotic arrest. Nat Commun. 2015;6:6891.
Ghasemi F, Black M, Vizeacoumar F, Pinto N, Ruicci KM, Le C, et al. Repurposing Albendazole: new potential as a chemotherapeutic agent with preferential activity against HPV-negative head and neck squamous cell cancer. Oncotarget. 2017;8:71512–9.
Liu H, Sun H, Zhang B, Liu S, Deng S, Weng Z, et al. (18)F-FDG PET imaging for monitoring the early anti-tumor effect of albendazole on triple-negative breast cancer. Breast Cancer. 2020;27:372–80.
Liu S, Liu H, Sun H, Deng S, Yue L, Weng Z, et al. (cRGD)2 peptides modified nanoparticles increase tumor-targeting therapeutic effects by co-delivery of albendazole and iodine-131. Anticancer Drugs. 2022;33:19–29.
Chen H, Weng Z, Xu C. Albendazole suppresses cell proliferation and migration and induces apoptosis in human pancreatic cancer cells. Anticancer Drugs. 2020;31:431–9.
Sethi G, Shanmugam MK, Arfuso F, Kumar AP. Role of RNF20 in cancer development and progression—a comprehensive review. Biosci Rep. 2018;38.
Wood A, Krogan NJ, Dover J, Schneider J, Heidt J, Boateng MA, et al. Bre1, an E3 ubiquitin ligase required for recruitment and substrate selection of Rad6 at a promoter. Mol Cell. 2003;11:267–74.
Hwang WW, Venkatasubrahmanyam S, Ianculescu AG, Tong A, Boone C, Madhani HD. A conserved RING finger protein required for histone H2B monoubiquitination and cell size control. Mol Cell. 2003;11:261–6.
So CC, Ramachandran S, Martin A. E3 Ubiquitin Ligases RNF20 and RNF40 are required for double-stranded break (DSB) repair: evidence for monoubiquitination of histone H2B Lysine 120 as a novel axis of DSB signaling and repair. Mol Cell Biol. 2019;39.
Moyal L, Lerenthal Y, Gana-Weisz M, Mass G, So S, Wang SY, et al. Requirement of ATM-dependent monoubiquitylation of histone H2B for timely repair of DNA double-strand breaks. Mol Cell. 2011;41:529–42.
Wegwitz F, Prokakis E, Pejkovska A, Kosinsky RL, Glatzel M, Pantel K, et al. The histone H2B ubiquitin ligase RNF40 is required for HER2-driven mammary tumorigenesis. Cell Death Dis. 2020;11:873.
Chernikova SB, Razorenova OV, Higgins JP, Sishc BJ, Nicolau M, Dorth JA, et al. Deficiency in mammalian histone H2B ubiquitin ligase Bre1 (Rnf20/Rnf40) leads to replication stress and chromosomal instability. Cancer Res. 2012;72:2111–9.
Jaaskelainen T, Makkonen H, Visakorpi T, Kim J, Roeder RG, Palvimo JJ. Histone H2B ubiquitin ligases RNF20 and RNF40 in androgen signaling and prostate cancer cell growth. Mol Cell Endocrinol. 2012;350:87–98.
Wang E, Kawaoka S, Yu M, Shi J, Ni T, Yang W, et al. Histone H2B ubiquitin ligase RNF20 is required for MLL-rearranged leukemia. Proc Natl Acad Sci USA. 2013;110:3901–6.
Sawin KE, LeGuellec K, Philippe M, Mitchison TJ. Mitotic spindle organization by a plus-end-directed microtubule motor. Nature. 1992;359:540–3.
She ZY, Zhong N, Yu KW, Xiao Y, Wei YL, Lin Y, et al. Kinesin-5 Eg5 is essential for spindle assembly and chromosome alignment of mouse spermatocytes. Cell Div. 2020;15:6.
Kapoor TM, Mitchison TJ. Eg5 is static in bipolar spindles relative to tubulin: evidence for a static spindle matrix. J Cell Biol. 2001;154:1125–33.
Garcia-Saez I, Skoufias DA. Eg5 targeting agents: From new anti-mitotic based inhibitor discovery to cancer therapy and resistance. Biochem Pharm. 2021;184:114364.
Kapoor TM, Mayer TU, Coughlin ML, Mitchison TJ. Probing spindle assembly mechanisms with monastrol, a small molecule inhibitor of the mitotic kinesin, Eg5. J Cell Biol. 2000;150:975–88.
Yang L, Jiang C, Liu F, You QD, Wu WT. Cloning, enzyme characterization of recombinant human Eg5 and the development of a new inhibitor. Biol Pharm Bull. 2008;31:1397–402.
Nakai R, Iida S, Takahashi T, Tsujita T, Okamoto S, Takada C, et al. K858, a novel inhibitor of mitotic kinesin Eg5 and antitumor agent, induces cell death in cancer cells. Cancer Res. 2009;69:3901–9.
Bayat Mokhtari R, Homayouni TS, Baluch N, Morgatskaya E, Kumar S, Das B, et al. Combination therapy in combating cancer. Oncotarget. 2017;8:38022–43.
Acknowledgements
This study was supported by BX002086 (VA merit), CA250383 (NIH/NCI), CA216746 (NIH/NCI) and Nebraska Research Initiative (NRI) to P.D and DK124095 and BX002761 (VA merit) to ABS. We also acknowledge NCI Cancer Center Support Grant P30 CA36727, NIH-1P50 CA 127297-01A2 for tissue arrays obtained. We also acknowledge Dr. Lynette M Smith, biostatistician, for analyzing the IC50 experiment data.
Funding
This study was supported by BX002086 (VA merit), CA250383 (NIH/NCI), CA216746 (NIH/NCI) and Nebraska Research Initiative (NRI) to P.D and DK124095 and BX002761 (VA merit) to ABS. We also acknowledge NCI Cancer Center Support Grant P30 CA36727, NIH-1P50 CA 127297-01A2 for tissue arrays obtained.
Author information
Authors and Affiliations
Contributions
PD and IF conceived the study and participated in the study design, performance, coordination, and manuscript writing. IF, RA, SB, SG, KP, MP and KWF carried out the assays and analysis. ABS revised the manuscript. All authors reviewed and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
All animal experiments were conducted with the approval of the UNMC Institutional Animal Care and Use Committee (IACUC; protocol #16-021-04FC). There is no human study.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fatima, I., Ahmad, R., Barman, S. et al. Albendazole inhibits colon cancer progression and therapy resistance by targeting ubiquitin ligase RNF20. Br J Cancer 130, 1046–1058 (2024). https://doi.org/10.1038/s41416-023-02570-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41416-023-02570-x