Abstract
Sustainable and effective means to control flying insect vectors are critically needed, especially with widespread insecticide resistance and global climate change. Understanding and controlling vectors requires accurate information about their movement and activity, which is often lacking. The Photonic Fence (PF) is an optical system that uses machine vision, infrared light, and lasers to identify, track, and interdict vectors in flight. The PF examines an insect’s outline, flight speed, and other flight parameters and if these match those of a targeted vector species, then a low-power, retina-safe laser kills it. We report on proof-of-concept tests of a large, field-sized PF (30 mL × 3 mH) conducted with Aedes aegypti, a mosquito that transmits dangerous arboviruses, and Diaphorina citri, a psyllid which transmits the fatal huanglongbing disease of citrus. In tests with the laser engaged, < 1% and 3% of A. aegypti and D. citri, respectfully, were recovered versus a 38% and 19% recovery when the lacer was silenced. The PF tracked, but did not intercept the orchid bee, Euglossa dilemma. The system effectively intercepted flying vectors, but not bees, at a distance of 30 m, heralding the use of photonic energy, rather than chemicals, to control flying vectors.
Similar content being viewed by others
Introduction
Flying insects that transmit human, animal, and plant pathogens have global impacts on human health, agriculture, and ecological stability1,2. Eighty percent of the world’s population is vulnerable to vector-borne diseases, which account for ca.17% of all infectious diseases and kill over 700,000 people per year, primarily children2. For example, malaria, a parasitic infection transmitted by Anopheles mosquitoes, infected 249 million people worldwide in 2016 and caused over 600,000 deaths, mostly young children2,3. Dengue, which causes 40,000 deaths per year2, is transmitted by Aedes mosquitoes as are other arboviruses, such as chikungunya fever, Zika Virus, West Nile Virus, and yellow fever2,4. The parasite that causes leishmaniasis is transmitted by female phlebotomine sandflies, which like mosquitoes, feed on blood to produce eggs5. It is estimated that 700,000 to 1 million new cases of leishmaniasis occur annually, primarily among the world’s poorest people6. The health of livestock, domestic animals and wildlife is also seriously affected by vector borne diseases, such as trypanosomiasis, Rift Valley Fever, and blue tongue virus7,8,9,10. With rapid globalization, ecological links within a native pathosystem can be replaced in a naïve area with new vectors and hosts, exacerbating efforts to limit disease spread11,12,13. Likewise, crop production suffers worldwide from plant diseases spread by flying insect vectors such as aphids, whiteflies, planthoppers, and psyllids14,15,16,17,18,19,20,21.
Control of insect vectors relies primarily on chemical insecticides, which are often the only practical control solution22,23,24. However, reliance on insecticides is costly and it negatively impacts the health of humans, non-target organisms, and the environment22,25,26,27,28. Alarmingly, most vector species are already resistant to available insecticides29,30,31,32,33,34,35,36,37, and insecticide resistance has now been reported in all major malaria vector species and against all classes of insecticide38,39,40.
Increasing global temperatures increases insect vector abundance and distribution, bringing outbreaks of vector-borne diseases to new areas, exacerbating control efforts in at-risk areas41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56, and promoting more insecticide use57,58,59. At higher temperatures and CO2 levels, insecticides decompose more quickly, and insects detoxify them more rapidly, which may decrease their efficacy as the climate warms60,61,62,63. Moreover, synergistic interactions between rising temperatures and increasing insecticide applications can aggravate insect pest outbreaks64,65. As the climate warms, the rapid evolution of vector resistance to available insecticides will be promoted by factors such as increases in vector activity levels, movement patterns, and generations per year33,42,51,66,67,68,69. There is also the troubling occurrence of increased vector resistance to pyrethroids, which are used to treat bed nets, in vector populations inhabiting agricultural areas where pyrethrins are used against crop pests, making it more difficult to control them with these materials in inhabited areas70,71,72,73,74. Developing and managing vector resistance programs to extend the life of available insecticides is among the most difficult and important challenges facing global public health and food security in the near term, especially as the climate warms and changes36,75,76,77,78,79.
The capability to accurately monitor the abundance and distribution of flying insect vectors within a target area is critical to implementing regional management plans aimed at reducing vector populations, managing pesticide resistance, and eliminating pathogen transmission80,81. Providing accurate estimates of vector abundance and distribution within a given area is contingent on obtaining reliable counts of vectors at light traps, CO2 traps, or other trapping devices82,83,84,85. However, efficiency of traps with respect to delivering reliable captures depends on several factors and trapping may not always provide accurate estimates of vector population size86,87,88. Still, examination of specimens caught in traps is the best, and sometimes only, way to identify and count individuals of a particular vector species. Retrieval of specimens from traps is required for genetic, morphological and biochemical analyses and examination, which are necessary steps for conducting fundamental studies on insecticide resistance in vector populations and for developing and implementing insecticide resistance management strategies33. Recent advances in sensor and computer vision, machine learning, and the Internet of Things technology have greatly improved the functionality and effectiveness of insect traps with respect to their capability to identify and classify vectors and to perform these tasks autonomously89,90,91,92,93,94,95,96. However, most insect traps in use today have the drawback of being labor- and time intensive to set-up, retrieve, and analyze, are difficult to use in unwelcoming terrain or over extensive sampling areas, and are subject to weather conditions and events97,98. This makes it difficult in many scenarios to accurately assess vector populations without incurring high costs84,99,100. In addition, traps sometimes fail to adequately sample important or infected vectors, which can lead to errors caused by sampling bias and misinterpretation of the data101. More effective vector monitoring and control approaches are urgently required to address insecticide resistance and prevent vector-borne diseases from increasing and spreading74,102.
An emerging technology for monitoring insect vectors is the use of electromagnetic radiation coupled with advanced optical imaging systems, signal processing technology, computer vision, and machine learning92,103,104,105,106,107,108,109,110. Advances in entomological radar and lidar have permitted successful survey and monitoring activities of insect fauna at high- to mid-altitudes111,112,113. This technology has provided important insights into insect migration and diurnal and seasonal flight differences as well as revealed the ecological and agricultural importance of insects inhabiting different elevational or altitudinal niches114,115. The introduction of harmonic radar, in which an insect is fitted with a transponder energized by the radar, permitted the tracking of individual insects at ground level116,117,118 or even using UAV drones to enhance tracking ability119. Near ground lidar tracking is now sufficiently sensitive to perform fine-scale observations such as the recording the anomalous daytime flight activity of mosquitoes during solar eclipses120 and quantifying insect pest abundance above rice paddies under different meteorological conditions121. Recently, a high resolution lidar scanning a 500 m transect outside a rural African village was able to quantify the daily dispersal of mosquitoes in a village and revealed that the distribution of each gender differed in relation to the village122. Over the course of that study, a quarter of a million insect observations were made, and several insect taxa were identified based on their wingbeat modulation signatures.
Infrared laser-based systems coupled with machine learning are now sensitive enough to determine the sex of individual mosquitoes in flight123,124. Because male and female mosquitoes produce different frequencies while in flight, the amplitude of the backscattered light received from each gender differs and the signal can be used as a basis for gender identification and discrimination. Sufficient image resolution permitted such systems to distinguish gravid from non-gravid female mosquitoes125. An infrared laser-based optical sensor performed continuous surveillance of flying insects for 80 days126. The device recorded light backscattered by insects transiting through its laser beam and compiled information about each insect’s wing beat frequency, transit time across the beam, and the ratio of its wings to body dimensions to identify the insects to specific taxonomic groups. Data from this survey enabled the determination of the aerial densities, circadian rhythms and peak activities of each taxonomic cluster of insects. These results demonstrate that optical sensors, coupled with machine learning, are a viable methodology for performing non-intrusive, automatic, measurements of insect population dynamics over extended periods of time.
Just as innovations in optical and machine learning systems are making tangible progress towards permitting real time detection, identification, and tracking of flying insects, combining this technology with photonics is providing the means to intercept and kill them127,128,129,130,131,132,133,134,135,136,137. Studies have demonstrated that lasers pulses can kill insects from as large as a roach (Blatella germanica)136 to as small as a whitefly (Bemisia tabaci)137. In lab tests, laser pulses were shown to be capable of killing flying mosquitoes (Anopheles stephensi, Culex pipiens), Asian citrus psyllid (Diaphorina citri) and, spotted wing drospophila (Drosophila suzukii)127,128,129,130,131. Aphids, which are among the most important vectors of plant viruses, are vulnerable to laser pulses while they feed on plants134,135. In this system, a robot bearing the photonic system moves down a row of crop plants, interrogates each plant, and pulses a laser to individual aphids attached to the stems and leaves.
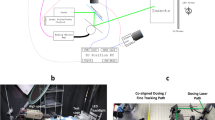
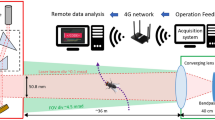
Here we report on the first tests of a field-scale sized all-optical system, the Photonic Fence, which is designed to identify and intercept small flying insect vectors over a wide area127,128,129 (Fig. 1). The Photonic Fence units, developed by Global Health Labs (formerly Global Good Fund), are mounted on two towers placed opposite of each other (Figs. 1, 2 and 3). Each unit is comprised of three independent modules which function sequentially. One module detects insect motion, and the others tracks and identifies the targeted insect. The initial detection of an insect is achieved by acquiring a video image of it when it crosses the virtual boundary of the system, which is referred to as the ‘active zone’. The active zone consists of a plane of infrared light (Fig. 3) projected by infrared lamps mounted on one Photonic Fence tower and reflected from a retroreflector on the other tower which is placed opposite of it at a defined distance (Fig. 3). Insects traversing the active zone are back illuminated by the infrared light reflected from the retroreflectors and thereby cast silhouettes. The first module, the ‘coarse tracking’ system (Fig. 2B), uses a pair of stereoscopic cameras to image the silhouettes and machine vision algorithms to provide real-time reconstruction of each insect’s silhouette and identify its three-dimensional (xyz) location within the active zone. If the insect’s size and speed fall within a pre-defined range, it is further interrogated by the second module, the ‘fine tracking’ system (Fig. 2B). This module uses a high-speed camera and a fast-scanning mirror to keep the targeted insect in the middle of the camera’s field of view. By comparing the target’s size, speed, and other parameters to those of vector species contained in a database, the system identifies the targeted insect. If the insect is a vector, then the third module (Fig. 2B) can direct a very brief pulse of a low-power, retina-safe laser to kill it. Sophisticated software integrates the module components, providing real-time, continuous coverage of the active zone. The laser system is designed so it will not fire if people or other animals enter the active zone127,128,129.
The screenhouse (40 m L × 3.05 m W × 3.66 m H) built to test the Photonic Fence at the USDA-Agricultural Research Service citrus grove in Fort Pierce, FL. (A) Exterior view. (B) Wide angle view of the interior showing the placement of Photonic Fence towers at both ends of the screenhouse. (C) Interior view from one Photonic Fence tower to the other showing the placement of traps for A. aegypti recovery. (D) View of yellow 3D traps arranged along recovery side of screenhouse during preliminary recovery tests. Note that the observer is located on the vector release side of the screenhouse and that the psyllids must fly across the active zone to reach the traps and potted host plants set up on the opposite side. See Fig. 10B for the actual set-up used during tests. (E) Screen house set-up for the open-air test to evaluate the Photonic Fence’s capability to track and target ambient levels of insects flying during dusk.
Diagrammatic representation of the Photonic Fence system: (A) Front view of the support tower showing the position of the tower frame, individual Photonic Fence units, reflector panel, communications and control computers, and laser generator. (B) Isometric view of an individual Photonic Fence unit showing position of infrared lamps, fine tracking system and laser aperture, and coarse tracking system apertures.
During development of the Photonic Fence, the capabilities of multiple types of lasers to kill anaesthetized mosquitoes (Anopheles stephansi) were evaluated and the primary optical parameters involved in causing thermal damage to internal organs and subsequent morbidity or mortality were identified127. Besides the laser’s wavelength (nm), these included beam (spot) diameter (mm), pulse duration (ms), and pulse energy (mJ). The tests demonstrated that anaesthetized mosquitoes could be killed by laser pulses to the thorax at various wavelengths, beam diameters, durations, and power conditions. Green and far-infrared wavelengths were found to be more effective in incapacitating mosquitoes than near- and mid-infrared wavelengths.
Follow-up studies were performed with vectors flying in small acrylic enclosures positioned in the active zone to evaluate and optimize the Photonic Fence’s tracking system and its integration with the laser system128,129. During tests with flying mosquitoes (A. stephansi), the system successfully derived individual mosquitoes’ velocity, and linear and angular acceleration before, during, and after the laser pulse from its xyz position, which was continuously monitored by the tracking system128. Spatial positioning of the mosquitoes was obtained by assigning each tracked mosquito a coordinate in image space based on its image centroid, from which the actual azimuth and elevation relative to the optical center of the camera could be calculated. To evaluate interception performance during the laser pulse, the fine tracking system was used to determine how much of the laser’s 2.5 mm beam diameter overlapped with the mosquito’s thorax and abdomen It was found that a tracking error of < 2 pixels for a minimum of 50 ms was required for the system to accurately target and hit the mosquito with the directed laser. The initial tests of this laboratory study were performed with the acrylic enclosures positioned 2 m from the Photonic Fence unit. After further calibration, the Photonic Fence was capable of targeting and killing all the mosquitoes (in this case both A. aegypti and C. pipiens) flying in an enclosure placed 30 m from it.
The Photonic Fence installation described in the current study had a large active zone measuring 30 m long × 3 m high × 0.3 m wide (Figs. 1 and 3). It was the largest and most complex design of the Photonic Fence to date and integrated the sub-systems that were tested and optimized previously with respect to laser control and delivery, target tracking, and target selection127,128,129 This version of the Photonic Fence was designed to maximize performance at the expense of size, weight, cost, and other system variables, with the expectation that those variables would be optimized during a later productization stage. The objective of the study was to determine if the Photonic Fence could detect, track, and intercept vectors across the entirety of an active zone of a size envisioned for deployment in the field.
The yellow fever mosquito, Aedes aegypti (L), was chosen as the representative human pathogen vector for this study because it and its congener Aedes albopictus (L), vector dangerous arboviruses, such as yellow fever, dengue, chikungunya, and Zika138,139,140. Once a forest-dwelling zoophilic mosquito, A. aegypti gradually adapted to changing weather and environmental conditions and became an anthropophilic, urban-dwelling species137,138,139,140,141. The females prefer to oviposit in containers holding stagnant water, such as flowerpots, discarded containers, tires, etc. and rest in habitations142. A. aegypti is 4–7 mm long and may fly over 100 m while searching for hosts and oviposition sites143,144. These adaptions have facilitated the spread and establishment of A. aegypti in new areas inhabited by immunologically naive human populations and led to a shift in the epidemiology of these viral diseases136,137,138,139. A further complication is that these mosquitoes can disseminate multiple viruses simultaneously145,146, which further shows that more effective means to surveil and control Aedes mosquitoes are needed, especially when managing arbovirus outbreaks147.
The Asian citrus psyllid, D. citri (Kuwayama), was chosen as a representative vector of a serious crop pathogen. This psyllid is an obligate phloem feeder of Citrus and closely related plants in the Rutaceae family. It vectors Candidatus Liberibacter asiaticus, a gram negative, phloem-limited bacterium that is the putative causal agent of huanglongbing disease of citrus148,149,150. Huanglongbing is the most devastating disease of citrus in the world: infected trees initially have mottled, misshaped leaves and green, bitter fruit and they typically die a few years later as there is currently no therapeutic treatment available against the pathogen151,152. Huanglongbing has seriously impacted major citrus-producing regions globally153,154,155,156. For example, citrus production in Florida, the largest orange juice producing state in the U.S., has decreased by over 70% since 2005, when the disease was first detected there153,156,157. Adult D. citri are typically 2.7–3.3 mm long158 and can disperse widely across landscapes159,160,161. Growers rely heavily on insecticides to control D. citri162, which consequently has resulted in the emergence of insecticide-resistant psyllid populations163,164,165,166,167. Because there are limited therapeutic treatments available for infected citrus trees168,169, new control measures are needed for D. citri. An earlier study demonstrated that the laser system of the Photonic Fenced could kill anaesthetized D. citri129. Dosing measurement showed that psyllid mortality occurred at a low energy level of 2 mJ, and that 90% mortality was reached at 15 mJ from pulses of a continuous wave 445 nm laser that lasted from 3 to 28 ms. The detection and tracking modules could be re-programed to track D. citri flying in a transparent acrylic chamber. Further testing of the integrated system demonstrated that it could detect, track and intercept D. citri at close range128. Other researchers have also shown that D. citri could be killed by laser pulses130.
The orchid bee, Euglossa dilemma (Friese), a Central American euglossine bee species established in southern Florida170,171, were used to test the Photonic Fence’s capability to discriminate between target and non-target insects. While they are exotic to Florida, euglossine bees are critical pollinators in many tropical plant communities in their native range172 and play a highly beneficial role in those regions. Males of this orchid bee were used as a representative non-target species because they don’t sting, making them easy to handle, are ca. 1.3 mm long, which is close in size to the honey bee (Apis mellifera L.)171, and are abundant in the test locality. Male orchid bees have a strong co-evolutionary relationship with certain orchid species, are highly attracted to certain floral volatiles173,174,175; and can be readily collected at scent baits176 (Fig. 4).
Photo of males of the orchid bee, E. dilemma, which were used as a representative non-target species. The males were attracted to a cotton swab which was dipped into a scent bait, p-methoxybenzene. The males collect the scent, store it in a specialized organ located on their rear legs, and then use it to produce a courtship pheromone. Photo by J. M. Patt.
Results
Initial mosquito tracking and interception tests
During the first 4 h of an initial mosquito tracking and interception test, the detection, interrogation, and tracking modes were active while the laser function was silenced. Following the release of 1000 A. aegypti in the screenhouse, with a human ‘agitator’ walking back and forth on the upwind side of the screenhouse exterior, a peak of just over 400 mosquitoes per minute were detected in the active space extending along the middle of the screenhouse (Fig. 5). At the 45-min point, the agitator took a break and left the vicinity of the screenhouse, and the number of mosquitoes detected per minute decreased to around 50 individuals per minute. When the agitator returned, the detection level again rose to greater than 300 individuals per minute. The agitator took two more breaks over the next 2 h, and each time the number of mosquitoes detected decreased to between 50 and 100 individuals per minute. The numbers then rebounded to between 200 and 280 individuals when the agitator returned. Four hours after the start of the test, the laser mode was engaged. During the final 60 min of the test the mosquito population within the screenhouse declined from laser-inflicted mortality and their detection levels dropped steadily to 50 or fewer individuals per minute (Fig. 6). A post-hoc analysis of the system’s responses showed that the mosquitoes were detected within the entire 180 square meter active space (Fig. 7). The laser typically engaged 1–2 mosquitoes per second but could engage up to 7 targets per second (Fig. 8).
Diagrammatic representation of the set-up for the initial 5.5-h long test of the Photonic Fence. A human ‘agitator’ walked along the outside of the screenhouse to help stimulate mosquito (A. aegypti) activity. Initially, the laser was disengaged, and the system measured the flight activity level of the mosquito population when the agitator was next to the screenhouse versus when they tool a rest break and were away from it. Towards the end of the test, the laser was engaged to test its effectiveness in reducing the mosquito population in the screenhouse.
The number of detections of flying A. aegyti over the 5.5 h-long initial test of the Photonic Fence. For this test, 1000 A. aegypti were released inside the test area. During the initial 4 honly the monitoring mode was active. Note that when the human agitator took a break and walked away from the screen house, mosquito activity declined. During the last hour of the test, the lethal laser mode was engaged, and the population of mosquitoes declined in the screen house.
Flying vector interception efficiency tests
Aedes aegypti
In a release and recovery test (Fig. 9A) with only the monitoring mode active, 38.3% of the 350 A. aegypti released at the beginning of the test on one side of the Photonic Fence were recaptured either on a human subject (61 total) or in the mosquito traps (73 total) 60 min following their release (Table 1; Fig. 10A). In the subsequent test with both the monitoring and laser modes active, only 3 (0.86%) of the 350 released mosquitoes were recaptured on the human subject while none were recaptured in the mosquito traps.
Diagrammatic representation of the set-up for testing the efficiency of the system’s ability to intercept flying insect vectors. Comparisons were made of vector recovery while only the monitoring mode was activated versus that with both the monitoring mode and laser activated. (A) Tests with mosquitoes, showing relative positions of cages for releasing A. aegypti, two pail traps with visual and olfactory attractants, two pan traps with seasoned water, and a human subject covered with a bee net and white overalls equipped with a 100 cm2 opening which exposed the human subject’s skin while seated during the test. (B) Tests with psyllids, showing relative positions of cages for releasing D. citri and yellow 3-D traps.
Results of the target discrimination tests in which there was a 100% laser pulse triggering when the pathogen vectors A. aegypti and Diaphorina citri were detected versus 0% laser pulse triggering when the pollinator E. dilemma was detected. Shown are silhouette images captured by the fine tracking system of the Photonic Fence of individual: (A) Yellow fever mosquitoes (A. aegypti), (B) Asian citrus psyllid (D. citri) and C. orchid bees (E. dilemma) in flight. Images show the last fine tracking frame prior to the target validation algorithm correctly setting a do-not-engage status. (D) Photo showing side-by-side comparison of mosquito and psyllid intercepted and killed by a laser pulse. Note the overall difference in the dimensions of each vector species.
Diaphorina citri
In the initial release and recovery test (Fig. 9B) with only the monitoring mode active, 18.7% (112 total) of the 600 D. citri released at the beginning of the test on one side of the Photonic Fence were recaptured in the 3-D yellow traps. In the following two tests with both the monitoring and laser modes active, 3.7% (22) and 2.3% (14) of the 600 released psyllids were recaptured in the 3-D yellow traps (Table 1; Fig. 10B).
Target discrimination test with bees
All 10 individual male orchid bees (E. dilemma) released in the screenhouse were recovered alive following a 60-min test period with both the monitoring mode and laser engaged (Fig. 10C).
Open-air test
The system detected very high numbers (> 1000/min) of ambient flying insects during the peak dusk activity period (Fig. 11). The release of 10,000 A. aegypti in the open section of the screen house at the peak dusk flight activity period did not produce a pronounced increase in insect detections, most likely due to the very high numbers of native crepuscular insects, that were active during the test period. Engagement of the laser during the initial 3 days of the test resulted in a pronounced decline in flying insect incidence relative to the fourth and subsequent days, when mosquitoes were released but the laser was not engaged (Fig. 11). When the laser was engaged during the first three nights of the test, the ratio of the baseline level of flying insects compared to the level of dusk-flying insects was 5% but when it was turned off during the remainder of the test, the ratio was 13%. The results showed that the system was capable of detecting and tracking enormous numbers of insects flying within the active zone.
Results of 10-day long open-air test with the middle section of screening removed from the screen house as shown in Fig. 1E. During the initial 96-h period, three releases were made in the screenhouse of 10,000 A. aegypti at the dusk, the peak period of daily ambient flying activity at the study site. The laser was engaged from the start of the test until 72 h had elapsed. After the laser was disengaged, a control test was conducted at dusk on day 5 in which 10,000 mosquitoes were released but the laser continued to be disengaged. During the last 4 days of the test, no further releases were conducted while the system continued to monitor the activity of ambient insects.
Flight elevation and speed observations
Each tracked insect trajectory through 3-dimentional space was timestamped and saved, along with the calculated size and sample target images from the fine tracking camera. The post-hoc evaluations of these flight data showed that the mosquito, A. aegypti, had a mean flight altitude of 1.3 m, which tapered off above 1.5 m. However, some individuals also flew near the screenhouse ceiling. (Fig. 12A). Asian citrus psyllids had no discernable flight altitude peak, but their flight tapered off at higher altitudes except for a peak close to the ceiling (Fig. 12B). Individual A. aegypti were observed flying at a typical velocity of 0.4–1 m/s (Fig. 13A) while individual male orchid bees (E. dilemma) were recorded flying at speeds ranging from 0.5 to 2.5 m/s (Fig. 13B).
Discussion
During the initial test with 1000 A. aegypti (Fig. 5), the system registered an increase in mosquito activity when the human ‘agitator’ walked along the upwind side of the exterior of the screenhouse and a decrease in flight activity when the agitator walked away from the screenhouse (Fig. 6). When the agitator was next to the screenhouse, 300–400 mosquitoes per minute were detected but this number fell to below 100 per minute when the agitator walked away from the screenhouse. Because the agitator was located outside and upwind of the mosquitoes in the tunnel, and the screening would have obscured their visual appearance, the observed changes in mosquito flight responses observed during this test were likely due primarily to the concentration of olfactory cues emanating from the agitator which changed as the agitator moved along or away from the screenhouse177. This result demonstrated the system’s sensitivity to detecting and recording changes in localized vector activity. Once the laser was engaged, mosquito activity rapidly declined, proving the effectiveness of the lethal mode of the system. The results of this test also showed that the system successfully tracked mosquitoes throughout the active zone (Fig. 7), showing that there were no areas where detection and tracking was deficient and that up to seven individuals per second could be lethally engaged (Fig. 8).
In the tests of interception efficiency, only 0.9% of A. aegypti were recovered on the human subject side of the screenhouse at the completion of the laser-enabled test as compared to a 38% recovery in the non-lethal monitoring control (Table 1A), demonstrating that the Photonic Fence was capable of effectively interdicting large numbers of flying mosquitoes. The system was less effective at interdicting flying Asian citrus psyllid than mosquitoes (Table 1B). Because the system was designed to intercept targets the size of mosquitoes, tracking, targeting, and hitting the psyllid, which exhibits a significantly smaller detected cross-sectional area, proved to be challenging to the system (Fig. 10D). Improved efficiency with respect to intercepting smaller-sized targets may be achieved by modifying the system through measures that improve image resolution and the optical field-of-view, such as adding more powerful optical lenses, decreasing the area of the active zone, or adjusting the spot size of the laser beam. Tests with free-flying orchid bees showed that the system could distinguish target from non-target species (Fig. 10C). This is important because in most potential applications, the system must eradicate only vectors, not non-target or beneficial insects. The open-air test demonstrated that the system could track the high numbers of ambient flying insects that were active at dusk at the study site, with as many as 1500 insects per minute detected (Fig. 11). When the laser was engaged, decreases were observed in the numbers of insects detected by the system, showing that the laser could operate effectively, even when challenged with large numbers of potential targets.
This proof-of-concept Photonic Fence hardware architecture comprised several features optimized for performance characteristics and data logging. These attributes will be atrophied during the product design phase to optimize the commercial device for cost, size, and power consumption. Each Photonic Fence subsystem has been designed with an eye towards off-the-grid operation to enable deployment in locations without reliable power. The electrical power budget for the product version of the Photonic Fence is 200 W, making it operable with the use of commercially available solar panels or energy storage systems.
The results demonstrated that a field-scale sized Photonic Fence system could reliably and accurately detect, track, and identify hundreds of individuals of two different flying insect vector species and then kill them with a brief pulse of a low-power laser at a distance of up to 30 m. The Photonic Fence tested here effectively prevented flying vectors from infiltrating a 90 m2 active zone; and it was sensitive enough to accurately discriminate targeted from non-targeted insect species, intercepting and killing the vector species while leaving the non-targeted insects unharmed. The Photonic Fence represents a significant advancement to the overall development of laser-based insect vector eradication technologies131,132,133,134,135,136. Photonic exposure now represents a new mode of flying vector control with a very different mode of action: pin-point thermal damage to tissue in targeted individuals127,128,129 in contrast to insecticides, which work by disrupting neurological transmissions178. The application of topical insecticides to a targeted treatment area results in selection for individuals that can detoxify the active ingredient of the insecticide, which, in turn, drives insecticide resistance in that population32,77,178. The thermal damage inflicted by the laser pulse either kills the insect outright or severely incapacitates it, so individuals intercepted by the pulsed laser do not further contribute to the genetic composition of the population, leaving no possibility of ‘laser resistance’ developing in vector populations exposed to laser pulses. The thermal damage inflicted on the target affects only the target, and so it does not produce negative effects on people and communities, non-target organisms, and the local environment as does the persistent application of insecticides. Given these capabilities, there are many potential applications of the Photonic Fence and similar photonic devices in the interdiction, surveillance and investigations of flying insect vectors. These are discussed below.
Applications of the Photonic Fence and similar photonic technologies
Vector interdiction
While the utility of the Photonic Fence specifically is discussed below, other types of optical and photonic systems, such as those mentioned above, could also potentially be used in these given situations. That the system was capable of intercepting large numbers of flying vectors throughout the 90 m2 active zone, demonstrated that the Photonic Fence could be used to establish ‘vector interception zones’ along the perimeters of areas in need of protection (Table 2). Strategic placement of vector interception zones would provide localized protection and help reduce insecticide reliance/resistance in vector populations at those locations. Further testing is needed to evaluate the efficacy and feasibility of the Photonic Fence in each of the application examples provided below.
In terms of protecting human health, the Photonic Fence could be used to establish vector interception zones in health care facilities and other areas where people congregate, such as schools, places of worship, transit stations, or markets. This was the original intent and inspiration for the Photonic Fence179. As the technology improves and matures, Photonic Fences could be deployed outdoors, around the perimeters of buildings, villages and neighborhoods that are highly vulnerable to flying vector infiltration or to create a barrier between protected areas and high-density vector habitats, such as wetlands180,181.
Vector interception zones established around the perimeters of open areas will not result in the total exclusion of vectors in that area. However, because many vectors typically fly close to the ground while searching for a host94,182,183,184, a 3 m high Photonic Fence established around the perimeter of a protected area could be effective in reducing vector infiltration into protected areas. Significantly diminishing vector infiltration numbers is helpful because decreasing the numbers of resistant individuals in local populations improves the effectiveness of both localized treatments and regional insecticide resistance management programs67,73,185. Reducing vector infiltration would also help maintain the effectiveness of personal protection measures such as indoor residual spraying and insecticide-treated bed nets186,187,188,189. Establishment of vector interception zones around vector refugia, such as wetlands, would help reduce the numbers of vectors that disperse to nearby inhabited areas180,181. Because of the overlap in the insecticides used in both agricultural areas and human health protection, insecticide resistant vector populations from agricultural production areas threaten nearby human habitations190,191. Establishing vector interception zones in these areas will serve the dual purpose of decreasing vector populations while reducing the density of resistant individuals in the vector population. Because cattle and other livestock are alternate hosts for certain vectors that also feed on human blood192,193,194,195, establishing vector interception zones between areas where livestock are kept and human habitations could also be an important protection and control strategy.
Because refugees, migrants, and displaced people are very vulnerable to contagious and vector-borne diseases196,197,198,199,200, the Photonic Fence could be used to help reduce the spread of vector borne diseases in areas experiencing humanitarian crises due to natural disasters, climate change, or armed conflict. The establishment of vector interception zones could provide protection to people in refugee camps and shelters and at forward-based hospitals, transportation, and logistical centers. Providing flying vector interception zones would help prevent the spread of vector borne diseases under these dire circumstances.
Vector-borne diseases are an important problem agriculture, particularly in the care of domesticated animals201 and crop plants20,202,203,204. The Photonic Fence could be used to establish vector interception zones around or in agricultural production structures that are vulnerable to infiltration by flying vectors, such as livestock and poultry housing facilities205,206,207, greenhouses208,209,210 and produce packinghouses211,212. The Photonic Fence could also be used to provide surveillance in ports-of-entry, packinghouses and similar situations where pest detection and eradication is critical to preventing their release or spread213,214. It could be emplaced on conveyances such as truck trailers and railroad cars that transfer livestock and produce to prevent flying vectors from entering or exiting the cargo area. Further development of the Photonic Fence system will permit its deployment around open agricultural production areas such as orchards, vineyards, crop fields and pastures to reduce the level of flying vector infiltration into these areas215,216,217. The ability to establish protective active zones will be particularly helpful to combat exotic invasive vectors that have a high capacity to kill crops, such as the Asian citrus psyllid transmitting Candidatus Liberibacter species in citrus groves151,152. A similar situation occurs when native insects vector an introduced phytopathogen, such as native spittlebug species transmitting the causal agent of Olive Quick Decline Syndrome in southern European olive groves218,219. While the Photonic Fence will not totally exclude vectors from infiltrating the perimeters of outdoor areas, it will none-the-less reduce vector populations in the targeted area and can be used in combination with other control measures to provide more comprehensive control and reduce insecticide inputs.
The Photonic Fence could also be used to help protect endangered species and maintain biodiversity in targeted areas. For example, vector interception zones could be established around target areas supporting populations of at-risk species that are vulnerable to vector-borne diseases220,221, newly revegetated or reintroduction areas222, or reservoirs of vectors that negatively impact vulnerable species in nearby areas223,224,225,226,227. As with livestock protection, flying vector interception zones could be positioned in zoos and rearing facilities for endangered species228,229,230,231. Conversely, livestock and domestic animals harbor vector-borne diseases that can be transmitted to wildlife and native species, sometimes resulting in catastrophic consequences232,233,234. Establishing vector interception zones between areas where livestock and domesticated animals are reared and adjacent areas supporting wildlife could help decrease vector movement between these areas.
As conceived, field deployed units of the Photonic Fence will need about 200W to operate and can utilize solar panels or batteries as remote power sources. The Photonic Fence shuts-off if a large object entered the active zone, such as a worker or animal129. Ideally, the system would be set-up such that the field of view is not restricted, especially horizontally. This may restrict its use in situations where a clear field of view is not achievable across the desired dimensions of the active zone. Further refinements will enable the tracking system to disregard objects such as tree branches or poles in the field of view. To optimize the system for each vector species of interest, certain tracking algorithm parameters (e.g. detection threshold) or dosing laser conditions (e.g. power, spot size) will need to be altered127,128,129. A database with the flight parameters of several targeted vector species will need to be devised for a particular area. This would require establishing the flight parameters of each vector species expected in the targeted areas129. The system showed a high level of sensitivity with respect to vector v. bee detection and tracking, so it is expected be able to discriminate between target and non-target flying insects in applied situations. However, there is a need for further testing to document that the system can differentiate between friend and foe under ambient conditions. The Photonic Fence is designed to be autonomous, making it vulnerable to theft of its components and housing. To reduce theft and vandalism, efforts must be made to obtain the acceptance and participation of residents and stakeholders in implementing a Photonic Fence system in their locality235,236,237.
Vector surveillance
Access to real-time, accurate vector monitoring information within targeted areas, though often unavailable, is a critical component in vector-borne disease prevention and control24. As such, there is an urgent need for improved entomological surveillance capabilities, not only to provide effective routine vector and pathogen monitoring238,239,240,241, but to address the mounting challenges to effective vector control from insecticide resistance and the effects of climate change on the biology of vector-borne disease pathosystems48,189,242,243,244,245. By itself, the tracking system of the Photonic Fence is an effective means of monitoring flying insect vectors, one which has a practical operating distance, real-time identification of species of interest, and highly specific targeting and interdiction capabilities. Because the tracking modules can provide information about the incidence, movement, and distribution of insect species of interest in real time, they could be implemented as a stand-alone, high-quality entomological monitoring tool for vector surveillance as well as for fundamental and applied entomological research.
Most of the potential surveillance applications of the Photonic Fence overlap the interdiction scenarios presented in Table 1. For example, the expansion of global air travel and seaborne trade enables insect vectors to move great distances in short periods of time11,246,247. The Photonic Fence monitoring system could work alone or with other sensory systems to enhance vector surveillance and biosanitation in ports-of-entry, packinghouses and similar situations where vector detection is critical to preventing their release or spread214,248. The accurate, real time vector surveillance information provided by the Photonic Fence could help advance local and regional vector surveillance capabilities by improving early detection of the onset of vector population growth and dispersal, and signal that proactive measures need to be initiated or escalated to address impeding outbreaks224,249. It can also be used to augment meteorological, remote sensing, and other geospatial data, including from mobile devices, to identify and delineate key vector habitats and refugia, flight corridors, and areas vulnerable to vector infiltration within the targeted landscape. In addition to providing data on existing situations on the ground, this information can be used to improve predictions about the spatial and temporal distribution patterns of vectors in targeted landscapes241,250,251,252,253,254,255,256,257,258.
While not addressed in this study, devices that collect vector specimens intercepted by the laser can be incorporated into the system; these, in turn, could provide information about the prevalence and distribution of insecticide-resistant genotypes within vector populations in the target location259,260. This information would help inform the development and management of area-wide insecticide resistance management programs244,261,262 as well as help with epidemiological forecasting263,264. For example, the Photonic Fence could be used to identify where different types of vector resistance to available active ingredients occur and therefore where next-generation bed nets and insecticides would be most effectively used244,265. The monitoring capabilities of the Photonic Fence could be part of Integrated Vector Surveillance networks that utilize different monitoring approaches to provide comprehensive assessments of vector abundance and distribution in targeted areas and identify key vector interdiction points266,267,268.
The rapid detection system afforded by the Photonic Fence could also help facilitate identifying the different climate change drivers that influence vector abundance and distribution, providing improved forecasting of vector frequency and distribution under conditions imposed by climate change45,269,270,271. The system could also provide a variety of monitoring functions that would measure the incidence, movement, and distribution of insect species of interest, providing a valuable tool for ecological monitoring and conservation efforts aimed at endangered species and other species of interest272,273.
Flight Behavior
The Photonic Fence provided detailed 3-dimensional information about the flight characteristics of individual insects, demonstrating that it can be used as a tool to conduct studies of insect flight behavior. Each tracked insect trajectory through 3-dimentional space is timestamped and saved, along with the calculated size and sample target images from the fine tracking camera. This data is then available for post-processing by the user. In-depth knowledge about vector flight behavior is fundamental to developing a comprehensive understanding of vector ecology, behavior, and epidemiology103,263. The ability to readily conduct 3-dimensional tracking of vector flight will facilitate understanding key vector behaviors, such as their responses to living hosts, traps, insecticide-treated bed nets, and attractant and repellent substances, the behavioral underpinnings of host location as well as the efficacy of traps, attract and kill devices and ‘push–pull’ strategies, and, identifying flight entry points into dwellings or structures 178,274,275,276,277. This information, in turn, will facilitate evaluating and developing other surveillance and control measures such as traps, screening, insecticide-treated bed nets, and spray programs in dwellings and residential areas278,279,280,281,282,283,284,285.
The Photonic Fence presents a new optical means of accurately detecting, monitoring and controlling flying insect vectors in real time as well as providing new capabilities to investigate their flight behavior and mechanics. In terms of vector interdiction, the Photonic Fence can provide ‘vector interception zones’ to protect sensitive areas and augment current vector abatement measures. Further trials are needed to test the efficacy and flexibility of the Photonic Fence in the various scenarios described in this paper. Specific identification of vector species and gender will require a developing a database using collected vector flight and environmental data. Optimization of tracking and interdiction capabilities for each vector or gender will require modification of parameters such as tracking algorithms to improve optical resolution capability. However, once configured, the vector detection and tracking units of the Photonic Fence can be used alone to provide effective surveillance of sensitive areas. This may be extended to monitor beneficial insects and species of interest in a given situation.
Methods and materials
Study site
The study was conducted in a screenhouse (40 mL × 3.05 m W × 3.66 mH) at the USDA-Agricultural Research Service citrus grove in Fort Pierce, FL (Fig. 1A, B). The screenhouse was equipped with water lines and a 220v electric line to power the Photonic Fence.
Insects
The eggs of the mosquito, A. aegypti, were obtained from colonies maintained by the USDA-ARS Laboratory in Gainesville, FL and from the University of Florida/IFAS Florida Medical Entomology Laboratory in Vero Beach, FL. The eggs and subsequent larvae were maintained in trays filled with RO water and supplied with standard rearing media containing dried liver and brewer’s yeast. Mosquitoes used in each test were from a single cohort. The rearing trays were kept in cages in an incubator set at 8 h L:16 h D photoperiod and held at 25 ± 1 °C temperature.
Adult Asian citrus psyllid (D. citri) were obtained from a research colony reared on orange jasmine (Murraya paniculata) seedlings and maintained in the controlled environment Insectary at the USDA Agricultural Research Laboratory in Fort Pierce, FL. The psyllid colonies were housed in screened cages containing a single potted orange jasmine seedling. The cages were kept on racks in the Insectary greenhouse and received supplemental lighting from an LED plant grow light. Both the immature and adult psyllids feed on the phloem sap of the orange jasmine seedlings. Only adults, 1–2-weeks post emergence in age, were used in the tests. Individuals for tests were collected 2–4 h prior the start of the tests and kept in ventilated plastic vials for transfer to the screenhouse. Both A. aegypti and D. citri are well-established at the test site in south Florida.
Photonic fence set-up
The Photonic Fence was mounted on two aluminum towers that were anchored in the soil and placed 30 m apart from one another along the centerline of the screenhouse (Fig. 1A–C). The front-facing side of each tower was divided longitudinally into two parts: one half was covered by a reflective screen panel while the other side contained two Photonic Fence units, mounted on the top and bottom of the tower, and support equipment (communications system, laser generator, computers), mounted mid-tower (Fig. 2A). Each individual Photonic Fence unit consisted of four 850 nm illumination projectors, two coarse tracking cameras (100fps frame rate), and a single fine tracking camera (1,500fps frame rate) and laser system (Fig. 2B). The towers were mirror images of each other, so that the light beams projected from one tower were reflected from the reflector panel on the opposite tower. In total, four units were used to create the 30 m long × 3 m high × 0.3 m wide active space of the detection, interrogation, and interception system (Fig. 3). The reflector panels consisted of wide-angle, exposed retroreflective lenses bonded to a fabric substrate, attached to a metal frame (Fig. 2A).
The laser system was comprised of a continuous wave, randomly polarized fiber laser whose output was boresight-aligned with the fine tracking camera field of view. Once an insect was approved for engagement by the target validation algorithm and the steerable optic had centered on it, the laser was pulsed for a duration of 25 ms. The power delivered to the target was 25W, at a wavelength of 1550 nm. To assist with location and recovery of insects killed in flight, the gravel floor of the screenhouse was covered with white polyester fabric which provided a light background and contrast for locating the insects that had fallen there after interception by the laser pulse (Fig. 10D). In addition, clean room adhesive mats were placed on the ground immediately below the active zone, which provided both visual contrast and helped secure fallen insects. These areas were visually inspected after each test to count the numbers of fallen insects.
Initial mosquito tracking and interception test
A 5.5-h long test was conducted to evaluate the capability of the Photonic Fence to detect, interrogate, and kill flying A. aegypti. At the start of the test, 1000 mosquitoes were released from three cages in the screenhouse. During the initial 4 h of the test, the detection and interrogation mode of the Photonic Fence was operational, but the laser was disengaged. To promote mosquito activity, a human ‘agitator’ walked slowly on the upwind side of the screenhouse (Fig. 5). One of the co-authors (AM) served as the agitator. All the methods were in accordance with relevant guidelines and regulations. After 30–45 min had passed, the agitator walked away from the screenhouse and then returned after a 15–20-min break to determine if the system could detect fluctuations in flight activity. During the last hour of the test, the laser was engaged to determine the system’s capability to intercept and kill flying mosquitoes while the agitator continued to walk outside. Post-hoc tests were performed to determine the system’s capability to detect flying mosquitoes across the active range of the system and the system’s laser engagement rate during the test.
Flying vector interception efficiency tests
To determine the capability of the system to intercept flying mosquitoes and psyllids, tests were conducted in which the insects were released from cages on one side of the screenhouse. The cages were placed on the floor and were spaced equidistant from each other along the length of the active space of the system. Tests were conducted with the laser engaged or silent to compare recovery levels of released insects.
For tests with A. aegypti, 350 mosquitoes were released per test. Mosquitoes that flew across the active area of the Photonic Fence were recovered either from: (1) a pair of open pan oviposition traps; (2) a pair of home-made pail traps; or, (3) a single human subject (Fig. 9A). The human subject sat in a chair in the center of the screenhouse on the screenhouse side opposite of the release side (Fig. 9A). A pan trap and bucket trap were placed on the floor 5 M on either side of the human subject (Figs. 1C and 9A). This arrangement provided a total of three mosquito recovery points for the tests. During the tests, neither the human subject nor traps were in the line-of-sight of the Photonic Fence (Figs. 1C and 9A). The oviposition trap consisted of a 10L white plastic pan containing 6 L of infused water that had seeped in straw for 48 h286. The pail trap consisted of a 6 L bucket and lid and had design features similar to other autocidal gravid ovitraps287,288,289. For example, the exterior of the pail and lid was painted black, an attractive color to Aedes290. The housing of a miniature CDC trap (BioQuip), containing a UV LED lamp and downdraft fan, was inserted into a fitted hole cut into the lid and secured with hot glue. Two scent baits were placed inside the pail: a sachet containing a commercial mosquito bait (Sweetscent, Biogents AG) and ca. 500 mL of crushed dry ice held in an open thermos container291. When the fan was operating, it suctioned clean air into the bucket, and then air carrying the scent baits was exhausted through two screened holes (9 cm2) cut into the lid. Mosquitoes attracted to the trap were suctioned into its interior when they became caught in the fan downdraft. The human subject wore white coveralls and a mosquito-proof head net. A 100 cm2 patch of fabric was removed from the one leg of the coverall that exposed the subject’s thigh while seated. During the 60-min-long test, the subject used a manual aspirator to collect mosquitoes when they alighted on the patch of exposed skin292.
For tests with D. citri, 600 psyllids were released per test. Psyllids were recovered in 3-D yellow traps designed especially to attract and trap D. citri by the Florida Department of Plant Industry293 (Fig. 1D). The traps were manufactured with a 3-D printer and were bright yellow in the human visual spectrum, a color that is highly attractive to D. citri294,296. In addition, the surface features of the traps helped retain the psyllids and motivate them to enter the trap interior293,297,298,299. Psyllids that were caught in the trap slid downwards into a vial of soapy water. At the conclusion of the tests, the vials were returned to the lab and the psyllids examined under magnification for counting and sexing. Ten yellow 3D psyllid traps were used for each test. The traps were hung from a nylon line supported by 99 cm high aluminum fence posts (Tractor Supply Company) inserted into the gravel floor of the screenhouse. The traps were placed were positioned in the center of each screenhouse hoop panel and ca. 25 cm from the south facing wall of the screenhouse. Because the release cages were placed on the north-facing side of the screen house, the psyllids, which are phototrophic297, tended to move to the sun-lit, south-facing side of the screenhouse where the traps were positioned. The screen house panels were also examined for the presence of psyllids that were not captured by the traps. Psyllids found on the screens were included in the total number of recovered psyllids.
Target discrimination test with male orchid bees
Male Euglossa dilemma were attracted to cotton swaps dipped in a scent bait, p-dimethoxybenzene300 (Sigma-Aldrich). The bees were collected with a net as they hovered near the scent bait (Fig. 4). The collections were made in a wooded residential neighborhood in Fort Pierce, FL, USA. The bees were collected in the morning, kept in individual plastic vials placed in a cool, dark storage box prior to the tests, and then used in the afternoon. At the start of the test, ten male E. dilemma were released in the screenhouse and allowed to fly freely for 60 min with the laser engaged.
Open air test
To test the Photonic Fence’s capability to detect and interrogate ambient numbers of insects flying during the peak dusk activity period, a test was conducted with the screen removed from the ten center support hoops of the screenhouse (Fig. 1E). This allowed native insects from the surrounding environment to fly across the active space of the Photonic Fence. The test was conducted over a 10-day period. The laser was engaged from the start of the test until 96 h had elapsed. During the initial 96-h period, three releases were made, 10 m upwind of the screenhouse, of 10,000 A. aegypti at the peak period of dusk flying activity (approximately 18:00 h Eastern Daylight Time). This test was conducted to determine (a) if a very large number of vectors could be targeted by the system when ambient insect levels were also very high, and (b) the natural flight elevation distribution of A. aegypti. A control test was conducted at dusk on day 5 in which 10,000 mosquitoes were released but the laser remained silenced. For the remaining 4 days, only monitoring and tracking of ambient insect levels was recorded.
Data availability
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.
References
Wilson, A. L. et al. The importance of vector control for the control and elimination of vector-borne diseases. PLoS Negl. Trop. Dis. 14, e0007831 (2020).
World Health Organization. Vector-borne diseases fact sheet. (World Health Organization, 2022).
World Health Organization. https://www.who.int/news-room/fact-sheets/detail/malaria (2023).
Leta, S. et al. Global risk mapping for major diseases transmitted by Aedes aegypti and Aedes albopictus. Int. J. Infect. Dis. 67, 25–35 (2018).
Ready, P. D. Biology of phlebotomine sand flies as vectors of disease agents. Annu. Rev. Entomol. 58, 227–250 (2013).
World Health Organization. Leismaniasis Fact Sheet. (World Health Organization, 2013).
Evum, U. P. C. V. V. Insight into trypanosomiasis in animals: various approaches for its diagnosis, treatment and control: A review. Asian J. Anim. Sci. 9, 172–186 (2015).
Miranda, M. Á. Case studies of vector-borne diseases in livestock: bluetongue virus. In Pests and Vector-Borne Diseases in the Livestock Industry 527–535 (Wageningen Academic Publishers, 2018).
Kar, S., Mondal, B., Ghosh, J., Mazumdar, S. M. & Mazumdar, A. Host preference of bluetongue virus vectors, Culicoides species associated with livestock in West Bengal, India: Potential relevance on bluetongue epidemiology. Acta Tropica 235, 106648 (2022).
Gomontean, B. et al. Diversity, Abundance and host blood meal analysis of Culicoides Latreille (Diptera: Ceratopogonidae) from cattle pens in different land use types from Thailand. Insects 14, 574 (2023).
Kilpatrick, A. M. Globalization, land use, and the invasion of West Nile virus. Science 334, 323–327 (2011).
Imperato, P. J. The convergence of a virus, mosquitoes, and human travel in globalizing the Zika epidemic. J. Commun. Health 41, 674–679 (2016).
Linthicum, K. J., Britch, S. C. & Anyamba, A. Rift Valley fever: an emerging mosquito-borne disease. Annu. Rev. Entomol. 61, 395–415 (2016).
Costa, A. S. Whitefly-transmitted plant diseases. Annu. Rev. Phytopathol. 14, 429–449 (1976).
Purcell, A. H. Insect vector relationships with procaryotic plant pathogens. Annu. Rev. Phytopathol. 20, 397–417 (1982).
Brown, J. K. & Czosnek, H. Whitefly Transmission of Plant Viruses. In Advances in Botanical Research Vol. 36 (eds Brown, J. et al.) 65–92 (Academic Press, 2002).
Ng, J. C. & Perry, K. L. Transmission of plant viruses by aphid vectors. Mol. Plant Pathol. 5, 505–511 (2004).
Weintraub, P. G. & Beanland, L. Insect vectors of phytoplasmas. Annu. Rev. Entomol. 51, 91–111 (2006).
Haapalainen, M. Biology and epidemics of Candidatus Liberibacter species, psyllid-transmitted plant-pathogenic bacteria. Ann. Appl. Biol. 165, 172–198 (2014).
Perilla-Henao, L. M. & Casteel, C. L. Vector-borne bacterial plant pathogens: interactions with hemipteran insects and plants. Front. Plant Sci. 7, 1163 (2016).
Moreno, A., Miranda, M. P. & Fereres, A. Psyllids as major vectors of plant pathogens. Entomol. Gen. 41, 419 (2021).
Tudi, M. et al. Agriculture development, pesticide application and its impact on the environment. Int. J. Environ. Res. Public Health 18, 1112 (2021).
van den Berg, H. et al. Recent trends in global insecticide use for disease vector control and potential implications for resistance management. Sci. Rep. 11, 23867 (2021).
World Health Organization. Global Vector Control Response 2017–2030 (2554) World Health Organization (2017).
Aktar, W., Sengupta, D. & Chowdhury, A. Impact of pesticides use in agriculture: Their benefits and hazards. Interdiscip. Toxicol. 2, 1–12 (2009).
Schmidt-Jeffris, R. A., Beers, E. H. & Sater, C. Meta-analysis and review of pesticide non-target effects on phytoseiids, key biological control agents. Pest Manag. Sci. 77, 4848–4862 (2021).
Serrão, J. E., Plata-Rueda, A., Martínez, L. C. & Zanuncio, J. C. Side-effects of pesticides on non-target insects in agriculture: A mini-review. Sci. Nat. 109, 17 (2022).
Schmidt-Jeffris, R. A. Non-target pesticide impacts on pest natural enemies: Progress and gaps in current knowledge. Curr. Opin. Insect Sci. 58, 101056 (2023).
Nauen, R. Insecticide resistance in disease vectors of public health importance. Pest Manag. Sci. 63, 628–633 (2007).
Labbé, P. et al. Evolution of resistance to insecticide in disease vectors. In Genetics and Evolution of Infectious Disease (ed. Tibayrenc, M.) 313–339 (Elsevier, 2017).
Nkya, T. E., Akhouayri, I., Kisinza, W. & David, J. P. Impact of environment on mosquito response to pyrethroid insecticides: Facts, evidences and prospects. Insect Biochem. Mol. Biol. 43, 407–416 (2013).
Bass, C. et al. The evolution of insecticide resistance in the peach potato aphid, Myzus persicae. Insect Biochem. Mol. Biol. 51, 41–51 (2014).
Simon, J. C. & Peccoud, J. Rapid evolution of aphid pests in agricultural environments. Curr. Opin. Insect Sci. 26, 17–24 (2018).
Balaska, S., Fotakis, E. A., Chaskopoulou, A. & Vontas, J. Chemical control and insecticide resistance status of sand fly vectors worldwide. PLoS Negl. Trop. Dis. 15, e0009586 (2021).
Juache-Villagrana, A. E. et al. Assessing the impact of insecticide resistance on vector competence: A review. Insects 13, 377 (2022).
Love, R. R., Sikder, J. R., Vivero, R. J., Matute, D. R. & Schrider, D. R. Strong positive selection in Aedes aegypti and the rapid evolution of insecticide resistance. Mol. Biol. Evol. 40, msad072 (2023).
Bass, C. & Nauen, R. The molecular mechanisms of insecticide resistance in aphid crop pests. Insect Biochem. Mol. Biol. 156, 103937 (2023).
Hemingway, J. et al. Averting a malaria disaster: Will insecticide resistance derail malaria control?. Lancet 387, 1785–1788 (2016).
Paine, M. J. & Brooke, B. Insecticide resistance and its impact on vector control. In Advances in Insect Control and Resistance Management (eds Horowitz, A. & Ishaaya, I.) 287–312 (Springer, 2016).
World Health Organization. Global report on insecticide resistance in malaria vectors: 2010–2016. (World Health Organization, 2018).
Lindgren, E., Andersson, Y., Suk, J. E., Sudre, B. & Semenza, J. C. Monitoring EU emerging infectious disease risk due to climate change. Science 336, 418–419 (2012).
Proestos, Y. et al. Present and future projections of habitat suitability of the Asian tiger mosquito, a vector of viral pathogens, from global climate simulation. Philos. Trans. R. Soc. B 370, 20130554 (2015).
Campbell, L. P. et al. Climate change influences on global distributions of dengue and chikungunya virus vectors. Philos. Trans. R. Soc. B 370, 20140135 (2015).
Metcalf, C. J. E. et al. Identifying climate drivers of infectious disease dynamics: Recent advances and challenges ahead. Proc. R. Soc. B Biol. Sci. 284, 20170901 (2017).
Caminade, C., McIntyre, K. M. & Jones, A. E. Impact of recent and future climate change on vector-borne diseases. Ann. N.Y. Acad. Sci. 1436, 157–173 (2019).
Rocklöv, J. & Dubrow, R. Climate change: An enduring challenge for vector-borne disease prevention and control. Nat. Immunol. 21, 479–483 (2020).
Wang, Y., Yim, S. H. L., Yang, Y. & Morin, C. W. The effect of urbanization and climate change on the mosquito population in the Pearl River Delta region of China. Int. J. Biometeorol. 64, 501–512 (2020).
Skendžić, S., Zovko, M., Živković, I. P., Lešić, V. & Lemić, D. The impact of climate change on agricultural insect pests. Insects 12, 440 (2021).
Semenza, J. C., Rocklöv, J. & Ebi, K. L. Climate change and cascading risks from infectious disease. Infect. Dis. Therapy 11, 1371–1390 (2022).
Kulkarni, M. A., Duguay, C. & Ost, K. Charting the evidence for climate change impacts on the global spread of malaria and dengue and adaptive responses: A scoping review of reviews. Global. Health 18, 1–18 (2022).
Mora, C. et al. Over half of known human pathogenic diseases can be aggravated by climate change. Nat. Clim. Change 12, 869–875 (2022).
Schneider, L., Rebetez, M. & Rasmann, S. The effect of climate change on invasive crop pests across biomes. Curr. Opin. Insect Sci. 50, 100895 (2022).
Tonnang, H. E., Sokame, B. M., Abdel-Rahman, E. M. & Dubois, T. Measuring and modelling crop yield losses due to invasive insect pests under climate change. Curr. Opin. Insect Sci. 50, 100873 (2022).
World Health Organization. World Malaria Report. (World Health Organization, 2023).
Edelson, P. J. et al. Climate change and the epidemiology of infectious diseases in the United States. Clin. Infect. Dis. 76, 950–956 (2023).
Zhu, L., Xue, Q., Ma, G. & Ma, C. S. Climate warming exacerbates plant disease through enhancing commensal interaction of co-infested insect vectors. J. Pest Sci. 96, 945–959 (2023).
Delcour, I., Spanoghe, P. & Uyttendaele, M. Literature review: Impact of climate change on pesticide use. Food Res. Int. 68, 7–15 (2015).
Rhodes, L. A. & McCarl, B. A. An analysis of climate impacts on herbicide, insecticide, and fungicide expenditures. Agronomy 10, 745 (2020).
Larsen, A. E. & McComb, S. Land cover and climate changes drive regionally heterogeneous increases in US insecticide use. Landsc. Ecol. 36, 159–177 (2021).
Matzrafi, M. Climate change exacerbates pest damage through reduced pesticide efficacy. Pest Manag. Sci. 75, 9–13 (2019).
Saha, P. et al. Prevalence of kdr mutations and insecticide susceptibility among natural population of Aedes aegypti in West Bengal. PLoS One 14, e0215541 (2019).
Mogilicherla, K. & Roy, A. Epigenetic regulations as drivers of insecticide resistance and resilience to climate change in arthropod pests. Front. Genetics 13, 1044980 (2023).
Adil, M., Abbas, G., Khan, R. N. & Abbas, F. Impact of Climate Change on Environmental Fate and Ecological Effects of Pesticides. In Strategizing Agricultural Management for Climate Change Mitigation and Adaptation (ed. Bandh, S. A.) 247–263 (Springer International Publishing, 2023).
Deutsch, C. A. et al. Increase in crop losses to insect pests in a warming climate. Science 361, 916–919 (2018).
Crossley, M. S. et al. Warmer temperatures trigger insecticide-associated pest outbreaks. Pest Manag. Sci. https://doi.org/10.1002/ps.7832 (2023).
Ziska, L. H. et al. Climate change, carbon dioxide, and pest biology, managing the future: Coffee as a case study. Agronomy 8, 152 (2018).
Dusfour, I. et al. Management of insecticide resistance in the major Aedes vectors of arboviruses: Advances and challenges. PLoS Negl. Trop. Dis. 13, e0007615 (2019).
Pu, J., Wang, Z. & Chung, H. Climate change and the genetics of insecticide resistance. Pest Manag. Sci. 76, 846–852 (2020).
Benelli, G., Wilke, A. B., Bloomquist, J. R., Desneux, N. & Beier, J. C. Overexposing mosquitoes to insecticides under global warming: A public health concern?. Sci. Total Environ. 762, 143069 (2021).
Gnankiné, O. et al. Insecticide resistance in Bemisia tabaci Gennadius (Homoptera: Aleyrodidae) and Anopheles gambiae Giles (Diptera: Culicidae) could compromise the sustainability of malaria vector control strategies in West Africa. Acta Tropica 128, 7–17 (2013).
Nkya, T. E., Mosha, F. W., Magesa, S. M. & Kisinza, W. N. Increased tolerance of Anopheles gambiae s.s. to chemical insecticides after exposure to agrochemical mixture. Tanzan. J. Health Res. https://doi.org/10.4314/thrb.v16i4.10 (2014).
Philbert, A., Lyantagaye, S. L. & Nkwengulila, G. A review of agricultural pesticides use and the selection for resistance to insecticides in malaria vectors. Adv. Entomol. 2, 47816 (2014).
Reid, M. C. & McKenzie, F. E. The contribution of agricultural insecticide use to increasing insecticide resistance in African malaria vectors. Malaria J. 15, 1–8 (2016).
Matiya, D. J., Philbert, A. B., Kidima, W. & Matowo, J. J. Dynamics and monitoring of insecticide resistance in malaria vectors across mainland Tanzania from 1997 to 2017: A systematic review. Malaria J. 18, 1–16 (2019).
Mnzava, A. P. et al. Implementation of the global plan for insecticide resistance management in malaria vectors: Progress, challenges and the way forward. Malaria J. 14, 1–9 (2015).
Ranson, H. & Lissenden, N. Insecticide resistance in African Anopheles mosquitoes: A worsening situation that needs urgent action to maintain malaria control. Trends Parasitol. 32, 187–196 (2016).
Kisinza, W. N. et al. Multiple insecticide resistance in Anopheles gambiae from Tanzania: A major concern for malaria vector control. Malaria J. 16, 1–10 (2017).
Sternberg, E. D. & Thomas, M. B. Insights from agriculture for the management of insecticide resistance in disease vectors. Evol. Appl. 11, 404–414 (2018).
Anoopkumar, A. N. & Aneesh, E. M. A critical assessment of mosquito control and the influence of climate change on mosquito-borne disease epidemics. Environ. Dev. Sustain. 24, 8900–8929 (2022).
Binns, M. R. & Nyrop, J. P. Sampling insect populations for the purpose of IPM decision making. Annu. Rev. Entomol. 37, 427–453 (1992).
Cotter, C. Evaluating and optimizing surveillance and response strategies for malaria elimination in the Asia Pacific region. (Doctoral dissertation, Acta Universitatis Upsaliensis, 2023).
Gibson, G. & Torr, S. J. Visual and olfactory responses of haematophagous Diptera to host stimuli. Med. Vet. Entomol. 13, 2–23 (1999).
Lothrop, H. D. & Reisen, W. K. Landscape affects the host-seeking patterns of Culex tarsalis (Diptera: Culicidae) in the Coachella Valley of California. J. Med. Entomol. 38, 325–332 (2001).
Muirhead-Thompson, R. C. Trap Responses of Flying Insects: The Influence of Trap Design on Capture Efficiency (Academic Press, 2012).
Brockerhoff, E. G. et al. Monitoring and surveillance of forest insects. In Forest Entomology and Pathology (eds Allison, J. et al.) 669–705 (Springer International Publishing, 2023).
Miller, J. R., Adams, C. G., Weston, P. A. & Schenker, J. H. Trapping of Small Organisms Moving Randomly: Principles and Applications to Pest Monitoring and Management (Springer, 2015).
Miller, J. R. Sharpening the precision of pest management decisions: Assessing variability inherent in catch number and absolute density estimates derived from pheromone-baited traps monitoring insects moving randomly. J. Econ. Entomol. 113, 2052–2060 (2020).
Onufrieva, K. S. & Onufriev, A. V. How to count bugs: Amethod to estimate the most probable absolute population density and its statistical bounds from a single trap catch. Insects 12, 932. https://doi.org/10.3390/insects12100932 (2021).
Manoukis, N. C. & Collier, T. C. Computer vision to enhance behavioral research on insects. Ann. Entomol. Soc. Am. 112, 227–235 (2019).
Lima, M. C. F., de Almeida Leandro, M. E. D., Valero, C., Coronel, L. C. P. & Bazzo, C. O. G. Automatic detection and monitoring of insect pests—a review. Agriculture 10, 161 (2020).
Preti, M., Verheggen, F. & Angeli, S. Insect pest monitoring with camera-equipped traps: Strengths and limitations. J. Pest Sci. 94, 203–217 (2021).
Kittichai, V. et al. Deep learning approaches for challenging species and gender identification of mosquito vectors. Sci. Rep. 11, 4838 (2021).
González-Pérez, M. I. et al. A novel optical sensor system for the automatic classification of mosquitoes by genus and sex with high levels of accuracy. Parasites Vectors 15, 1–11 (2022).
Johnson, B. J., Manby, R. & Devine, G. J. The use of automated traps to assess the efficacy of insecticide barrier treatments against abundant mosquitoes in remote environments. J. Med. Entomol. 59, 384–389 (2022).
Susetyo, J. Insects pest trap monitoring system using Internet of Things based sensors. Jurnal Internasional ETJ 8, 2342–2349 (2023).
Cannet, A. et al. An annotated wing interferential pattern dataset of dipteran insects of medical interest for deep learning. Sci. Data 11, 4 (2024).
Bidlingmayer, W. L. The measurement of adult mosquito population changes-some considerations. J. Am. Mosq. Control Assoc. 1, 328–348 (1985).
Reisen, W. K., Lothrop, H. D. & Lothrop, B. Factors influencing the outcome of mark-release-recapture studies with Culex tarsalis (Diptera: Culicidae). J. Med. Entomol. 40, 820–829 (2003).
Petrovskaya, N., Petrovskii, S. & Murchie, A. K. Challenges of ecological monitoring: Estimating population abundance from sparse trap counts. J. R. Soc. Interface 9, 420–435 (2012).
Sikaala, C. H. et al. A cost-effective, community-based, mosquito-trapping scheme that captures spatial and temporal heterogeneities of malaria transmission in rural Zambia. Malaria J. 13, 1–13 (2014).
McDermott, E. G. & Mullens, B. A. The dark side of light traps. J. Med. Entomol. 55, 251–261 (2018).
Buchwald, A. G., Hayden, M. H., Dadzie, S. K., Paull, S. H. & Carlton, E. J. Aedes-borne disease outbreaks in West Africa: A call for enhanced surveillance. Acta Trop. 209, 105468 (2020).
Spitzen, J. & Takken, W. Keeping track of mosquitoes: A review of tools to track, record and analyse mosquito flight. Parasites Vectors 11, 1–11 (2018).
Voloshin, V. et al. Diffuse retro-reflective imaging for improved video tracking of mosquitoes at human baited bednets. R. Soc. Open Sci. 7, 191951. https://doi.org/10.1098/rsos.191951 (2020).
Kirkeby, C. et al. Advances in automatic identification of flying insects using optical sensors and machine learning. Sci. Rep. 11, 1555 (2021).
Rydhmer, K. et al. Automating insect monitoring using unsupervised near-infrared sensors. Sci. Rep. 12, 2603 (2022).
Rhodes, M. W., Bennie, J. J., Spalding, A., French-Constant, R. H. & Maclean, I. M. Recent advances in the remote sensing of insects. Biol. Rev. 97, 343–360 (2021).
Siddiqui, A. A. I., & Kayte, C. Convolution Neural Network-based Mosquito Classification System. Proceedings of the 3rd International Conference on ICT for Digital, Smart, and Sustainable Development 449, 60 (European Alliance for Innovation, 2023).
van Klink, R. et al. Emerging technologies revolutionise insect ecology and monitoring. Trends Ecol. Evol. 37, 872. https://doi.org/10.1016/j.tree.2022.06.001 (2022).
Wang, Y., Zhao, C., Dong, D. & Wang, K. Real-time monitoring of insects based on laser remote sensing. Ecol. Indic. 151, 110302 (2023).
Repasky, K. S. et al. Optical detection of honeybees by use of wing-beat modulation of scattered laser light for locating explosives and land mines. Appl. Opt. 45, 1839–1843 (2006).
Brydegaard, M. & Jansson, S. Advances in entomological laser radar. J. Eng. 2019, 7542–7545 (2019).
Noskov, A., Bendix, J. & Friess, N. A review of insect monitoring approaches with special reference to radar techniques. Sensors 2021, 1474. https://doi.org/10.3390/s21041474 (2021).
Bauer, S. et al. From agricultural benefits to aviation safety: Realizing the potential of continent-wide radar networks. BioScience 67, 912–918 (2017).
Shamoun-Baranes, J., Nilsson, C., Bauer, S. & Chapman, J. Taking radar aeroecology into the 21st century. Ecography 42, 847–851 (2019).
Osborne, J. L. et al. A landscape-scale study of bumble bee foraging range and constancy, using harmonic radar. J. Appl. Ecol. 36, 519–533 (1999).
Maggiora, R., Saccani, M., Milanesio, D. & Porporato, M. An innovative harmonic radar to track flying insects: The case of Vespa velutina. Sci. Rep. 9, 11964 (2019).
Makinson, J. C. et al. Harmonic radar tracking reveals random dispersal pattern of bumblebee (Bombus terrestris) queens after hibernation. Sci. Rep. 9, 4651 (2019).
Lavrenko, A. et al. Autonomous swarm of UAVs for tracking of flying insects with harmonic radar in 2021 IEEE 93rd Vehicular Technology Conference 1–5 (Institute of Electrical and Electronics Engineers, 2021).
Brydegaard, M. et al. Lidar reveals activity anomaly of malaria vectors during pan-African eclipse. Sci. Adv. 6, eaay5487 (2020).
Zhu, S. et al. Insect abundance over Chinese rice fields in relation to environmental parameters, studied with a polarization-sensitive CW near-IR lidar system. Appl. Phys. B 123, 1–11 (2017).
Jansson, S. et al. Real-time dispersal of malaria vectors in rural Africa monitored with lidar. Plos One 16, e0247803 (2021).
Genoud, A. P., Basistyy, R., Williams, G. M. & Thomas, B. P. Optical remote sensing for monitoring flying mosquitoes, gender identification and discussion on species identification. Appl. Phys. B 124, 1–11 (2018).
Genoud, A. P., Gao, Y., Williams, G. M. & Thomas, B. P. A comparison of supervised machine learning algorithms for mosquito identification from backscattered optical signals. Ecol. Inf. 58, 101090 (2020).
Genoud, A. P., Gao, Y., Williams, G. M. & Thomas, B. P. Identification of gravid mosquitoes from changes in spectral and polarimetric backscatter cross sections. J. Biophotonics 12, e201900123 (2019).
Genoud, A. P., Torsiello, J., Belson, M. & Thomas, B. P. Entomological photonic sensors: Estimating insect population density, its uncertainty and temporal resolution from transit data. Ecol. Inf. 61, 101186 (2021).
Keller, M. D. et al. Laser induced mortality of Anopheles stephensi mosquitoes. Sci. Rep. 6, 20936 (2016).
Keller, M. D. et al. Optical tracking and laser-induced mortality of insects during flight. Sci. Rep. 10, 1–11 (2020).
Mullen, E. R., Rutschman, P., Pegram, N., Patt, J. M. & Adamczyk, J. J. Laser system for identification, tracking, and control of flying insects. Opt. Express 24, 11828–11838 (2016).
Kalile, M. O., Magalhaes, D., Ragni, M. & Fancelli, M. Efeitos da radiação eletromagnética laser em Diaphorina citri. Sitientibus Série Ciencias Fısicas 15, 15–26 (2019).
Hu, P. S. et al. Knocking down free-flight adult mosquitoes via dynamic tracking. OSA Contin. 2, 2028–2037 (2019).
Rakhmatulin, I. Raspberry PI for kill mosquitoes by laser. SSRN Electron. J. https://doi.org/10.2139/ssrn.3772579 (2021).
Rakhmatulin, I. Detect caterpillar, grasshopper, aphid and simulation program for neutralizing them by laser. SSRN Electron. J. https://doi.org/10.2139/SSRN.3831722 (2021).
Gaetani, R. et al. Sustainable laser-based technology for insect pest control. Scientific Reports 11, 11068 (2021).
Lacotte, V. et al. A pesticide-free robotic control of aphids as crop pests. AgriEngineering 4, 903–921 (2022).
Rakhmatulin, I., Lihoreau, M. & Pueyo, J. Selective neutralisation and deterring of cockroaches with laser automated by machine vision. Oriental Insects 57, 728–745 (2023).
Zaidem, A. et al. New biocompatible technique based on the use of a laser to control the whitefly Bemisia tabaci. Photonics 10, 636 (2023).
Lwande, O. W. et al. Globe-trotting Aedes aegypti and Aedes albopictus: risk factors for arbovirus pandemics. Vector-Borne Zoonotic Dis. 20, 71–81 (2020).
Madewell, Z. J. Arboviruses and their vectors. South. Med. J. 113, 520 (2020).
Näslund, J. et al. Emerging mosquito-borne viruses linked to Aedes aegypti and Aedes albopictus: global status and preventive strategies. Vector-Borne Zoonotic Dis. 21, 731–746 (2021).
Kache, P. A. et al. Bridging landscape ecology and urban science to respond to the rising threat of mosquito-borne diseases. Nat. Ecol. Evol. 6, 1601–1616 (2022).
Xia, S. et al. Larval sites of the mosquito Aedes aegypti formosus in forest and domestic habitats in Africa and the potential association with oviposition evolution. Ecol. Evol. 11, 16327–16343 (2021).
Juarez, J. G. et al. Dispersal of female and male Aedes aegypti from discarded container habitats using a stable isotope mark-capture study design in South Texas. Sci. Rep. 10, 1–12 (2020).
Moore, T. C. & Brown, H. E. Estimating Aedes aegypti (Diptera: Culicidae) flight distance: Meta-data analysis. J. Med. Entomol. 59, 1164–1170 (2022).
Nuckols, J. T. et al. Evaluation of simultaneous transmission of chikungunya virus and dengue virus type 2 in infected Aedes aegypti and Aedes albopictus (Diptera: Culicidae). J. Med. Entomol. 52, 447–451 (2015).
Göertz, G. P., Vogels, C. B., Geertsema, C., Koenraadt, C. J. & Pijlman, G. P. Mosquito co-infection with Zika and chikungunya virus allows simultaneous transmission without affecting vector competence of Aedes aegypti. PLoS Negl. Trop. Dis. 11, e0005654 (2017).
Thompson, R., Martin Del Campo, J. & Constenla, D. A review of the economic evidence of Aedes-borne arboviruses and Aedes-borne arboviral disease prevention and control strategies. Exp. Rev. Vaccines 19, 143–162 (2020).
Halbert, S. E. & Manjunath, K. L. Asian Citrus Psyllids (Sternorrhyncha: Psyllidae) and greening disease of citrus: A literature review and assessment of risk in Florida. Florida Entomol. 87, 330–353 (2004).
Grafton-Cardwell, E. E., Stelinski, L. L. & Stansly, P. A. Biology and management of Asian citrus psyllid, vector of the huanglongbing pathogens. Annu. Rev. Entomol. 58, 413–432 (2013).
Hall, D. G., Richardson, M. L., Ammar, E. D. & Halbert, S. E. Asian citrus psyllid, Diaphorina citri, vector of citrus huanglongbing disease. Entomologia Experimentalis et Applicata 146, 207–223 (2013).
da Graҫa, J. V. Citrus greening disease. Ann. Rev. Phytopathol. 29, 109–136 (1991).
Bové, J. M. Huanglongbing: A destructive, newly-emerging, century-old disease of citrus. J. Plant Pathol. 88, 7–37 (2006).
Graham, J., Gottwald, T. & Setamou, M. Status of huanglongbing (HLB) outbreaks in Florida, California and Texas. Trop. Plant Pathol. 45, 265–278 (2020).
Bassanezi, R. B. et al. Overview of citrus huanglongbing spread and management strategies in Brazil. Trop. Plant Pathol. 45, 251–264 (2020).
Zhou, C. The status of citrus Huanglongbing in China. Trop. Plant Pathol. 45, 279–284 (2020).
Singerman, A. & Rogers, M. E. The economic challenges of dealing with citrus greening: The case of Florida. J. Integr. Pest Manag. 11, 3 (2020).
Luckstead, J. & Devadoss, S. Trends and issues facing the US citrus industry. Choices 36, 1–10 (2021).
Pérez-Valencia, L. I. & Moya-Raygoza, G. Body size variation of Diaphorina citri (Hemiptera: Psyllidae) through an elevation gradient. Ann. Entomol. Soc. Am. 108, 800–806 (2015).
Tsai, J. H. & Liu, Y. H. Biology of Diaphorina citri (Homoptera: Psyllidae) on four host plants. J. Econ. Entomol. 93, 1721–1725 (2000).
Zorzenon, F. P., Tomaseto, A. F., Daugherty, M. P., Lopes, J. R. & Miranda, M. P. Factors associated with Diaphorina citri immigration into commercial citrus orchards in São Paulo State, Brazil. J. Appl. Entomol. 145, 326–335 (2021).
Sétamou, M., Tarshis-Moreno, A. & Patt, J. M. Source or Sink? The role of residential host plants in Asian citrus psyllid infestation of commercial citrus groves. J. Econ. Entomol. 115, 438–445 (2022).
Li, S., Wu, F., Duan, Y., Singerman, A. & Guan, Z. Citrus greening: Management strategies and their economic impact. HortScience 55, 604–612 (2020).
Tiwari, S., Mann, R. S. & Rogers, M. E. Insecticide resistance in field populations of Asian citrus psyllid in Florida. Pest Manag. Sci. 67, 1258–1268 (2011).
Tiwari, S., Pelz-Stelinski, K. & Stelinski, L. L. Effect of Candidatus Liberibacter asiaticus infection on susceptibility of Asian citrus psyllid, Diaphorina citri, to selected insecticides. Pest Manag. Sci. 67, 94–99 (2011).
Naeem, A. et al. Monitoring of insecticide resistance in Diaphorina citri Kuwayama (Hemiptera: Psyllidae) from citrus groves of Punjab, Pakistan. Crop Protect. 86, 62–68 (2016).
Tian, F., Mo, X., Rizvi, S. A. H., Li, C. & Zeng, X. Detection and biochemical characterization of insecticide resistance in field populations of Asian citrus psyllid in Guangdong of China. Sci. Rep. 8, 1–11 (2018).
Chen, X. D., Neupane, S., Gossett, H., Pelz-Stelinski, K. S. & Stelinski, L. L. Insecticide rotation scheme restores insecticide susceptibility in thiamethoxam-resistant field populations of Asian citrus psyllid, Diaphorina citri Kuwayama (Hemiptera: Liviidae), in Florida. Pest Manag. Sci. 77, 464–473 (2021).
Mendonça, L. B. P., Zambolim, L. & Badel, J. L. Bacterial citrus diseases: Major threats and recent progress. J. Bacteriol. Mycol. 5, 4–2017 (2017).
Barbieri, H. B., Fernandes, L. S., Pontes, J. G. D. M., Pereira, A. K. & Fill, T. P. An overview of the most threating diseases that affect worldwide citriculture: Main features, diagnose, and current control strategies. Front. Nat. Prod. 2, 1045364 (2023).
Skov, C. & Wiley, J. Establishment of the neotropical orchid bee Euglossa viridissima (Hymenoptera: Apidae) in Florida. Florida Entomol. 88, 225–227 (2005).
Mullins, A. Green orchid bee Euglossa dilemma Friese (Insecta: Hymenoptera: Apidae). University of Florida/ Institute of Food and Agriculture Science Extension Bulletin EENY576. (2013).
Williams, N. H. & Dodson, C. H. Selective attraction of male euglossine bees to orchid floral fragrances and its importance in long distance pollen flow. Evolution 26, 84–95 (1972).
Dressler, R. L. Pollination by euglossine bees. Evolution 1, 202–210 (1968).
Dressler, R. L. Biology of the Orchid Bees (Euglossini). Ann. Rev. Ecol. Syst. 13, 373–394 (1982).
Henske, J., Saleh, N. W., Chouvenc, T., Ramírez, S. R. & Eltz, T. Function of environment-derived male perfumes in orchid bees. Curr. Biol. 33, 2075–2080 (2023).
Opedal, Ø. H., Martins, A. A. & Marjakangas, E. L. A database and synthesis of euglossine bee assemblages collected at fragrance baits. Apidologie 51, 519–530 (2020).
Dormont, L., Mulatier, M., Carrasco, D. & Cohuet, A. Mosquito attractants. J. Chem. Ecol. 47, 351–393 (2021).
Liu, N. Insecticide resistance in mosquitoes: Impact, mechanisms, and research directions. Annu. Rev. Entomol. 60, 537–559 (2015).
https://www.ted.com/talks/nathan_myhrvold_could_this_laser_zap_malaria
Charlwood, J. D., Vij, R. & Billingsley, P. F. Dry season refugia of malaria-transmitting mosquitoes in a dry savannah zone of east Africa. Am. J. Trop. Med. Hyg. 62, 726–732 (2000).
Kahamba, N. F. et al. Using ecological observations to improve malaria control in areas where Anopheles funestus is the dominant vector. Malaria J. 21, 1–15 (2022).
Giles, M. T. & Wilkes, T. J. The effect of high fences on the dispersal of some West African mosquitoes (Diptera: Culicidae). Bull. Entomol. Res. 68, 401–408 (1978).
Faiman, R., Kirstein, O., Moncaz, A., Guetta, H. & Warburg, A. Studies on the flight patterns of foraging sand flies. Acta Trop. 120, 110–114 (2011).
Burkot, T. R. et al. Barrier screens: A method to sample blood-fed and host-seeking exophilic mosquitoes. Malaria J. 12, 1–9 (2013).
Benelli, G. & Beier, J. C. Current vector control challenges in the fight against malaria. Acta Trop. 174, 91–96 (2017).
Pates, H. & Curtis, C. Mosquito behavior and vector control. Annu. Rev. Entomol. 50, 53–70 (2005).
Norris, L. C. et al. Adaptive introgression in an African malaria mosquito coincident with the increased usage of insecticide-treated bed nets. Proc. Natl. Acad. Sci. 112, 815–820 (2015).
Glunt, K. D. et al. Empirical and theoretical investigation into the potential impacts of insecticide resistance on the effectiveness of insecticide-treated bed nets. Evol. Appl. 11, 431–441 (2018).
Sougoufara, S., Ottih, E. C. & Tripet, F. The need for new vector control approaches targeting outdoor biting Anopheline malaria vector communities. Parasites Vectors 13, 1–15 (2020).
Philbert, A., Lyantagaye, S. L. & Nkwengulila, G. Farmers’ pesticide usage practices in the malaria endemic region of North-Western Tanzania: Implications to the control of malaria vectors. BMC Public Health 19, 1–11 (2019).
Matowo, N. S. et al. Participatory approaches to raise awareness among subsistence farmers in Tanzania about the spread of insecticide resistance in malaria vectors and the possible link to improper agricultural pesticide use. Malaria J. 21, 277–294 (2022).
Seyoum, A., Balcha, F., Balkew, M., Ali, A. & Gebre-Michael, T. Impact of cattle keeping on human biting rate of anopheline mosquitoes and malaria transmission around Ziway, Ethopia. East Afr. Med. J. 79, 485–490 (2002).
Killeen, G. F. & Smith, T. A. Exploring the contributions of bed nets, cattle, insecticides and excitorepellency to malaria control: A deterministic model of mosquito host-seeking behaviour and mortality. Trans. R. Soc. Trop. Med. Hyg. 101, 867–880 (2007).
Asale, A., Duchateau, L., Devleesschauwer, B., Huisman, G. & Yewhalaw, D. Zooprophylaxis as a control strategy for malaria caused by the vector Anopheles arabiensis (Diptera: Culicidae): A systematic review. Infect. Dis. Poverty 6, 1–14 (2017).
Bett, B. K., Grace, D., & McDermott, J. J. Africa's growing risk of diseases that spread from animals to people. International Food Policy Blog 7, Africa’s growing risk of diseases that spread from animals to people | IFPRI : International Food Policy Research Institute (2020).
Ivers, L. C. & Ryan, E. T. Infectious diseases of severe weather-related and flood-related natural disasters. Curr. Opin. Infect. Dis. 19, 408–414 (2006).
Aagaard-Hansen, J., Nombela, N. & Alvar, J. Population movement: A key factor in the epidemiology of neglected tropical diseases. Trop. Med. Int. Health 15, 1281–1288 (2010).
Lam, E., McCarthy, A. & Brennan, M. Vaccine-preventable diseases in humanitarian emergencies among refugee and internally-displaced populations. Human Vaccines Immunotherapeutics 11, 2627–2636 (2015).
Bardosh, K. L., Ryan, S. J., Ebi, K., Welburn, S. & Singer, B. Addressing vulnerability, building resilience: Community-based adaptation to vector-borne diseases in the context of global change. Infect. Dis. Poverty 6, 1–21 (2017).
Braam, D. H., Jephcott, F. L. & Wood, J. L. N. Identifying the research gap of zoonotic disease in displacement: A systematic review. Global Health Res. Policy 6, 1–12 (2021).
Pagès, N. & Cohnstaedt, L. W. Mosquito-borne diseases in the livestock industry. In Pests and Vector-Borne Diseases in the Livestock Industry (eds Garros, C. et al.) 129–144 (Wageningen Academic Publishers, 2018).
Purcell, A. H. & Rodrigo, P. P. Almeida Insects as vectors of disease agents. Encycl. Plant Crop Sci. 10, 1–5 (2005).
Harris, K. F. & Maramorosch, K. Vectors of Plant Pathogens 482 (Academic Press, 2013).
Eigenbrode, S. D., Bosque-Pérez, N. A. & Davis, T. S. Insect-borne plant pathogens and their vectors: Ecology, evolution, and complex interactions. Annu. Rev. Entomol. 63, 169–191 (2018).
Kobayashi, S. et al. The role of neighboring infected cattle in bovine leukemia virus transmission risk. J. Veterinary Med. Sci. 77, 861–863 (2015).
Kohara, J., Takeuchi, M., Hirano, Y., Sakurai, Y. & Takahashi, T. Vector control efficacy of fly nets on preventing bovine leukemia virus transmission. J. Veterinary Med. Sci. 80, 1524–1527 (2018).
Patel, A., Jenkins, M., Rhoden, K. & Barnes, A. N. A systematic review of zoonotic enteric parasites carried by flies, cockroaches, and dung beetles. Pathogens 11, 90 (2022).
Hanafi, A. Integrated production and protection today and in the future in greenhouse crops in the Mediterranean region. Proc. VI International Symposium on Protected Cultivation in Mild Winter Climate: Product and Process Innovation 614, 755-765 (2002).
Knapp, M., Palevsky, E. & Rapisarda, C. Insect and mite pests. In Integrated Pest and Disease Management in Greenhouse Crops. Plant Pathology in the 21st Century Vol. 9 (eds Gullino, M. et al.) 101–146 (Springer, 2020).
Kruidhof, H. M. & Elmer, W. H. Cultural methods for greenhouse pest and disease management. In Integrated Pest and Disease Management in Greenhouse Crops. Plant Pathology in the 21st Century Vol. 9 (eds Gullino, M. et al.) 285–330 (Springer, 2020).
Beuchat, L. R. & Ryu, J. H. Produce handling and processing practices. Emerg. Infect. Dis. 3, 459 (1997).
Morse, J. G. & Hoddle, M. S. Invasion biology of thrips. Annu. Rev. Entomol. 51, 67–89 (2006).
Zettler, J. L. Quarantine Issues and Research Developments in (eds. EJ Donahaye, S. Navarro, C. Bell, D. Jaya, R. Noyes, and TW Phillips) Proc. of the International Conference on Controlled Atmosphere and Fumigation in Stored Products 439–453 (2007).
Martin, R. R., Constable, F. & Tzanetakis, I. E. Quarantine regulations and the impact of modern detection methods. Annu. Rev. Phytopathol. 54, 189–205 (2016).
Favret, C. & Voegtlin, D. J. Migratory aphid (Hemiptera: Aphididae) habitat selection in agricultural and adjacent natural habitats. Environ. Entomol. 30, 371–379 (2001).
Reynolds, D. R., Chapman, J. W. & Harrington, R. The migration of insect vectors of plant and animal viruses. Adv. Virus Res. 67, 453–517 (2006).
Sétamou, M., Alabi, O. J., Tofangsazi, N. & Grafton-Cardwell, E. COPF: Citrus orchard perimeter fencing as a strategy for reducing Asian citrus psyllid (Hemiptera: Liviidae) infestation. J. Appl. Entomol. 142, 959–966 (2018).
Cornara, D. et al. Spittlebugs as vectors of Xylella fastidiosa in olive orchards in Italy. J. Pest Sci. 90, 521–530 (2017).
Morelli, M. et al. Xylella fastidiosa in olive: A review of control attempts and current management. Microorganisms 9, 1771 (2021).
Heywood, V. H. & Iriondo, J. M. Plant conservation: Old problems, new perspectives. Biol. Conserv. 113, 321–335 (2003).
Smith, K. F., Sax, D. F. & Lafferty, K. D. Evidence for the role of infectious disease in species extinction and endangerment. Conserv. Biol. 20, 1349–1357 (2006).
Messing, R. H., Tremblay, M. N., Mondor, E. B., Foottit, R. G. & Pike, K. S. Invasive aphids attack native Hawaiian plants. Biol. Invas. 9, 601–607 (2007).
Ancheta, J. & Heard, S. B. Impacts of insect herbivores on rare plant populations. Biol. Conserv. 144, 2395–2402 (2011).
Hatcher, M. J., Dick, J. T. & Dunn, A. M. Disease emergence and invasions. Funct. Ecol. 26, 1275–1287 (2012).
Heard, M. J. et al. The threat of disease increases as species move toward extinction. Conserv. Biol. 27, 1378–1388 (2013).
Roy, K., Jaenecke, K. A. & Peck, R. W. Ambrosia beetle (Coleoptera: Curculionidae) communities and frass production in ʻŌhiʻa (Myrtales: Myrtaceae) infected with Ceratocystis (Microascales: Ceratocystidaceae) fungi responsible for rapid ʻŌhiʻa death. Environ. Entomol. 49, 1345–1354 (2020).
Roy, K., Granthon, C., Peck, R. W., & Atkinson, C. T. Effectiveness of rapid'ōhi'a death management strategies at a focal disease outbreak on Hawai'i Island. Technical Report HCSU-099, University of Hawai’i at Hilo, Hawai’i Cooperative Studies Unit (2021).
Heym, E. C., Kampen, H., Krone, O., Schäfer, M. & Werner, D. Molecular detection of vector-borne pathogens from mosquitoes collected in two zoological gardens in Germany. Parasitol. Res. 118, 2097–2105 (2019).
la Martínez-de Puente, J. et al. Mosquitoes in an urban zoo: identification of blood meals, flight distances of engorged females, and avian malaria infections. Front. Veterinary Sci. 7, 460 (2020).
Werner, D. & Kampen, H. Zoos and wildlife parks: A laboratory for the study of mosquito-borne wildlife diseases. In Ecology and Control of Vector-borne Diseases (eds Gutiérrez-López, R. et al.) 944–947 (Wageningen Academic Publishers, 2022).
McGregor, B. L. et al. Using zoos as sentinels for re-emerging arboviruses: vector surveillance during an outbreak of epizootic hemorrhagic disease at the Minnesota Zoo. Pathogens 12, 140 (2023).
Tchouassi, D. P., Torto, B., Sang, R., Riginos, C. & Ezenwa, V. O. Large herbivore loss has complex effects on mosquito ecology and vector-borne disease risk. Transbound. Emerg. Dis. 68, 2503–2513 (2021).
Daszak, P., Cunningham, A. A. & Hyatt, A. D. Emerging infectious diseases of wildlife–threats to biodiversity and human health. Science 287, 443–449 (2000).
Stuchin, M., Machalaba, C. C., & Karesh, W. B. Vector-Borne Diseases: Animals and Patterns in Forum on Microbial Threats; Board on Global Health; Health and Medicine Division; National Academies of Sciences, Engineering, and Medicine. Global Health Impacts of Vector-Borne Diseases: Workshop Summary. (National Academies Press, 2016).
Acevedo, V., Amador, M., Félix, G. & Barrera, R. Operational aspects of the centers for disease control and prevention autocidal gravid ovitrap. J. Am. Mosq. Control Assoc. 32, 254 (2016).
Meek, P. D. et al. Camera trap theft and vandalism: Occurrence, cost, prevention and implications for wildlife research and management. Remote Sens. Ecol. Conserv. 5, 160–168 (2019).
Uhler, J. et al. A comparison of different Malaise trap types. Insect Conserv. Divers. 15, 666–672 (2022).
Gu, W., Unnasch, T. R., Katholi, C. R., Lampman, R. & Novak, R. J. Fundamental issues in mosquito surveillance for arboviral transmission. Trans. R. Soc. Trop. Med. Hyg. 102, 817–822 (2008).
Low, R. D. et al. Building international capacity for citizen scientist engagement in mosquito surveillance and mitigation: The GLOBE program’s GLOBE observer mosquito habitat mapper. Insects 13, 624 (2022).
Bhuvaneswari, A., Shriram, A. N., Raju, K. H. K. & Kumar, A. Mosquitoes, lymphatic filariasis, and public health: A systematic review of Anopheles and Aedes surveillance strategies. Pathogens 12, 1406 (2023).
Carney, R. et al. Integrating global citizen science platforms to enable next-generation surveillance of invasive and vector mosquitoes. Insects 13, 675 (2022).
Aregbesola, O. Z., Legg, J. P., Sigsgaard, L., Lund, O. S. & Rapisarda, C. Potential impact of climate change on whiteflies and implications for the spread of vectored viruses. J. Pest Sci. 92, 381–392 (2019).
Trebicki, P. Climate change and plant virus epidemiology. Virus Res. 286, 198059 (2020).
Cohen, J. M., Okumu, F. & Moonen, B. The fight against malaria: Diminishing gains and growing challenges. Sci. Transl. Med. 14, eabn3256 (2022).
Li, D. et al. Increasing risk of aphids spreading plant viruses in maize fields on both sides of China’s Heihe-Tengchong line under climate change. Pest Manag. Sci. 78, 3061–3070 (2022).
Tatem, A. J., Hay, S. I. & Rogers, D. J. Global traffic and disease vector dispersal. Proc. Natl. Acad. Sci. 103, 6242–6247 (2006).
Rezza, G. Dengue and chikungunya: Long-distance spread and outbreaks in naïve areas. Pathog. Global Health 108, 349–355 (2014).
Zettler, J. L. Quarantine Issues and Research Developments in (eds. E. J. Donahaye, S. Navarro, C. Bell, D. Jaya, R. Noyes, & T. W, Phillips) Proceedings of the International Conference on Controlled Atmosphere and Fumigation in Stored Products 439–453 (2007).
Lozano-Fuentes, S. et al. Emerging information technologies to provide improved decision support for surveillance, prevention, and control of vector-borne diseases. In Efficient Decision Support Systems: Practice and Challenges in Biomedical Related Domain (ed. Jao, C.) 89–114 (InTech-Open Access Publisher, 2011).
Kitron, U. Landscape ecology and epidemiology of vector-borne diseases: Tools for spatial analysis. J. Med. Entomol. 35, 435–445 (1998).
Kalluri, S., Gilruth, P., Rogers, D. & Szczur, M. Surveillance of arthropod vector-borne infectious diseases using remote sensing techniques: a review. PLoS Pathog. 3, e116 (2007).
Ellis, B. R. & Wilcox, B. A. The ecological dimensions of vector-borne disease research and control. Cadernos de Saúde Pública 25, S155–S167 (2009).
Reisen, W. K. Landscape epidemiology of vector-borne diseases. Annu. Rev. Entomol. 55, 461–483 (2010).
Hartfield, K. A., Landau, K. I. & Van Leeuwen, W. J. Fusion of high resolution aerial multispectral and LiDAR data: Land cover in the context of urban mosquito habitat. Remote Sens. 3, 2364–2383 (2011).
Sousa, L. et al. An information system for preventing and combating mosquito-borne diseases with social networks. Inf. Syst. 75, 26–42 (2018).
Lorenz, C. et al. Predicting Aedes aegypti infestation using landscape and thermal features. Sci. Rep. 10, 21688 (2020).
Aldosery, A. et al. MEWAR: Development of a cross-platform Mobile application and web dashboard system for real-time mosquito surveillance in Northeast Brazil. Front. Public Health 9, 754072 (2021).
Bennett, K. L. et al. The role of heterogenous environmental conditions in shaping the spatiotemporal distribution of competing Aedes mosquitoes in Panama: Implications for the landscape of arboviral disease transmission. Biol. Invas. 23, 1933–1948 (2021).
Deming, R. et al. Spatial variation of insecticide resistance in the dengue vector Aedes aegypti presents unique vector control challenges. Parasites Vectors 9, 1–10 (2016).
Bennett, K. L., McMillan, W. O. & Loaiza, J. R. The genomic signal of local environmental adaptation in Aedes aegypti mosquitoes. Evol. Appl. 14, 1301–1313 (2021).
World Health Organization. Manual for monitoring insecticide resistance in mosquito vectors and selecting appropriate interventions. (World Health Organization, 2022).
Devillers, J. et al. Integrated plan of insecticide resistance surveillance in mosquito vectors in France. Insects 14, 457 (2023).
Peterson, A. T., Martínez-Campos, C., Nakazawa, Y. & Martínez-Meyer, E. Time-specific ecological niche modeling predicts spatial dynamics of vector insects and human dengue cases. Trans. R. Soc. Trop. Med. Hyg. 99, 647–655 (2005).
Lana, R. M., Carneiro, T. G., Honório, N. A. & Codeco, C. T. Seasonal and nonseasonal dynamics of Aedes aegypti in Rio de Janeiro, Brazil: Fitting mathematical models to trap data. Acta Trop. 129, 25–32 (2014).
Killeen, G. F. Control of malaria vectors and management of insecticide resistance through universal coverage with next-generation insecticide-treated nets. Lancet 395, 1394–1400 (2020).
Al-Eryani, S. M. et al. Public health impact of the spread of Anopheles stephensi in the WHO Eastern Mediterranean Region countries in Horn of Africa and Yemen: Need for integrated vector surveillance and control. Malaria J. 22, 187 (2023).
Salomón, O. D. & Quintana, M. G. Eco-epidemiological studies to develop integrated vector surveillance of leishmaniasis vectors in the Americas. One Health Implement. Res. 2, 45–55 (2022).
Musah, A. et al. Coalescing disparate data sources for the geospatial prediction of mosquito abundance, using Brazil as a motivating case study. Front. Trop. Dis. 4, 1039735 (2023).
Medlock, J. M. & Leach, S. A. Effect of climate change on vector-borne disease risk in the UK. Lancet Infect. Dis. 15, 721–730 (2015).
Bartlow, A. W. et al. Forecasting zoonotic infectious disease response to climate change: Mosquito vectors and a changing environment. Veterinary Sci. 6, 40 (2019).
Franklinos, L. H., Jones, K. E., Redding, D. W. & Abubakar, I. The effect of global change on mosquito-borne disease. Lancet Infect. Dis. 19, e302–e312 (2019).
Crowl, T. A., Crist, T. O., Parmenter, R. R., Belovsky, G. & Lugo, A. E. The spread of invasive species and infectious disease as drivers of ecosystem change. Front. Ecol. Environ. 6, 238–246 (2008).
Medeiros, M. J. et al. The importance of insect monitoring to conservation actions in Hawaii. Proc. Hawaiian Entomol. Soc. 45, 149–166 (2013).
Bried, J. et al. A framework to integrate habitat monitoring and restoration with endangered insect recovery. Environ. Manag. 54, 1385–1398 (2014).
Willcox, B. K., Robson, A. J., Howlett, B. G. & Rader, R. Toward an integrated approach to crop production and pollination ecology through the application of remote sensing. PeerJ 6, e5806 (2018).
Tonelli, G. B., Binder, C., Margonari, C. & Andrade Filho, J. D. Sand fly behavior: Much more than weak-flying. Memórias do Instituto Oswaldo Cruz 116, e210230 (2021).
Joshi, A. & Miller, C. Review of machine learning techniques for mosquito control in urban environments. Ecol. Inf. 61, 101241 (2021).
Faiman, R., Cuno, R. & Warburg, A. Control of phlebotomine sand flies with vertical fine-mesh nets. J. Med. Entomol. 46, 820–831 (2009).
Lewis-Rosenblum, H., Martini, X., Tiwari, S. & Stelinski, L. L. Seasonal movement patterns and long-range dispersal of Asian citrus psyllid in Florida citrus. J. Econ. Entomol. 108, 3–10 (2015).
Martini, X., Hoffmann, M., Coy, M. R., Stelinski, L. L. & Pelz-Stelinski, K. S. Infection of an insect vector with a bacterial plant pathogen increases its propensity for dispersal. PLoS One 10, e0129373 (2015).
Kröner C, et al. 3D tracking of mosquitoes: A field compatible technique to understand malaria vector behaviour in Imaging and Applied Optics 2016, OSA Technical Digest paper TW5A.4. (Optica Publishing Group, 2016).
Samuel, M. et al. Community effectiveness of indoor spraying as a dengue vector control method: A systematic review. PLoS Negl. Trop. Dis. 11, e0005837 (2017).
Manrique-Saide, P. et al. The TIRS trial: protocol for a cluster randomized controlled trial assessing the efficacy of preventive targeted indoor residual spraying to reduce Aedes-borne viral illnesses in Merida, Mexico. Trials 21, 1–19 (2020).
Buckner, E. A. et al. A field efficacy evaluation of In2Care mosquito traps in comparison with routine integrated vector management at reducing Aedes aegypti. J. Am. Mosq. Control Assoc. 37, 242–249 (2021).
Barrera, R. New tools for Aedes control: Mass trapping. Curr. Opin. Insect Sci. 52, 100942 (2022).
Allan, S. A. & Kline, D. L. Evaluation of organic infusions and synthetic compounds mediating oviposition in Aedes albopictus and Aedes aegypti (Diptera: Culicidae). J. Chem. Ecol. 21, 1847–1860 (1995).
Roiz, D. et al. Trapping the tiger: Efficacy of the novel BG-Sentinel 2 with several attractants and carbon dioxide for collecting Aedes albopictus (Diptera: Culicidae) in Southern France. J. Med. Entomol. 53, 460–465 (2016).
Cilek, J. E., Knapp, J. A. & Richardson, A. G. Comparative efficiency of biogents gravid Aedes trap, Cdc autocidal gravid ovitrap, and CDC gravid trap in Northeastern Florida. J. Am. Mosq. Control Assoc. 33, 103–107 (2017).
Zhu, D. et al. Modifying the autocidal gravid ovitrap (AGO) with a powered suction fan and additional lures to increase the collections of released Aedes aegypti and a natural population of Ae. albopictus (Diptera: Culicidae). J. Vector Ecol. 44, 282–284 (2019).
Hoel, D. F. et al. Efficacy of ovitrap colors and patterns for attracting Aedes albopictus at suburban field sites in north-central Florida. J. Am. Mosq. Control Assoc. 27, 245–251 (2011).
Degener, C. M. et al. Field trials to evaluate the effectiveness of the Biogents®-Sweetscent lure in combination with several commercial mosquito traps and to assess the effectiveness of the biogents-mosquitaire trap with and without carbon dioxide. J. Am. Mosq. Control Assoc. 35, 32–39 (2019).
Barnard, D. R. et al. Measurement of landing mosquito density on humans. Acta Trop. 136, 58–67 (2014).
Snyder, J. et al. The development and evaluation of insect traps for the Asian citrus psyllid, Diaphorina citri (Hemiptera: Psyllidae), vector of citrus huanglongbing. Insects 13, 295 (2022).
Wenninger, E. J., Stelinski, L. L. & Hall, D. G. Roles of olfactory cues, visual cues, and mating status in orientation of Diaphorina citri Kuwayama (Hemiptera: Psyllidae) to four different host plants. Environ. Entomol. 38, 225–234 (2009).
Patt, J. M. et al. Multimodal cues drive host-plant assessment in Asian citrus psyllid (Diaphorina citri). Environ. Entomol. 40, 1494–1502 (2011).
Allan, S. A., George, J., Stelinski, L. L. & Lapointe, S. L. Attributes of yellow traps affecting attraction of Diaphorina citri (Hemiptera: Liviidae). Insects 11, 452 (2020).
Yasuda, K., Kawamura, F. & Oishi, T. Location and preference of adult Asian citrus psyllid, Diaphorina citri (Homoptera: Psyllidae) on Chinese box orange jasmine, Murraya exotica L. and flat lemon, Citrus depressa. Jpn. J. Appl. Entomol. Zool. 49, 146 (2005).
Patt, J. M. et al. Efficacy of an autodisseminator of an entomopathogenic fungus, Isaria fumosorosea, to suppress Asian citrus psyllid, Diaphorina citri, under greenhouse conditions. Biol. Control 88, 37–45 (2015).
George, J. et al. A multimodal attract-and-kill device for the Asian citrus psyllid Diaphorina citri (Hemiptera: Liviidae). Insects 11, 870 (2020).
Cancino, A. D. M. & Damon, A. Fragrance analysis of euglossine bee pollinated orchids from Soconusco, south-east Mexico. Plant Species Biol. 22, 127–132 (2007).
Acknowledgements
The authors thank William H. Gates III for his support and sponsorship through Global Health Labs (formerly Global Good Fund I, LLC). Additional funding for this study was provided by the USDA Agricultural Research Service. We are grateful to A. Tarshis-Moreno for her assistance with insect rearing, setting up and conducting the tests, and data collection, S. Mayo for his assistance in coordinating screen house construction, A. Hill for assistance with obtaining D. citri, Prof. S. Ramirez at University of California, Davis, for providing p-dimethoxybenzene to collect orchid bees, to the USDA-ARS-CMAV laboratory in Gainesville, FL and University of Florida-IFAS Medical Entomology Laboratory in Vero Beach, FL for providing A. aegypti eggs, to W. Meikle and R. Neidz for reviewing a draft of the manuscript, and to two anonymous reviewers whose critiques greatly improved the manuscript. Mention of trade names or commercial products in this publication is solely for the purpose of providing specific information and does not imply recommendation or endorsement by Global Health Labs or the USDA for its use.
Funding
Agricultural Research Service, William H. Gates III.
Author information
Authors and Affiliations
Contributions
All authors devised the experiments and reviewed the outcomes. A.M., B.N., M.M., and P.R. designed, integrated, and operated the experimental systems, including the optoelectronic hardware, tracking algorithms, and overall software and control architecture. J.M.P. wrote the manuscript and A.M. and M.N. reviewed it.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Patt, J.M., Makagon, A., Norton, B. et al. An optical system to detect, surveil, and kill flying insect vectors of human and crop pathogens. Sci Rep 14, 8174 (2024). https://doi.org/10.1038/s41598-024-57804-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-57804-6
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.