Abstract
This study investigated whether the progression of liver fibrosis affects the prevalence of sarcopenia and incidence of decreased gait speed in older patients with chronic liver disease (CLD). Patients with CLD aged ≥ 60 years were classified into low, intermediate, and high fibrosis 4 (FIB-4) index groups according to the degree of liver fibrosis. The prevalence of sarcopenia and incidence of decreased gait speed (< 1.0 m/s) were compared among the three groups. Logistic regression analysis was performed to investigate factors affecting the risk of decreased gait speed. No significant difference was observed in the prevalence of sarcopenia among the three groups, but the incidence of decreased gait speed significantly differed (p = 0.029). When analyzed individually, a significant difference in decreased gait speed incidence was observed between the high and low FIB-4 index groups (p = 0.014). In logistic regression analysis, the progression of liver fibrosis (odds ratio: 1.32, 95% confidence interval: 1.13–1.55) and lower extremity muscle strength (LEMS) (odds ratio: 0.92, 95% confidence interval: 0.88–0.97) were significantly associated with decreased gait speed. As liver fibrosis progresses in older patients with CLD, it becomes important to focus on not only skeletal muscle mass and grip strength, but also gait speed and LEMS.
Similar content being viewed by others
Introduction
Worldwide, 1.5 billion people experience chronic liver disease (CLD), and the incidence of CLD and cirrhosis has increased by 13% since 20001. The incidence of CLD increases and the prognosis worsens with age2. CLD is a condition in which various factors cause liver fibrosis, leading to liver injury and cirrhosis3. The financial burden of CLD increases with a decline in patient quality of life and activities of daily living (ADL) during disease progression4,5,6. In Japan, the number of older patients with CLD is increasing because of the aging population, as well as advances in treatment7,8.
Sarcopenia is induced by aging, nutritional status, cachexia, and various diseases9. In recent years, sarcopenia has become an important topic in the field of care prevention, particularly for the growing older population10. The European Working Group on Sarcopenia in Older People (EWGSOP) and Asian Working Group for Sarcopenia (AWGS) recommend the diagnosis and classification of the severity of sarcopenia based on skeletal muscle mass, muscle strength, and physical function. Recently, new criteria for sarcopenia have been published in the EWGSOP 2 and AWGS 2019 guidelines11,12.
Sarcopenia is a strong risk factor for mortality in older patients with CLD13,14,15. The prevalence of sarcopenia increases with the severity of cirrhosis, an advanced state of CLD14. Therefore, it is important to clarify the association between the development of liver fibrosis and sarcopenia in the process of CLD leading to cirrhosis. However, there are few reports on the association between the progression of liver fibrosis and sarcopenia in CLD. Specifically, there are no reports on the association between the progression of liver fibrosis and physical function measures such as gait speed in CLD, although skeletal muscle mass tends to decrease in patients with advanced liver fibrosis16 and that liver fibrosis severity is associated with lower grip strength17. The Japanese Society of Hepatology (JSH) has established criteria for assessing sarcopenia; however, these criteria only include skeletal muscle mass and grip strength18,19.
The use of criteria that focus on not only skeletal muscle mass and muscle strength but also physical function measures, such as gait speed, can provide new insights into the presence and severity of sarcopenia in older patients with CLD leading to cirrhosis and the relationship between the progression of liver fibrosis and physical function. An understanding of the relationship between the progression of liver fibrosis and gait speed in older patients with CLD would be useful in the prevention and improvement of sarcopenia and decline in physical function. Therefore, the purpose of this study was to examine the association between the progression of liver fibrosis and sarcopenia in older patients with CLD using the latest sarcopenia criteria. In addition, the association between the progression of liver fibrosis and gait speed as a physical function measure was determined.
Methods
Study design and patients
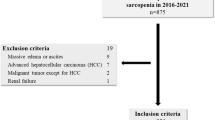
Data for this study were collected retrospectively from medical records. Patients aged ≥ 60 years with CLD, who were admitted to the Department of Gastroenterology at Hiroshima University Hospital between April 2014 and June 2018 and referred to the Department of Rehabilitation Medicine, were included in this study. A total of 123 consecutive patients were included in this study. All patients had been hospitalized due to CLD, including hepatocellular carcinoma, diagnosed by hepatologists in our University Hospital before the assessment of physical function and rehabilitation therapy by a physical therapist. This study excluded patients with pacemaker insertions, for whom body composition assessments were not indicated, as well as those with neuromuscular diseases, excessive pain, or dementia that impeded the assessment of physical function. Three patients were excluded because of missing measurement data due to neuromuscular diseases, excessive pain, or dementia (Fig. 1). The study was conducted in accordance with Declaration of Helsinki and the Ethical Guidelines for Medical and Health Research Involving Human Subjects enacted by the Ministry of Health, Labour and Welfare of Japan (https://www.mhlw.go.jp/content/001077424.pdf), and approved by the Ethical Review Committee of Hiroshima University Hospital (Permit No. E-583-1). All patients were verbally informed that their medical records and charts might be used for research purposes. Data were obtained during routine medical care and reviewed retrospectively. The need to obtain informed consent from the study participants was waived by the Ethical Review Committee of Hiroshima University Hospital since this is a retrospective analysis. Patients who were eligible for this study had the opportunity to refuse to participate in the study by opting out.
Data on patient characteristics, blood analysis, body composition, and physical function were collected. Assessment of patient characteristics and hematological tests were performed on admission, whereas body composition and physical function were assessed on the day of initiation of physical therapy. Skeletal muscle mass and physical function were assessed by a physical therapist in the rehabilitation room.
Clinical and laboratory assessments
Patient age, sex, height, weight, body mass index, and blood data [hemoglobin (HGB), platelets (PLT), total bilirubin (T-BIL), aspartate aminotransferase (AST), alanine aminotransferase (ALT), albumin (ALB), prothrombin time (PT), ammonia, and hemoglobin A1c] were extracted from the medical records. Diagnoses of chronic hepatitis, liver cirrhosis, encephalopathy, ascites, hepatocellular carcinoma (HCC), hypertension (HT), dyslipidemia (DL), diabetes mellitus (DM), chronic heart failure (CHF), and cardiovascular disease (CVD) were also recorded.
Liver fibrosis assessments
The indices of liver fibrosis used in this study as a scoring system with serum markers included AST-platelet ratio index (APRI) and fibrosis 4 (FIB-4) index20,21,22,23.
The FIB-4 index was used as a representative biomarker to determine the pathological progression of CLD and to assess the degree of liver fibrosis. The FIB-4 index was calculated using the following formula24,25,26:
The FIB-4 index provides information regarding the risk of fibrosis24. The patients in this study were categorized into three groups based on the FIB-4 index in accordance with previous studies27,28: low (FIB-4 index < 2.0), intermediate (2.0 ≤ FIB-4 index ≤ 2.67), and high (FIB-4 index > 2.67) FIB-4 index groups.
In addition, to verify the validity of the FIB-4 index, we calculated the APRI, which is another indicator of liver fibrosis, using the following formula:
Body composition assessments
The skeletal muscle mass was measured using a bioelectrical impedance assessment (BIA) device (InBody720; InBody Co., Ltd., Seoul, Korea). The sum of extremity muscle mass was calculated as Appendicular skeletal muscle mass (ASM). The skeletal muscle mass index (SMI) was calculated as ASM divided by the square of height12,18,19.
To measure intracellular and extracellular water content, the ratio of extracellular water to total body water (ECW/TBW) was calculated.
Muscle strength assessments
Upper and lower extremity muscle strength were assessed. Upper extremity muscle strength was measured using grip strength based on the AWGS 2019 criteria. Grip strength was measured in 0.5 kg units using a grip strength meter (Smedley-type grip strength meter; Matsuyoshi Medical Instruments Co., Tokyo, Japan). Patients were instructed to stand with their elbows extended and their upper limbs lowered. Two measurements were obtained for each upper extremity, and the average of the highest grip strength values obtained for each side was used in the analyses.
Lower extremity muscle strength (LEMS) assessed the knee extensor force (KEF) using a belt-mounted handheld dynamometer (μTas F-1; Anima, Tokyo, Japan). Patients were instructed to sit in the end-seated position with an upright trunk and one lower leg secured to the belt, with the knee joint flexed at 90º. The maximum isometric knee extension strength was then measured. Two measurements were taken on each side, and the average of the maximum values obtained for each side was used in the analyses. To account for differences in body size, KEF was divided by the patient’s body weight or the lower extremity muscle mass29,30. The KEF/weight was multiplied by 100 at the time of its input, as described in a previous study31.
Gait speed assessment
The participants were instructed to walk for 16 m. The first 3 m allowed them to pick up speed while the final 3 m allowed them to slow down. The time required to walk the middle 10 m was measured using a stopwatch, and the gait speed was calculated and used in the analyses. The patients were not permitted to use a walking aid and were instructed to walk normally at a comfortable pace11,12. The time required to walk 10 m was measured twice, and the average value was used in the analyses.
According to the latest EWGSOP 2 and AWGS 2019 diagnostic criteria for sarcopenia, the cutoff value for a comfortable gait speed is < 1.0 m/s11,12, and we defined decreased gait speed as a speed of < 1.0 m/s.
Diagnosis of sarcopenia
The criteria for sarcopenia defined by AWGS 201912 and JSH (second version)18,19 were used in this study. The AWGS 2019 diagnostic criteria for skeletal muscle mass loss and decreased muscle strength or gait speed correspond to sarcopenia. The AWGS 2019 criteria for loss of skeletal muscle mass include an SMI of < 7.0 kg/m2 in men and < 5.7 kg/m2 in women, while the criteria for muscle weakness include a grip strength of < 28 kg in men and < 18 kg in women, with a decreased gait speed of < 1.0 m/s12.
The JSH (second version) diagnostic criteria for loss of skeletal muscle mass and muscle weakness correspond to sarcopenia. The JSH criteria for loss of skeletal muscle mass are defined as an SMI of < 7.0 kg/m2 in men and < 5.7 kg/m2 in women, while the criteria for muscle weakness include a grip strength of < 28 kg in men and < 18 kg in women18,19.
Statistical analysis
Continuous variables are presented as median (interquartile range) and categorical variables are presented as number (frequency). Among the three FIB-4 index groups, continuous variables were compared using the Kruskal–Wallis test, while categorical variables were compared using Fisher's exact test. The incidence of decreased gait speed (< 1.0 m/s) was compared among the three FIB-4 index groups using Fisher’s exact test with the Bonferroni correction. Furthermore, we reviewed prior studies to determine the percentage of gait speed divided by 0.2 m/s for each Fib4 index grouping32,33. The association between the FIB-4 index and gait speed was investigated using Spearman’s correlation coefficient for each FIB-4 index grouping. Factors associated with decreased gait speed (< 1.0 m/s) were analyzed by logistic regression analysis using the forced entry method. The explanatory variables were age, sex, presence of skeletal muscle mass loss, FIB-4 index, presence of HCC, and KEF/weight. The patients with CLD were further subclassified into five categories according to etiology: hepatitis B virus (HBV), hepatitis C virus (HCV), alcoholic liver injury (Alc), nonalcoholic fatty liver disease (NAFLD), and others. Multiple comparisons were made using the Steel–Dwass test for continuous variables and Fisher’s exact test for nominal variables.
JMP version 17 statistical software (SAS Institute Inc., Cary, NC, USA) was used to conduct the statistical analyses in this study. All hypothesis tests were two-tailed. Statistical significance was set at p < 0.05, with p < 0.016 only for analyses with the Bonferroni correction.
Results
Patient characteristics
Age, sex, and BMI did not significantly differ between the three FIB-4 index groups. Similarly, the three groups showed no significant difference in the prevalence of comorbidities, such as HT, DL, DM, CHF, and CVD. The FIB-4 index (p < 0.001), APRI (p < 0.001), incidence of liver cirrhosis (p < 0.001), and liver reserve capacity (p < 0.001) were significantly different among the three FIB-4 index groups. There were significant differences in HGB (p = 0.042), PLT (p < 0.001), T-BIL (p < 0.001), AST (p < 0.001), ALB (p = 0.013), and PT (p < 0.001) among the three groups (Table 1).
Body composition, physical function, and sarcopenia
The prevalence of sarcopenia, determined using the AWGS 2019 criteria, was 17% among older patients with CLD in this study. The prevalence of sarcopenia according to the JSH (second version) and AWGS 2019 criteria was not significantly different among the three groups. However, there was a significant difference in the incidence of decreased gait speed among the three groups (Table 2). In multivariate analysis, the high FIB-4 index group showed a significantly higher incidence of decreased gait speed (41%) than did the low FIB-4 index group (17%; p = 0.014; Fig. 2). Figure 1 shows the incidence of decreased gait speed in older patients with chronic liver disease, classified according to the FIB-4 index. In total, 5.56% and 17.46% patients in the low and high FIB-4 index groups, respectively, showed a gait speed of 0.6–0.8 m/s, 8.33% and 23.81% patients in the low and high FIB-4 index groups, respectively, showed a gait speed of 0.8–1.0 m/s, and 52.78% and 31.75% patients in the low and high FIB-4 index groups, respectively, showed a gait speed of 1.0–1.2 m/s (Fig. 2). The high FIB-4 index group demonstrated a significant negative correlation between the FIB-4 index and gait speed (ρ = − 0.24; p = 0.047; Fig. 3). In contrast, no significant correlation was observed in the low (ρ = 0.01; p = 0.939) and intermediate (ρ = − 0.16; p = 0.514) FIB-4 index groups.
Incidence of decreased gait speed in older patients with chronic liver disease, classified according to the fibrosis 4 (FIB-4) index. The incidence of decreased gait speed in older patients with chronic liver disease classified using the FIB-4 index was 17% in the low FIB-4 index group (FIB-4 index < 2.0), 22% in the medium FIB-4 index group (2.0 < FIB-4 index < 2.67), and 41% in the high FIB-4 index group (> 2.67). The decreased gait speed was significantly higher in the high FIB-4 index group compared to the low FIB-4 index group (p = 0.014). In total, 5.56% and 17.46% patients in the low and high FIB-4 index groups, respectively, show a gait speed of 0.6–0.8 m/s, 8.33% and 23.81% patients in the low and high FIB-4 index groups, respectively, show a gait speed of 0.8–1.0 m/s, and 52.78% and 31.75% patients in the low and high FIB-4 index groups, respectively, show a gait speed of 1.0–1.2 m/s.
Relationship between the fibrosis 4 (FIB-4) index and gait speed. The relationship between the FIB-4 index and gait speed in older patients with chronic liver disease is shown. There is no significant correlation between gait speed and the FIB-4 index in the low FIB-4 (FIB-4 index < 2.0) and intermediate FIB-4 (2.0 ≤ FIB-4 index ≤ 2.67) index groups (ρ = 0.01; p = 0.939, ρ = − 0.16; p = 0.514). In the high FIB-4 index group (FIB-4 index > 2.67), there is a significant correlation between gait speed and the FIB-4 index. (ρ = − 0.24; p = 0.047).
Multivariate analysis of decreased gait speed
The FIB-4 index (odds ratio [OR]: 1.32, 95% CI 1.13–1.55) and KEF/weight (OR 0.92, 95% CI 0.88–0.97) were significantly associated with decreased gait speed (< 1.0 m/s), independent of age, sex, presence of HCC, and skeletal muscle mass (Table 3).
Body composition, physical function, and sarcopenia by etiology
Supplementary Table S1 shows results of the multiple comparisons test for the characteristics of patients with CLD, categorized according to etiology. A significant difference was observed in the proportion of etiological factors between men and women (p < 0.05; HBV, 77% and 23%; HCV, 59% and 41%; Alc, 100% and 0%; NAFLD, 37% and 63%, respectively). Patients with NAFLD also had a significantly higher BMI than those with HCV (p < 0.05) and Alc (p < 0.05). In contrast, there were no significant differences in decreased gait speed and prevalence of sarcopenia when classified by etiology.
Discussion
In the present study, we investigated whether the progression of liver fibrosis affects sarcopenia and gait speed as a physical function measure in older patients with CLD. The results showed that the prevalence of sarcopenia did not change with the progression of liver fibrosis, although the incidence of decreased gait speed (< 1.0 m/s) was significantly changed. Multivariate analysis revealed that the risk of decreased gait speed was associated with liver fibrosis and LEMS, regardless of age, sex, presence of hepatocellular carcinoma, and skeletal muscle mass.
The prevalence of sarcopenia was 17% among the older patients with CLD in the present study according to the latest AWGS 2019 diagnostic criteria, which include physical function as well as skeletal muscle mass, and there was no difference in the prevalence of sarcopenia according to the degree of progression of liver fibrosis. Similarly, using the JSH (second version) diagnostic criteria, we found no significant difference in the prevalence of sarcopenia according to the degree of progression of liver fibrosis. In a meta-analysis of patients with cirrhosis, the prevalence of sarcopenia as defined by skeletal muscle mass only was 37.5%14. In a study of patients with CLD, including hepatitis and cirrhosis, the prevalence of sarcopenia as defined by skeletal muscle mass only was 25%34. In the present study, which also included hepatitis and cirrhosis, approximately 30% of patients were diagnosed with sarcopenia when assessed by skeletal muscle mass only, using the AWGS2019 and JSH (second version) criteria. This result was consistent with the findings of a previous study34. Most sarcopenia studies involving CLD assessed the presence of sarcopenia on the sole basis of skeletal muscle mass14,34,35. However, if the criteria include physical function measures, particularly gait speed, the percentage of patients diagnosed with sarcopenia is likely to be lower, as observed in the present study. In addition, there are various methods and sites for evaluating skeletal muscle mass, and a unified method for diagnosis is needed in the future. In the present study, skeletal muscle mass was evaluated using BIA, and there is a possibility that water content affected the results. In liver disease, fluid retention becomes a concern as the disease progresses36,37,38.
In the present study, there was a difference in the incidence of decreased gait speed depending on the progression of liver fibrosis in older patients with CLD. Although it has been reported that the development of liver fibrosis affects skeletal muscle loss39, to our knowledge, this is the first study to show that the degree of liver fibrosis is associated with decreased gait speed. Gait speed is an important diagnostic criterion for sarcopenia in the EWGSOP and AWGS guidelines11,12. The overall mean gait speed in the present study was 1.10 m/s, and 31% of patients had a speed of < 1.0 m/s. In a cohort study of 2,000 community-dwelling older people in Asia, the average gait speed of all subjects was 1.14 ± 0.25 m/s. The values of the present study are equivalent to or slightly smaller than those in the previous large-scale study40. Nishikawa et al. reported a gait speed of < 1.0 m/s in approximately 10% of Japanese patients with CLD32; this was a smaller percentage than that observed in the present study. We believe that the study by Nishikawa et al.32 was influenced by the fact that it included a greater number of younger patients with an average age of 66 years (22–94 years) than did the present study. A decreased gait speed occurs with aging, particularly after the age of 60 years41. Decreased gait speed plays a crucial role in determining the prognosis of older individuals42,43, and it is also a significant factor linked to mortality in patients with liver cirrhosis44. Each increase of 0.1 m/s in gait speed is associated with a 12% reduction in the risk of death43. Gait speed is also a predictor of ADL such as bathing and dressing43,45. If the disease progresses, CLD causes greater physical dysfunction and ADL limitations than does osteoarthritis, diabetes mellitus, or cardiac disease46. Therefore, it is very important to focus on not only skeletal muscle mass but also gait speed in terms of prognosis and ADL in patients with CLD.
The risk of decreased gait speed in older patients with CLD was found to be associated with the decline in LEMS and the development of liver fibrosis, independent of age, sex, the presence of HCC, and skeletal muscle mass. In the three FIB-4 index groups, differences were observed in blood data such as HGB and ALB. Gait speed has been influenced by LEMS in the general older population47, and it is likely to be similarly relevant in older patients with CLD. In terms of gait speed and LEMS, there is no correlation between muscle strength and gait speed in robust older people, although there is a correlation in frail older people48. In general, worsening liver fibrosis leads to blood cell loss and decreased protein synthesis. In patients with frailty and sarcopenia, HGB and ALB levels are reportedly associated with decreased gait speed49,50,51. Therefore, the decrease in HGB and ALB due to the development of liver fibrosis may have affected decreased gait speed in the present study. In addition, skeletal muscle mass was not related to decreased gait speed in the present study. Physical function, including gait speed, correlates with muscle strength rather than skeletal muscle mass in patients with cirrhosis52. Additionally, skeletal muscle mass measured using BIA and gait speed are not correlated in patients with severe liver disease53. These conclusions support our finding of no relationship between decreased gait speed and skeletal muscle mass in the present study. We believe that not only skeletal muscle mass and grip strength but also LEMS should be used to evaluate gait speed in older patients with CLD. The effect of interventions on gait speed in patients with CLD is unclear. As such. it is important to pay attention to exercise physiology, physical activity, and diet in future intervention studies54.
There are some limitations in the present study. First, this was a single-center, cross-sectional study. Second, the sample size was small and susceptible to bias; thus, the findings may not be generalizable to other populations. Third, patients with CLD of various etiologies were included. The differences in etiologies might have affected the incidence and mechanisms of physical dysfunction. Fourth, the age of the patients, progression of liver disease, and rate of cirrhosis differed from those in previous studies. Therefore, future studies should longitudinally examine the effect of the progression of liver fibrosis on physical function in patients with CLD according to the etiology of CLD, with patients ranging from those with a recent diagnosis of hepatitis to those with progression to cirrhosis.
Conclusions
In older patients with CLD, there was no difference in the prevalence of sarcopenia, according to criteria that included gait speed, as liver fibrosis progressed, although there was a significant difference in the incidence of decreased gait speed. The risk of decreased gait speed was associated with a decline in LEMS and progression of liver fibrosis, independent of skeletal muscle mass. It is important to consider not only skeletal muscle mass but also gait speed and LEMS, which is not a diagnostic criterion for sarcopenia.
Data availability
As the data contain potentially sensitive information, the data are not publicly available. Qualified researchers may apply for access to a minimal dataset by contacting Kenichi Fudeyasu, first author, fudeyasu@hiroshima-u.ac.jp or Kai Ushio, corresponding author, ushiosista@hiroshima-u.ac.jp.
References
Moon, A. M., Singal, A. G. & Tapper, E. B. Contemporary epidemiology of chronic liver disease and cirrhosis. Clin. Gastroenterol. Hepatol. 18, 2650–2666 (2020).
Maeso-Díaz, R. & Gracia-Sancho, J. Aging and chronic liver disease. Semin. Liver Dis. 40, 373–384 (2020).
Tsochatzis, E. A., Bosch, J. & Burroughs, A. K. Liver cirrhosis. Lancet 383, 1749–1761 (2014).
Ohashi, K. et al. Relationship between pre-sarcopenia and quality of life in patients with chronic liver disease: A cross-sectional study. Eur. J. Gastroenterol. Hepatol. 31, 1408–1413 (2019).
Li, L. et al. The association of liver function and quality of life of patients with liver cancer. BMC Gastroenterol. 19, 66 (2019).
Rakoski, M. O. et al. Burden of cirrhosis on older Americans and their families: Analysis of the health and retirement study. Hepatology 55, 184–191 (2012).
Frith, J., Jones, D. & Newton, J. L. Chronic liver disease in an ageing population. Age Ageing 38, 11–18 (2008).
Stahl, E. C., Haschak, M. J., Popovic, B. & Brown, B. N. Macrophages in the aging liver and age-related liver disease. Front. Immunol. 9, 2795 (2018).
Cruz-Jentoft, A. J. et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 39, 412–423 (2010).
Clegg, A., Young, J., Iliffe, S., Rikkert, M. O. & Rockwood, K. Frailty in elderly people. Lancet 381, 752–762 (2013).
Cruz-Jentoft, A. J. et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 48, 16–31 (2019).
Chen, L. K. et al. Asian Working Group for Sarcopenia: 2019 Consensus update on sarcopenia diagnosis and treatment. J. Am. Med. Dir. Assoc. 21, 300-307.e2 (2020).
Kim, G., Kang, S. H., Kim, M. Y. & Baik, S. K. Prognostic value of sarcopenia in patients with liver cirrhosis: A systematic review and meta-analysis. PLoS One 12, e0186990 (2017).
Tantai, X. et al. Effect of sarcopenia on survival in patients with cirrhosis: A meta-analysis. J. Hepatol. 76, 588–599 (2022).
Harimoto, N. et al. Clinical outcomes of living liver transplantation according to the presence of sarcopenia as defined by skeletal muscle mass, hand grip, and gait speed. Transplant. Proc. 49, 2144–2152 (2017).
Lee, Y. H. et al. Sarcopaenia is associated with NAFLD independently of obesity and insulin resistance: Nationwide surveys (KNHANES 2008–2011). J. Hepatol. 63, 486–493 (2015).
Gabr, S. A. & Alghadir, A. H. Handgrip strength and vitamin D as predictors of liver fibrosis and malnutrition in chronic hepatitis C patients. Dis. Markers 2021, 6665893 (2021).
Nishikawa, H. et al. Japan Society of Hepatology guidelines for sarcopenia in liver disease (1st edition): Recommendation from the working group for creation of sarcopenia assessment criteria. Hepatol. Res. 46, 951–963 (2016).
Nishikawa, H. et al. Reduced handgrip strength predicts poorer survival in chronic liver diseases: A large multicenter study in Japan. Hepatol. Res. 51, 957–967 (2021).
Vallet-Pichard, A. et al. FIB-4: An inexpensive and accurate marker of fibrosis in HCV infection. comparison with liver biopsy and fibrotest. Hepatology 46, 32–36 (2007).
Shah, A. G. et al. Comparison of noninvasive markers of fibrosis in patients with nonalcoholic fatty liver disease. Clin. Gastroenterol. Hepatol. 7, 1104–1012 (2009).
Wai, C. T. et al. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology 38, 518–526 (2003).
Tapper, E. B. et al. Simple non-invasive biomarkers of advanced fibrosis in the evaluation of non-alcoholic fatty liver disease. Gastroenterol. Rep. (Oxf) 2, 276–280 (2014).
Sterling, R. K. et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology 43, 1317–1325 (2006).
Xiao, G. et al. Comparison of laboratory tests, ultrasound, or magnetic resonance elastography to detect fibrosis in patients with nonalcoholic fatty liver disease: A meta-analysis. Hepatology 66, 1486–1501 (2017).
Ioannou, G. N. et al. Increased risk for hepatocellular carcinoma persists up to 10 years after HCV eradication in patients with baseline cirrhosis or high FIB-4 scores. Gastroenterology 157, 1264-1278.e4 (2019).
Kaya, E. et al. Simple noninvasive scores are clinically useful to exclude, not predict, advanced fibrosis: A study in Turkish patients with biopsy-proven nonalcoholic fatty liver disease. Gut Liver 14, 486–491 (2020).
McPherson, S. et al. Age as a confounding factor for the accurate non-invasive diagnosis of advanced NAFLD fibrosis. Am. J. Gastroenterol. 112, 740–751 (2017).
Andersen, H., Nielsen, S., Mogensen, C. E. & Jakobsen, J. Muscle strength in type 2 diabetes. Diabetes 53, 1543–1548 (2004).
Nomura, T., Ishiguro, T., Ohira, M., Oka, H. & Ikeda, Y. Isometric knee extension force in Japanese type 2 diabetic patients without apparent diabetic polyneuropathy: Data from the multicenter survey of the isometric lower extremity strength in type 2 diabetes study. SAGE Open Med. 7, 2050312118823412 (2019).
Dutkiewicz, R., Zetterberg, H., Andreasson, U., Blennow, K. & Nellgård, B. Dementia and CSF-biomarkers for Alzheimer’s disease predict mortality after acute hip fracture. Acta Anaesthesiol. Scand. 64, 93–103 (2020).
Nishikawa, H. et al. Walking speed: Japanese data in chronic liver diseases. J. Clin. Med. 9, 166 (2020).
Stimpson, K. H., Heitkamp, L. N., Horne, J. S. & Dean, J. C. Effects of walking speed on the step-by-step control of step width. J. Biomech. 68, 78–83 (2018).
Nakamura, A., Yoshimura, T., Sato, T. & Ichikawa, T. Diagnosis and pathogenesis of sarcopenia in chronic liver disease using liver magnetic resonance imaging. Cureus 14, e24676 (2022).
Beer, L. et al. MRI-defined sarcopenia predicts mortality in patients with chronic liver disease. Liver Int. 40, 2797–2807 (2020).
Wu, T. J., Huang, J. J. & Lin, C. Y. Effects of fluid retention on the measurement of body composition using bioelectric impedance. J. Formos. Med. Assoc. 93, 939–943 (1994).
Kikuchi, N. et al. Evaluation of skeletal muscle mass in patients with chronic liver disease shows different results based on bioelectric impedance analysis and computed tomography. Ann. Nutr. Metab. 78, 336–344 (2022).
Son, S. W., Song, D. S., Chang, U. I. & Yang, J. M. Definition of sarcopenia in chronic liver disease. Life (Basel) 11, 349 (2021).
Kang, M. K., Park, J. G., Lee, H. J. & Kim, M. C. Association of low skeletal muscle mass with advanced liver fibrosis in patients with non-alcoholic fatty liver disease. J. Gastroenterol. Hepatol. 34, 1633–1640 (2019).
Kim, M. & Won, C. W. Sarcopenia is associated with cognitive impairment mainly due to slow gait speed: Results from the Korean Frailty and Aging Cohort Study (KFACS). Int. J. Environ. Res. Public Health 16, 1491 (2019).
Himann, J. E., Cunningham, D. A., Rechnitzer, P. A. & Paterson, D. H. Age-related changes in speed of walking. Med. Sci. Sports Exerc. 20, 161–166 (1988).
Landi, F. et al. Disability, more than multimorbidity, was predictive of mortality among older persons aged 80 years and older. J. Clin. Epidemiol 63, 752–759 (2010).
Studenski, S. et al. Gait speed and survival in older adults. JAMA 305, 50–58 (2011).
Soto, R. et al. Frailty and reduced gait speed are independently related to mortality of cirrhotic patients in long-term follow-up. Ann. Hepatol. 25, 100327 (2021).
Perera, S. et al. Gait speed predicts incident disability: A pooled analysis. J. Gerontol. A Biol. Sci. Med. Sci. 71, 63–71 (2015).
Katsura, T. et al. A retrospective cohort study on the risk assessment of newly certificated long-term care need of elderly individuals in a community: Basic checklist and specific health checkup. J. Rural Med. 12, 68–84 (2017).
Purser, J. L., Pieper, C. F., Poole, C. & Morey, M. Trajectories of leg strength and gait speed among sedentary older adults: Longitudinal pattern of dose response. J. Gerontol. A Biol. Sci. Med. Sci. 58, M1125–M1134 (2003).
Buchner, D. M., Larson, E. B., Wagner, E. H., Koepsell, T. D. & de Lateur, B. J. Evidence for a non-linear relationship between leg strength and gait speed. Age Ageing 25, 386–391 (1996).
Yamamoto, M. et al. Lower albumin levels are associated with frailty measures, trace elements, and an inflammation marker in a cross-sectional study in Tanushimaru. Environ. Health Prev. Med. 26, 25 (2021).
Liu, Q. et al. Hemoglobin level is negatively associated with sarcopenia and its components in Chinese aged 60 and above. Front. Public Health 11, 1081843 (2023).
Hirani, V. et al. Low hemoglobin concentrations are associated with sarcopenia, physical performance, and disability in older Australian men in cross-sectional and longitudinal analysis: The Concord Health and Ageing in Men Project. J. Gerontol. A Biol. Sci. Med. Sci. 71, 1667–1675 (2016).
Wang, C. W. et al. A comparison of muscle function, mass, and quality in liver transplant candidates: Results from the functional assessment in liver transplantation study. Transplantation 100, 1692–1698 (2016).
Itoh, S. et al. Skeletal muscle mass assessed by computed tomography correlates to muscle strength and physical performance at a liver-related hospital experience. Hepatol. Res. 46, 292–297 (2016).
West, J., Gow, P. J., Testro, A., Chapman, B. & Sinclair, M. Exercise physiology in cirrhosis and the potential benefits of exercise interventions: A review. J. Gastroenterol. Hepatol. 36, 2687–2705 (2021).
Acknowledgements
The authors would like to thank Dr. Hirofumi Wakaki, Professor at Hiroshima University, and Dr. Keisuke Fukui, Associate Professor at Hiroshima University, for their guidance in statistical analyses.
Funding
Funding was provided by the Japanese Society of Physical Therapy (Grant No. H30-A59), Japan Society for the Promotion of Science (Grant No. JP19K19831).
Author information
Authors and Affiliations
Contributions
K.F., T.N., T.K., D.I., Y.N., and Y.M. conceived the concept and design of the study. K.F., T.K., D.I., Y.N., A.N., A.H., E.M., and S.O. collected the data. K.F., K.U., T.N., Y.N., and A.H. analyzed and interpreted the data. K.F., K.U., Y.N., E.M., and A.H. prepared the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Fudeyasu, K., Ushio, K., Nomura, T. et al. Advanced liver fibrosis is associated with decreased gait speed in older patients with chronic liver disease. Sci Rep 14, 6809 (2024). https://doi.org/10.1038/s41598-024-57342-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-57342-1
Keywords
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.