Abstract
We aimed to evaluate the association between systemic sclerosis (SSc) and major cerebrovascular/cardiovascular risks through a systematic approach. Databases were systematically searched from their inception to October 10, 2023 for studies comparing cerebrovascular/cardiovascular event rates between patients with SSc and controls. The primary outcome was the stroke risk in patients with SSc. Secondary outcomes included risk of myocardial infarction (MI), cardiovascular disease (CVD), peripheral vascular disease (PVD), and venous thromboembolism (VTE). Seventeen studies with 6,642,297 participants were included. SSc was associated with a significantly increased risk of stroke (HR, 1.64; 95% confidence interval [CI], 1.35–2.01), CVD (HR, 2.12; 95% CI, 1.36–3.3), MI (HR, 2.15; 95% CI, 1.23–3.77), VTE (HR, 2.75; 95% CI, 1.77–4.28), and PVD (HR, 5.23; 95% CI, 4.25–6.45). Subgroup analysis revealed a significantly increased stroke risk in the non-Asian group (HR, 1.55; 95% CI, 1.26–1.9), while the Asian group displayed a higher but not statistically significant risk (HR, 1.86; 95% CI, 0.97–3.55). The study found that SSc is associated with a significantly increased risk of cerebrovascular/cardiovascular events. These findings highlight the importance of vasculopathy in SSc and suggest the need for enhanced clinical monitoring and preventive measures in this high-risk population.
Similar content being viewed by others
Introduction
Systemic sclerosis (SSc), also known as scleroderma, is an autoimmune disorder characterized by inflammation, vasculopathy, and excessive collagen deposition1,2. Although the thickened, hardened skin is the hallmark of SSc, the disease also frequently affects other organs, including the gastrointestinal tract and lungs3,4,5. A previous meta-analysis has reported a pooled prevalence of SSc of 17.6 per 100,000 and a pooled incidence rate of 1.4 per 100,000 person-years, with noticeable regional disparities; notably, studies from North America reported substantially higher estimates than those of other regions6. The disease predominantly affects females, with both pooled incidence and prevalence in females being fivefold higher than those in males6. While skin involvement frequently marks the clinical onset of SSc, vascular system dysfunction including vascular injury, vascular remodeling, and endothelial dysfunction represent hallmark pathological changes7,8. Systemic vasculopathy can result in various microvascular and macrovascular diseases, thereby leading to multi-organ disorders. Microvascular disease leads to Raynaud’s phenomenon, cutaneous telangiectasia, pulmonary arterial hypertension, scleroderma renal crisis, and gastrointestinal involvement7,8,9. Conversely, macrovascular disease also appears prevalent, with emerging evidence alluding to an increased risk of atherosclerotic events, including stroke, myocardial infarction (MI), and peripheral vascular disease (PVD) in patients with SSc9,10,11.
The risk of cerebrovascular and cardiovascular complications attributed to SSc remains debated12,13. Proposed explanations center around accelerated atherosclerosis triggered by chronic inflammation, endothelial dysfunction, and imbalance between vasoconstrictive and vasodilatory mediators12,13. Furthermore, SSc vasculopathy could independently precipitate vascular occlusive events14,15. Extensive nationwide cohort studies have demonstrated that SSc is independently linked to an increased likelihood of experiencing an ischemic stroke or cardiovascular disease (CVD)16,17,18. Consistently, several meta-analyses have reported the associations of SSc with cerebrovascular and cardiovascular complications10,19,20. However, the shortcomings of the earlier evidence stemmed from the inclusion of a restricted number of studies for investigating the associations10,19,20. Furthermore, these studies did not investigate the source of heterogeneity10,19,20. Recently, several studies have delved into the relationship between SSc and cerebrovascular/CVDs16,21,22,23,24. These recent studies could provide more solid evidence. In this context, our meta-analysis aimed to synthesize the existing literature on the association between SSc and major cerebrovascular and cardiovascular outcomes. The relationship between SSc and risk of stroke was the primary outcome, whereas the associations of SSc with CVD (e.g., MI), venous thromboembolism (VTE), and other cardiac-related complications (e.g., heart failure) were the secondary outcomes. Elucidating the relationship between SSc and cerebrovascular/CVDs has significant implications for prognosis, screening, prevention, and management. Our findings will help guide clinicians caring for patients with SSc and highlight areas warranting further research.
Methods
This review was conducted and documented following the MOOSE guidelines, with details of the protocol registration available in PROSPERO (Number: CRD42023471039).
Search strategy and data sources
An exhaustive and systematic literature search was performed across multiple electronic databases, including Medline, Embase, Cochrane Library, and Google Scholar, spanning from their inception to October 10, 2023. The search strategy was meticulously designed to combine terms specifically associated with SSc (e.g., “scleroderma” and “systemic sclerosis”) with terms indicative of cerebrovascular or CVD (e.g., “coronary artery disease” or “stroke” or “ischemic heart disease” or “myocardial infarction” or “peripheral vascular disease” or “venous thromboembolism”). Adjunctively, to ensure a comprehensive capture of all applicable studies, the reference lists of the articles selected for inclusion, as well as relevant review articles, were manually screened. The search strategy for Medline is summarized in Supplemental Table 1. A similar search strategy was also applied for other databases.
Two independent authors initially screened all identified records from the literature search, first assessing titles and abstracts against predefined criteria. Potentially relevant studies were subsequently subjected to a full-text review. Each author made independent decisions on study inclusion, and any discrepancies were discussed in an attempt to reach consensus. If disagreements persisted, a third expert was consulted. During the selection process, no limitations were placed on the publication year or sample size.
Inclusion and exclusion criteria
Eligibility for study inclusion was defined as follows: (1) peer-reviewed articles that explicitly reported cerebrovascular/cardiovascular risks (e.g., stroke or MI) among patients diagnosed with SSc and compared these findings to either a control group or the wider general population; and (2) studies that provided hazard ratios (HRs) with 95% confidence intervals (CIs). The following were the exclusion criteria: (1) case reports, case series, review articles, opinion letters, and editorials; (2) any study that lacked a comparative control group or devoid of the necessary quantitative data for analysis; (3) full texts not available; and (4) case–control or cross-sectional studies were not included as they only reported the cumulative risk of cerebrovascular/cardiovascular events (e.g., they provided risk ratios or odds ratios) and not the occurrence of events over time (i.e., HRs).
Data collection
A pair of independent reviewers, well-versed in the study topic, performed data extraction from the selected studies, employing a standardized and pre-approved data collection template. In instances of disagreements or discrepancies between them, a consensus was sought either through discussion or, if required, mediation by a third senior reviewer. Essential data points extracted encompassed details, including the first author’s name, publication year, total sample size, characteristics of the study population, specific cardiovascular outcomes evaluated (e.g., MI or stroke), and the presented risk estimates with their respective 95% CIs. For studies that provided both unadjusted and adjusted data, we only collected adjusted data for analysis. We gathered the data from the period with the longest follow-up when studies presented identical data across various follow-up periods.
Outcomes and definitions
The stroke risk in patients with SSc was the primary outcome for this meta-analysis. This outcome was defined as the occurrence of cerebrovascular accidents, including ischemic stroke, transient ischemic attack, or hemorrhage stroke. Moreover, several secondary outcomes were assessed, including the risk of MI, CVD, PVD, VTE, and cardiac-related complications (e.g., heart failure, atrial fibrillation, and pacemaker implantation). The specific definition and criteria for these events were based on the individual definitions provided by each study included in the meta-analysis. As definitions may vary slightly across studies, we took care to ensure consistency in our interpretation and reporting of results.
Quality assessment of included studies
A rigorous assessment of each study’s methodological quality was performed using the Newcastle–Ottawa Scale (NOS). This assessment critiqued studies across the following three broad domains: the selection process of study groups, the comparability of groups, and the accuracy in ascertaining exposure or outcome data. A maximum score of 9 points denoted the pinnacle of study quality, whereas studies scoring below 6 points were categorized as being of lower methodological quality.
Statistical analyses
To generate pooled risk estimates (e.g., HRs) with 95% CIs, a random-effects model was employed, anticipating potential heterogeneity across the included studies. A random-effects model was used that weighed studies based on both within-study and between-study variance, not just on sample size. This approach ensures that study weights are not solely proportional to sample sizes, with between-study variance playing a significant role in the weighting process. The degree of heterogeneity was quantified using the I2 statistic, with values exceeding 50% signaling significant heterogeneity. To delve deeper into sources of variability, subgroup analysis on the primary outcome (e.g., stroke risk) was performed on the basis of ethnicity (e.g., non-Asian vs. Asian). In addition, to avoid potential patient duplication across studies, a subgroup analysis was performed, including only the single largest cohort study from each country. This subgroup analysis eliminates scenarios in which the same patient can be counted multiple times across different hospitals or insurance datasets within a country. The possibility of publication bias was assessed using funnel plots for outcomes encompassing more than 10 studies. To evaluate the robustness of the results, sensitivity analysis was performed. All statistical analyses were performed using the RevMan software. A p-value of < 0.05 was considered statistically significant.
Results
Selection process and characteristics of studies
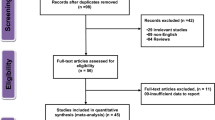
From an initial pool of 463 articles identified for the meta-analysis, 56 duplicates were removed, leaving 407 unique articles (Fig. 1). These were preliminarily screened on the basis of titles and abstracts, thereby narrowing the selection to 28 articles for a detailed full-text review. Upon rigorous assessment against specific inclusion and exclusion criteria, 11 articles were excluded for various reasons, resulting in a final set of 17 articles, encompassing a vast participant pool of 6,642,297 individuals, which were deemed suitable and included in the meta-analysis16,17,18,21,22,23,24,25,26,27,28,29,30,31,32,33,34.
The characteristics of studies are summarized in Table 1. We thoroughly examined 17 studies spanning diverse geographic regions, including Canada, Denmark, Taiwan, Belgium, Switzerland, the USA, and Copenhagen. The studies collectively encapsulated a broad participant pool, with the number of patients with SSc ranging from as few as 78 in the study by Kurmann et al.30 to as much as 8947 in the study by Thormann et al.33. The mean age of participants in these studies varied, with mean ages ranging from 35 years in the study by Thormann et al.33. to 60.9 years in the study by Ying et al.16. A noteworthy observation across these studies was the prominence of female participants, with percentages frequently exceeding 70%, such as the 91% reported in the study by Kurmann et al.30. Additionally, the follow-up duration varied across the studies, with the shortest being 1 year in the study by Thormann et al.33, and the longest extending up to 14 years in the study by Aviña-Zubieta et al.25.
Quality of studies
Table 1 summarize the quality of studies by using the NOS. Approximately 53% (9 of 17) of the studies showcased high-quality research methodologies, as indicated by scores ranging from 8 to 9. On the other hand, approximately 41% (7 of 17) of the studies were of moderate quality, with NOS scores between 6 and 7. A small portion, approximately 12% (2 of 17) of the studies, namely Hesselvig 2018 and Michel 202029,31, were identified as having low quality, with scores below 6. This lower score was attributed to potential limitations in participant selection, comparability of groups, or outcome measurement, thereby introducing a greater risk of bias.
Results of meta-analysis
Primary outcome
Among the 17 studies included in our meta-analysis, 11 reported data on the association between SSc and stroke risk16,17,18,21,23,25,26,30,31,33,34. Collectively, these studies encompassed 1,157,175 participants. The pooled HR for stroke in patients with SSc compared with the general population was 1.64 (95% CI, 1.35–2.01; p < 0.00001; I2 = 82%) (Fig. 2), suggesting a significant association between SSc and an increased stroke risk. Subgroup analysis indicated a significant increase in risk within the non-Asian subgroup (HR, 1.55; 95% CI, 1.26–1.9; p < 0.0001; I2 = 77%). In contrast, while individuals from the Asian subgroup (all from Taiwan)17,21,24,27,28,34 demonstrated a trend toward an increased stroke risk, this trend was not statistically significant (HR, 1.86; 95% CI, 0.97–3.55; p = 0.06; I2 = 90%) (Fig. 3).
To evaluate the stability of the outcome, a subgroup analysis was conducted using only the largest cohort study from each country. Therefore, six studies16,21,23,25,31,33 with the largest sample sizes from distinct regions were analyzed for the primary outcome. The combined HR for stroke in patients with SSc compared to the general population was found to be 1.46 (95% CI, 1.18–1.81; p = 0.0005; I2 = 78%) (Fig. 4), indicating a consistent result.
Secondary outcomes
Seven studies provided data on CVD risk in patients with SSc. Involving a total of 5,524,044 participants, the combined HR for CVD was 2.12 (95% CI, 1.36–3.3; p = 0.0009; I2 = 98%)18,22,24,25,29,30,33. This suggests that patients with SSc have a significant increased risk of CVD compared with those without the condition (Fig. 5). Consistently, data from seven studies that included 116,023 participants showed a pooled HR of 2.15 (95% CI, 1.23–3.77; p = 0.008; I2 = 96%) for MI in patients with SSc, indicating a significant association between SSc and an elevated MI risk (Fig. 6)18,23,25,26,27,30,33. Additionally, patients with SSc were observed to have a heightened VTE risk (HR, 2.75; 95% CI, 1.77–4.28; p < 0.00001; I2 = 77%; 47,533 participants) (Fig. 7)23,26,28,32. A significantly increased PVD risk was identified among the SSc cohort (HR, 5.23; 95% CI, 4.25–6.45; p < 0.00001; I2 = 0%; 26,040 participants) (Fig. 8)18,26,30.
Regarding other cardiac-related events (Fig. 9)23,26,30, heart failure was more prevalent among patients with SSc, with a HR of 2.68 (95% CI: 2.28 to 3.15, p < 0.00001, I2 = 14%, 24,377 participants). Atrial fibrillation (AF) occurrence was also higher in the SSc group (HR: 1.62 95% CI: 1.4 to 1.89, p < 0.00001, I2 = 0%, 24,143 participants). The risk for pacemaker implantation was elevated among patients with SSc when compared to controls (HR: 1.83 95% CI: 1.32 to 2.53, p = 0.0003, I2 = 6%, 24,143 participants).
Sensitivity analysis
Our sensitivity analysis, aimed at ensuring the robustness of our results, confirmed the consistency of all findings. The omission of any single study from the analysis did not materially alter the overall results. This consistency underscores the reliability of our conclusions and the absence of undue influence by any individual study.
Funnel plot results
For stroke risk, an evaluation of the funnel plot showcased an asymmetrical dispersion of the included studies, hinting at the presence of significant publication bias (Fig. 10). As there are fewer than 10 studies, funnel plots were not assessed for other outcomes.
Discussion
Cerebrovascular/CVDs (e.g., MI and stroke) remain dominant contributors to global morbidity and mortality35. This systematic review and meta-analysis of 17 studies reported that patients with SSc were at a significantly increased risk of stroke, with a pooled HR of 1.64 (95% CI, 1.35–2.01). Additionally, CVD and MI risks were increased in patients with SSc. Regarding atherosclerotic complications, SSc conferred an added risk of VTE (HR, 2.75) and PVD (HR, 5.23). Patients with SSc had more prevalence of other cardiac manifestations, including heart failure, AF, and pacemaker implantation, than controls. Overall, this meta-analysis demonstrates that SSc is associated with an increased cerebrovascular and CVD risk across a wide spectrum of vascular complications. The elevated risk of both macrovascular events highlights systemic vasculopathy as a central feature of the SSc pathophysiology. Our findings support increased clinical surveillance and consideration of preventative strategies in this high-risk population.
Several meta-analyses have evaluated the association between SSc and stroke risk. Two previous meta-analyses, published in 2016 and 2019, reported a significant association between SSc and stroke risk10,19. However, these meta-analyses had limitations as they each reported their main findings on the basis of only four studies. Additionally, these meta-analyses used the cumulative measure of risk (e.g., risk ratio or odds ratio) to examine their association but did not consider the risk of the event over time. A recent meta-analysis assessed stroke risk over time in patients with SSc by including five studies with a total of 67,085 participants20. However, only five studies were available for the analysis of the association between SSc and stroke risk in that meta-analysis20, which may have impaired the robustness of their findings. In contrast, our meta-analysis included eleven studies with a total of 1,157,175 participants, demonstrating an HR of 1.64. Furthermore, several studies have uncovered racial disparities in SSc presentation36,37. Accordingly, another advantage of our meta-analysis lies in the inclusion of a more diverse range of studies, encompassing both Asian and non-Asian populations, which provides more robust evidence.
Our meta-analysis of seven studies with over 116,000 participants noted that patients with SSc had a significantly increased MI risk compared with controls without SSc. The pooled HR was 2.15, indicating that patients with SSc had more than double the risk of experiencing MI. Several mechanisms may explain the observed association between SSc and MI. First, SSc is characterized by chronic inflammation, autoimmunity, and vascular injury1,2, which accelerate atherosclerosis and coronary artery disease. Second, endothelial dysfunction and impaired vascular repair, the hallmarks of SSc, also promote atherosclerosis38,39. Third, the progression of peripheral microvasculopathy, a key element in SSc pathogenesis, may further contribute to arterial stiffening and myocardial damage over time40. Our findings are consistent with a previous meta-analysis demonstrating increased subclinical atherosclerosis, as measured by carotid intima–media thickness, in patients with SSc compared with that in healthy controls41. Moreover, clinical studies have reported higher angiographically confirmed coronary artery disease prevalence in asymptomatic SSc42,43. The elevated MI risk in SSc remained significant after adjusting for traditional cardiovascular risk factors in several included studies25,26,27, suggesting that disease-related mechanisms mediate much of the increased MI risk.
VTE carries a significant risk of morbidity and mortality, with studies indicating a 30-day mortality rate ranging from 11 to 30%44,45,46. Virchow’s triad is the three principal risk factors for venous thrombosis, including venous stasis, heightened blood coagulability, and vascular wall damage47. In patients with SSc, augmented damage to the vascular wall may be observed, as implied by vasculopathy and vascular injury, which are hallmark features of SSc1,2. Consistently, our meta-analysis reported a significantly increased risk of VTE among patients with SSc, with a pooled HR of 2.75 compared with individuals without SSc. Our finding is consistent with that of a previous meta-analysis that reported a pooled risk ratio of VTE in patients with SSc being 2.5148. The distinction between our study and the previous one48 is that we accounted for the potential occurrence of events over time by utilizing the HR. While the mechanisms linking SSc and VTE require further studies, clinicians should be cognizant of the heightened thrombotic risk in this population. Anticoagulation may be considered for VTE prophylaxis in patients with SSc with additional risk factors. Moreover, patients should be educated on promptly reporting VTE symptoms for timely diagnosis and treatment.
Our meta-analysis provides an updated and comprehensive synthesis of the relationship between SSc and major cerebrovascular and cardiovascular outcomes. Involving 17 studies and over 6 million participants, this represents the most extensive meta-analysis on this topic to date. This meta-analysis makes several notable contributions to the literature. First, it provides a contemporary update, incorporating recent large-scale studies published over the past 5 years. Second, it assesses a comprehensive range of clinically relevant cardiovascular outcomes beyond just stroke risk. Third, the expansive dataset of over 6 million participants lends reliability and helps minimize the influence of individual studies. Fourth, subgroup analyses by ethnicity offer initial insights on geographic variations in SSc-related vascular risk. The focus on synthesizing hard clinical endpoints, which represent tangible adverse outcomes in patients, rather than surrogate markers of subclinical vascular dysfunction is the principal novel aspect of this study. Most of the previous literature centered on subtle changes in vascular physiology. Our findings reaffirm vasculopathy as a central SSc feature that warrants greater clinical attention by demonstrating consistent elevations across an array of overt clinical events.
This meta-analysis had several limitations that merit consideration. First, publication bias was a concern, as suggested by the asymmetric funnel plot for the outcome of stroke. It was plausible that small studies demonstrating no significant associations may be underrepresented. Second, inherent to all meta-analyses, the robustness of our conclusions depended on the methodological rigor of the original studies. As all studies were conducted in a retrospective study design, the evidence may be impaired. Third, the reliability of our analyses was restricted by the scarce availability of pertinent clinical details. Data on SSc disease duration, subtype, antibody profiles, severity, and pharmacological treatments were largely unavailable. Without this granular information, elucidating the mechanisms underlying the association between SSc and cerebrovascular/CVD remains challenging. Finally, the statistical power for analyzing some outcomes was limited, as exemplified by the wide confidence intervals or significant heterogeneity. To enable robust conclusions, larger scale prospective studies with adequate adjustment for confounders are needed.
Conclusion
This meta-analysis, encompassing 17 studies, underscores that patients with SSc face an augmented risk across various vascular complications, including stroke, CVD, MI, VTE, and PVD. Furthermore, these patients exhibited a higher prevalence of other cardiac-related manifestations, including heart failure, AF, and an increased risk of pacemaker implantations. These findings emphasize systemic vasculopathy as a pivotal component of the SSc pathophysiology. Considering these insights, future studies should delve deeper into the mechanistic underpinnings of these associations, explore potential interventions, and assess the efficacy of preventative strategies tailored for this high-risk population.
Data availability
The original contributions presented in this study are included in this article/Supplementary material, further inquiries can be directed to the corresponding authors.
References
Denton, C. P. & Khanna, D. Systemic sclerosis. Lancet 390, 1685–1699 (2017).
Truchetet, M. E., Brembilla, N. C. & Chizzolini, C. Current concepts on the pathogenesis of systemic sclerosis. Clin. Rev. Allergy Immunol. 64, 262–283 (2023).
Luquez-Mindiola, A., Atuesta, A. J. & Gómez-Aldana, A. J. Gastrointestinal manifestations of systemic sclerosis: An updated review. World J. Clin. Cases 9, 6201 (2021).
Lee, K.-I. et al. Cardiovascular manifestations of systemic sclerosis: An overview of pathophysiology, screening modalities, and treatment options. Cardiol. Rev. 31, 22–27 (2023).
Perelas, A., Silver, R. M., Arrossi, A. V. & Highland, K. B. Systemic sclerosis-associated interstitial lung disease. Lancet Respir. Med. 8, 304–320 (2020).
Bairkdar, M. et al. Incidence and prevalence of systemic sclerosis globally: A comprehensive systematic review and meta-analysis. Rheumatology 60, 3121–3133 (2021).
Kawaguchi, Y. & Kuwana, M. Pathogenesis of vasculopathy in systemic sclerosis and its contribution to fibrosis. Curr. Opin. Rheumatol. 35, 309–316 (2023).
Ren, H. et al. Further insight into systemic sclerosis from the vasculopathy perspective. Biomed. Pharmacother. 166, 115282 (2023).
Kavian, N. & Batteux, F. Macro- and microvascular disease in systemic sclerosis. Vasc. Pharmacol. 71, 16–23 (2015).
Ungprasert, P., Sanguankeo, A. & Upala, S. Risk of ischemic stroke in patients with systemic sclerosis: A systematic review and meta-analysis. Mod. Rheumatol. 26, 128–131 (2016).
Ungprasert, P. et al. Risk of coronary artery disease in patients with systemic sclerosis: A systematic review and meta-analysis. Clin. Rheumatol. 33, 1099–1104 (2014).
Ngian, G.-S., Sahhar, J., Wicks, I. P. & Van Doornum, S. Cardiovascular disease in systemic sclerosis-an emerging association?. Arthritis Res. Ther. 13, 1–10 (2011).
Cannarile, F. et al. Cardiovascular disease in systemic sclerosis. Ann. Transl. Med. 3, 8 (2015).
Lescoat, A. et al. Ulnar artery occlusion and severity markers of vasculopathy in systemic sclerosis: A multicenter cross-sectional study. Arthritis Rheumatol. 71, 983–990 (2019).
Mori, A. et al. Central retinal arterial and venous occlusion in a patient with systemic sclerosis and a review of the related literature. Am. J. Intern. Med. 9, 214–218 (2021).
Ying, D. et al. Increased risk of ischemic stroke in systemic sclerosis: A national cohort study of US veterans. J. Rheumatol. 47, 82–88 (2020).
Chiang, C. H. et al. Systemic sclerosis and risk of ischaemic stroke: A nationwide cohort study. Rheumatology (Oxford, England) 52, 161–165 (2013).
Man, A. et al. The risk of cardiovascular disease in systemic sclerosis: A population-based cohort study. Ann. Rheum. Dis. 72, 1188–1193 (2013).
Hong, Y. et al. Multiple sclerosis and stroke: A systematic review and meta-analysis. BMC Neurol. 19, 1–11 (2019).
Cen, X., Feng, S., Wei, S., Yan, L. & Sun, L. Systemic sclerosis and risk of cardiovascular disease: A PRISMA-compliant systemic review and meta-analysis of cohort studies. Medicine 99, e23009 (2020).
Huang, Y.-C. & Weng, M.-Y. Elevated risk of young stroke in patients with systemic sclerosis: A population-based study. Formosan J. Rheumatol. 35, 28–34 (2021).
Conrad, N. et al. Autoimmune diseases and cardiovascular risk: A population-based study on 19 autoimmune diseases and 12 cardiovascular diseases in 22 million individuals in the UK. Lancet 400, 733–743 (2022).
Sun, G. et al. Gender- and age-specific rates of heart failure and other adverse cardiovascular outcomes in systemic sclerosis. Rheumatology (Oxford, England) 61, 4374–4383 (2022).
Yen, T. H. et al. The risk of major adverse cardiovascular events in patients with systemic sclerosis: A nationwide, population-based cohort study. Rheumatology (Oxford, England) https://doi.org/10.1093/rheumatology/kead464 (2023).
Aviña-Zubieta, J. A. et al. Early Cardiovascular disease after the diagnosis of systemic sclerosis. Am. J. Med. 129, 324–331 (2016).
Butt, S. A. et al. Cardiovascular manifestations of systemic sclerosis: A Danish nationwide cohort study. J. Am. Heart Assoc. 8, e013405 (2019).
Chu, S.-Y. et al. Increased risk of acute myocardial infarction in systemic sclerosis: A nationwide population-based study. Am. J. Med. 126, 982–988 (2013).
Chung, W.-S. et al. Systemic sclerosis increases the risks of deep vein thrombosis and pulmonary thromboembolism: A nationwide cohort study. Rheumatology 53, 1639–1645 (2014).
Hesselvig, J. H. et al. Localized scleroderma, systemic sclerosis and cardiovascular risk: A Danish Nationwide Cohort Study. Acta Dermato-Venereol. 98, 361–365 (2018).
Kurmann, R. D. et al. Cardiovascular risk factors and atherosclerotic cardiovascular events among incident cases of systemic sclerosis: Results from a population-based cohort (1980–2016). Mayo Clin. Proc. 95, 1369–1378 (2020).
Michel, A., González-Pérez, A., Sáez, M. E. & García Rodríguez, L. A. Risk of bleeding events among patients with systemic sclerosis and the general population in the UK: A large population-based cohort study. Clin. Rheumatol. 39, 19–26 (2020).
Schoenfeld, S. R., Choi, H. K., Sayre, E. C. & Aviña-Zubieta, J. A. Risk of pulmonary embolism and deep venous thrombosis in systemic sclerosis: A general population-based study. Arthritis Care Res. 68, 246–253 (2016).
Thormann, A., Magyari, M., Koch-Henriksen, N., Laursen, B. & Sørensen, P. S. Vascular comorbidities in multiple sclerosis: A nationwide study from Denmark. J. Neurol. 263, 2484–2493 (2016).
Tseng, C. H., Huang, W. S., Lin, C. L. & Chang, Y. J. Increased risk of ischaemic stroke among patients with multiple sclerosis. Eur. J. Neurol. 22, 500–506 (2015).
Moraes-Silva, I. C., Rodrigues, B., Coelho-Junior, H. J., Feriani, D. J. & Irigoyen, M. C. Myocardial infarction and exercise training: Evidence from basic science. Adv. Exp. Med. Biol. 999, 139–153 (2017).
Jaeger, V. K. et al. Racial differences in systemic sclerosis disease presentation: A European Scleroderma Trials and Research group study. Rheumatology (Oxford, England) 59, 1684–1694 (2020).
Silver, R. M., Bogatkevich, G., Tourkina, E., Nietert, P. J. & Hoffman, S. Racial differences between blacks and whites with systemic sclerosis. Curr. Opin. Rheumatol. 24, 642–648 (2012).
Patnaik, E., Lyons, M., Tran, K. & Pattanaik, D. Endothelial dysfunction in systemic sclerosis. Int. J. Mol. Sci. 24, 14385 (2023).
Chhabra, N. Endothelial dysfunction—A predictor of atherosclerosis. Internet J. Med. Update 4, 33–41 (2009).
Pagkopoulou, E. et al. Peripheral microcirculatory abnormalities are associated with cardiovascular risk in systemic sclerosis: A nailfold video capillaroscopy study. Clin. Rheumatol. 40, 4957–4968 (2021).
Tyrrell, P. N. et al. Rheumatic disease and carotid intima-media thickness: A systematic review and meta-analysis. Arterioscler. Thromb. Vasc. Biol. 30, 1014–1026 (2010).
Tarek, E.-G., Yasser, A. E. & Gheita, T. Coronary angiographic findings in asymptomatic systemic sclerosis. Clin. Rheumatol. 25, 487–490 (2006).
Mok, M. et al. Coronary atherosclerosis using computed tomography coronary angiography in patients with systemic sclerosis. Scand. J. Rheumatol. 38, 381–385 (2009).
Naess, I. A. et al. Incidence and mortality of venous thrombosis: A population-based study. J. Thromb. Haemost. 5, 692–699 (2007).
Cushman, M. et al. Deep vein thrombosis and pulmonary embolism in two cohorts: The longitudinal investigation of thromboembolism etiology. Am. J. Med. 117, 19–25 (2004).
Heit, J. A. et al. Predictors of survival after deep vein thrombosis and pulmonary embolism: A population-based, cohort study. Arch. Intern. Med. 159, 445–453 (1999).
Malone, P. & Agutter, P. The aetiology of deep venous thrombosis. J. Assoc. Physicians 99, 581–593 (2006).
Ungprasert, P., Srivali, N. & Kittanamongkolchai, W. Systemic sclerosis and risk of venous thromboembolism: A systematic review and meta-analysis. Mod. Rheumatol. 25, 893–897 (2015).
Funding
This research was funded by Chi Mei Medical Center, Tainan, Taiwan, grant number CMOR11203.
Author information
Authors and Affiliations
Contributions
Conceptualization and literature search: I-Wen Chen and Wei-Ting Wang; methodology: Yi-Chen Lai; Trial selection: Chien-Ming Lin and Ping-Hsin Liu; Data analysis: Kuo-Chuan Hung; Data extraction: Kuo-Chuan Hung and Su-Zhen Wu; Writing—original draft preparation: Kuo-Chuan Hung and I-Wen Chen; Writing—review and editing: Kuo-Chuan Hung and I-Wen Chen. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chen, IW., Wang, WT., Lai, YC. et al. Association between systemic sclerosis and risk of cerebrovascular and cardiovascular disease: a meta-analysis. Sci Rep 14, 6445 (2024). https://doi.org/10.1038/s41598-024-57275-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-57275-9
Keywords
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.