Abstract
The total cerebral small vessel disease (SVD) score is a proposed comprehensive index of SVD severity in the brain. However, data on lifestyle-related risk factors affecting SVD scores are limited. We conducted a cross-sectional study with 858 neurologically healthy adults who underwent brain magnetic resonance imaging (MRI). Information on clinical and lifestyle-related risk factors was obtained from health screenings. The SVD score (0–4) was calculated from the presence of lacunes, cerebral microbleeds, moderate to severe white matter lesions, and basal ganglia perivascular spaces on MRI. Subjects were divided into two groups by SVD score; potential risk factors and their joint effects in the two groups were assessed by logistic regression. Biologic interactions were estimated using the synergy index. After adjustment for possible confounders, the adjusted odds ratio for moderate to severe SVD scores (SVD score ≥ 2) was 1.12 (95% confidence interval (CI) 1.08–1.16) for age per year, 1.33 (95% CI 1.02–1.74) for body mass index per standard deviation, 3.39 (95% CI 1.90–6.03) for hypertension, 2.31 (95% CI 1.14–4.69) for diabetes, and 2.35 (95% CI 1.10–5.02) for smoking. Hypertension and current smoking had a synergistic effect on the risk of moderate to severe SVD (OR 10.59, 95% CI 3.97–28.3; synergy index 4.03, 95% CI 1.17–28.30), and the combination of hypertension and diabetes had an additive effect on the risk of moderate to severe SVD (OR 9.48, 95% CI 3.80–23.66; synergy index 2.12, 95% CI 0.68–6.67). Therefore, combined strategies for managing hypertension, smoking, and diabetes may be effective for preventing SVD.
Similar content being viewed by others
Introduction
Stroke is the second-leading cause of death worldwide, and a recent systematic analysis of 188 studies from low-income to high-income countries suggested that more than 90% of the stroke burden is attributable to modifiable risk factors [1]. The same study showed that the 10 most important global risk factors were high systolic blood pressure (SBP), a diet low in fruit, a high body mass index (BMI), a diet high in sodium, smoking, a diet low in vegetables, ambient PM2.5 pollution, household air pollution from solid fuels, a diet low in whole grains, and high fasting glucose. However, the population attributable fraction of these risk factors for stroke varies between countries due to differences in socioeconomic factors, populations, life expectancies, and access to health and social services. Therefore, country-specific approaches are needed to reduce the stroke burden.
In Japan, stroke is the third-leading cause of death, with approximately 110,000 people dying from stroke every year [2]. Stroke is also a major cause of disability, confinement to bed and dementia, which are major contributors to health care costs in Japan, a country with the most rapidly aging population in the world [2]. In a Japan-specific approach, a health-screening program using brain magnetic resonance imaging (MRI) is being used for the earlier detection of small vessel diseases (SVDs) to prevent stroke and dementia. Among the modifiable risk factors for stroke, we previously reported that hypertension and current smoking were associated with cerebral microbleeds (CMBs), a known MRI marker of SVD. In addition, we showed that the combination of hypertension and current smoking had a synergistic additive effect on the occurrence of CMBs [3].
Although the clinical implications of markers of SVD, including CMBs, lacunes, white matter hyperintensity (WMH), and perivascular spaces (PVS), have been separately studied, these findings often coexist and are interrelated [4]. Recently, the Maastricht collaborative group developed a pragmatic method of estimating the total SVD burden in patients with lacunar stroke by summing all features present on MRI to yield a “total SVD score” ranging from 0 to 4 [5]. The SVD score, which is a proposed comprehensive index of SVD severity in the brain, was shown to be a good predictor of recurrent stroke in Caucasian and Chinese patients [6]. Staals et al. reported that older age, male sex, hypertension, and smoking were associated with a higher SVD score in Caucasian populations [7]. However, studies evaluating the modifiable risk factors for the SVD score are limited, especially in Asian populations.
Here, we aimed to identify the potential risk factors for SVD scores, their interactions and their additive and synergistic effects on SVD scores in a healthy Japanese population.
Methods
Study design and subjects
This study was conducted as part of the Kashima Scan Study, which aims to investigate age-related brain changes on MRI as previously reported [3, 8,9,10,11]. This study aimed to identify the risk factors for these brain changes. Briefly, the Kashima Scan Study enrolled individuals who were aged ≥30 years, did not have any disability in instrumental activities of daily living, had the ability to independently make visits for current health screening tests of the brain, voluntarily provided written informed consent, did not have a history of neurological disorders or brain injuries, and had complete MRI images were available. Data were collected on the individuals’ clinical characteristics, such as age, gender, years of education, history of stroke and ischemic heart diseases, family history of stroke, smoking status, and presence of hypertension, diabetes mellitus, and hyperlipidemia. A total of 1237 consecutive adults underwent health screening tests of the brain at their own expense between December 2005 and November 2010. They were asked to respond to a questionnaire on lifestyle-related factors via mail within 1 month of the health screening tests, with 891 subjects providing responses (72.0%). We confirmed that the number of subjects with CMBs was similar between respondents and nonrespondents [3]. After excluding 33 subjects who had a history of stroke (n = 7) or ischemic heart disease (n = 26), 858 subjects (376 men, 482 women; age, 33–84 years) were included in this cross-sectional analysis. This study was approved by the human ethics review board of Saga University (18-2).
Cardiovascular risk assessment
Hypertension was defined as SBP ≥140 mmHg and/or diastolic blood pressure (DBP) ≥90 mmHg or use of anti-hypertensive medication. Diabetes mellitus was defined as fasting serum glucose level ≥126 mg/dl, hemoglobin A1c level ≥6.5% (NGSP), or the use of anti-diabetic medication. Dyslipidemia was defined as fasting serum total cholesterol level ≥220 mg/dl and/or fasting serum triglyceride level ≥150 mg/dl and/or the use of anti-hyperlipidemic agents. Patients who were smokers at the time of analysis were classified as current smokers. Information regarding the duration of education was collected for each subject.
Brain MRI and SVD burden
MRI was performed using a 1.5-T scanner (EXCELART Vantage, version 7.0; Toshiba Medical Systems, Tokyo, Japan). Gradient-echo T2*-weighted MRI, axial T1-weighted imaging, fluid-attenuated inversion recovery (FLAIR) imaging, and fast spine-echo T2-weighted imaging were performed. For axial T1-weighted imaging, the following parameters were used: repetition time (TR), 550 ms; echo time (TE), 15 ms; flip angle (FA), 80°; section thickness, 7 mm; gap width, 1.4 mm; matrix, 256 × 352 mm2; and field of view, 220 × 220 mm2. For axial fast spin-echo T2-weighted imaging, the following parameters were used: TR, 4000 ms; TE, 108 ms; FA, 90°; section thickness, 7 mm; gap width, 1.4 mm; matrix, 352 × 400 mm2; and field of view, 220 × 220 mm2. For axial FLAIR imaging, the following parameters were used: TR, 10,000 ms; inversion time, 2500 ms; TE, 96 ms; FA, 90°; section thickness, 7 mm; gap width, 1.4 mm; matrix, 224 × 336 mm2; and field of view, 220 × 220 mm2. For axial gradient-echo T2*-weighted imaging, the following parameters were used: TR, 735 ms; TE, 20 ms; flip angle, 30°; section thickness, 7 mm; gap width, 1.4 mm; matrix, 224 × 320 mm2; and field of view, 220 × 220 mm2.
All raters of MRI findings were blinded to the clinical data. The components of the total SVD score, including lacunes (of presumed vascular origin), CMBs, WMH and periventricular hyperintensity (PVH), and basal ganglia (BG)-PVS, were classified according to the international consensus criteria of the Standards for Reporting Vascular Changes on Neuroimaging [12]. Lacunes were defined as focal, sharply demarcated lesions >3 mm in diameter showing high intensity on T2-weighted imaging and low intensity on T1-weighted imaging [12]. They were distinguished from PVS by their larger size, spheroid shape, and surrounding hyperintensity on FLAIR. CMBs (size <10 mm) were rated using the Microbleed Anatomic Rating Scale and a recent guideline [12,13,14]. White matter changes on T2-weighted imaging and FLAIR imaging were assessed for WMH and PVH using the Fazekas scale [12, 15] because both are variables in the total SVD score [5, 7]. For WMH, grade 1 = punctuate; grade 2 = early confluence; and grade 3 = confluent. For PVH, grade 1 = caps or lining; grade 2 = bands; and grade 3 = irregular extension into the deep white matter. BG-PVS were rated on axial T2-weighted MRI using a validated visual rating scale [7, 8]. PVS were defined as small, sharply delineated structures with signal intensity very similar or identical to that of cerebrospinal fluid, measuring <3 mm, and following the course of perforating or medullary vessels. For BG-PVS, a score of 0 = no PVS, 1 = 1–10 PVS, 2 = 11–20 PVS, 3 = 21–40 PVS, and 4 = 40 or more PVS. The number of PVS in each score refers to the number on one side of the brain. After reviewing all relevant slices for the anatomical area being assessed, the scores for the slice and the side with the greatest number of PVS were recorded. When the assessment of PVS was influenced by changes in confluent white matter, we estimated the closest PVS score based on the noninvolved white matter [16]. Interrater reliability for lacunes, CMBs, WMH, and PVH was determined as Cohen’s kappa values as follows: the presence of CMBs, 0.71; the presence of lacunes, 0.58; grade ≥2 on the Fazekas scale for WMH, 0.58; and grade ≥2 on the Fazekas scale for PVH, 0.45. Intrarater reliability values for these findings were determined from 50 randomly selected scans that were scored twice. All intrarater kappa values were between 0.66 and 0.87. For PVS, interrater reliability testing (n = 100 randomly selected scans) demonstrated a weighted Fleiss-Cohen kappa coefficient of 0.56. Intrarater reliability for BG-PVS was determined from 50 randomly selected scans scored twice 8 weeks apart and with the investigator blinded to the initial ratings. The weighted Fleiss-Cohen kappa coefficient was 0.74.
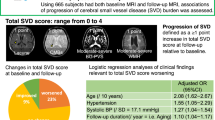
Rating of SVD score
Based on a recent scoring system [5, 7, 17], scores were assigned as follows: the presence of lacunes or CMBs was defined as the presence of one or more foci (1 point for each if present); the presence of moderate to severe PVH or WMH was defined as either irregular PVH extending into the deep white matter (Fazekas score 3) and/or (early) confluent deep WMH (Fazekas score 2 or 3) [15] (1 point if present); and the presence of moderate to severe BG-PVS was defined as grade 2–4 PVS (1 point if present). Therefore, the total SVD score ranged from 0 to 4. In the analyses, subjects were divided into two groups: a none to mild group (SVD score <2) and a moderate to severe group (SVD score ≥2).
Statistical analyses
We used the χ2 test and t-test to compare baseline characteristics between the 2 groups. The associations of lifestyle-related risk factors with an SVD score ≥2 were examined using univariate (model 1) and multiple logistic regression models (model 2, 3), and the odds ratios (ORs) and confidence intervals (CIs) of moderate to severe SVD risk were computed for each risk factor. The selection of potential confounders was based on a priori consideration of their association with the SVD score. Model 2 was adjusted for age and sex. Model 3 was additionally adjusted for BMI, a history of hypertension, diabetes, dyslipidemia, current smoking status, a heavy alcohol drinking habit (30 g ethanol/day), and years of education. The joint effects of hypertension and diabetes with lifestyle-related risk factors were examined. Interaction on the multiplicative scale was assessed by comparing multiplicative models using the log-likelihood ratio test. Interaction on the additive scale was assessed by calculating the synergy index (S) using the algorithm reported by Anderson et al. [18] S was calculated as [RR11 – 1]/[(RR10 – 1) + (RR01 – 1)], where RR11 is the relative risk of both risk factors being present, RR10 is the relative risk of the first risk factor being present and the second risk factor being absent, and RR01 is the relative risk of the first risk factor being absent and the second risk factor being present. S ≠ 1 indicates the presence of an additive interaction. The statistical analysis was performed with the Statistical Analysis System version 9.4 (SAS Institute) and Microsoft Excel (version 2010, Microsoft Corp). P < 0.05 was considered statistically significant.
Results
The majority of study subjects had an SVD score of 0. The numbers of subjects with SVD scores of 0, 1, 2, 3, and 4 were 607 (70.8%), 177 (20.6%), 49 (5.7%), 18 (2.1%), and 7 (0.8%), respectively. The characteristics of the study subjects stratified by SVD score are shown in Table 1. The subjects with moderate to severe scores were more likely to be older, to be male, have higher BMI, have hypertension, have diabetes mellitus, and be less educated than those in the none to mild score group.
Univariate analysis showed that moderate to severe SVD scores were significantly associated with age (OR: 1.13, 95% CI 1.09–1.17), male sex (OR: 1.67, 95% CI 1.03–2.70), BMI (OR: 1.42, 95% CI 1.13–1.79), hypertension (OR: 5.67, 95% CI 3.32–9.67), diabetes mellitus (OR: 3.44, 95% CI 1.83–6.46), and lower education levels (OR: 0.64, 95% CI 0.50–0.81). After adjusting for possible confounding factors in model 3, moderate to severe SVD scores were significantly associated with age (adjusted OR: 1.12, 95% CI 1.08–1.16), BMI (adjusted OR: 1.33, 95% CI 1.02–1.74), hypertension (adjusted OR: 3.39, 95% CI 1.90–6.03), diabetes mellitus (adjusted OR: 2.31, 95% CI 1.14–4.69), and current smoking (adjusted OR: 2.35, 95% CI 1.10–5.02) (Table 2).
The joint effects of current smoking and hypertension tended to be stronger than the sum of the independent effects of each factor for moderate to severe SVD scores, and the synergy index for this combination was statistically significant (adjusted OR 10.59, 95% CI 3.97–28.3; synergy index 4.03, 95% CI 1.17–13.8) (Table 3). Although there were no significant synergistic effects, the joint effects of hypertension and diabetes tended to be larger than the sum of the independent effects (adjusted OR 9.48, 95% CI 3.80–23.66; synergy index 2.12, 95% CI 0.68–6.67). In contrast, the joint effects of hypertension and obesity, diabetes and current smoking, diabetes and obesity, and current smoking and obesity were not larger than the sums of the independent effects.
Discussion
The present study demonstrated significant associations between moderate to severe SVD scores and modifiable risk factors among a healthy Japanese population. Similar to an earlier study in a Caucasian population, the total SVD score was associated with age, hypertension, and smoking [7]. In addition, our analyses showed that a higher BMI and diabetes mellitus were also associated with moderate to severe SVD scores. The most important finding in this study was that there was a joint effect of hypertension and current smoking and a joint effect of hypertension and diabetes mellitus on the risk of moderate to severe SVD scores. These findings suggest that modifying these risk factors may be effective for preventing SVD progression, which might be associated with future stroke or dementia.
While previous studies have identified independent modifiable risk factors for SVD [7, 11, 19], they did not examine the effects of interactions between the risk factors. To the best of our knowledge, this is the first study to show the significant joint effects of hypertension and smoking and hypertension and diabetes on SVD scores. Studies have reported the additive effects of smoking and hypertension on endothelial dysfunction and vascular damage [20, 21], although the exact pathophysiological mechanism remains unclear. The effects of hypertension and smoking on the decrease in endothelial nitric oxide synthase activity and nitric oxide production may lead to endothelial dysfunction and impaired vasodilation [22]. In addition, a recent study reported that asymptomatic intracranial lesions, including CMBs and lacunar infarction, were associated with arterial stiffness, a prominent biomarker for endothelial dysfunction [23]. The synergistic effect of the interaction between current smoking status and hypertension on SVD score suggests that the combination of these risk factors increases the probability of developing SVD. Therefore, combined strategies for managing blood pressure and smoking may be effective for reducing the SVD score and contributing to preventing stroke and dementia.
We found that diabetes, independently and in combination with hypertension, was significantly associated with the risk of moderate to severe SVD, despite finding no statistically significant association between the presence of cerebral microbleeds and diabetes in our previous study [3]. This discrepancy may be due to the SVD score being a more comprehensive measure of SVD severity. Given that diabetes is a well-known significant risk factor for both large vessel disease and SVD [24], it is plausible that diabetes is associated with the SVD score. Several studies have reported that hypertension and type 2 diabetes independently increase the risk of stroke and that the combination of these factors drastically increases the risk even further [25, 26]. Although the pathogenesis of SVD is not fully understood, hyperglycemia may cause toxic effects, such as oxidative stress and glycated products, leading to microvascular damage [27].
Previous studies have reported that ischemic stroke patients with a total SVD score ≥3 have a significantly higher risk of recurrent ischemic stroke [6] and mortality [28]. However, we chose a total SVD score cut-off value of 2 in the present study because the study participants were healthy individuals who had no previous history of stroke, and we therefore expected few subjects to have a total SVD score ≥3. The cut-off score is crucial to the use of the SVD score as a tool for the prevention for stroke and dementia, and therefore, we conducted additional analyses to evaluate the associations of lifestyle-related factors with an SVD score ≥1 (n = 251) compared with an SVD score of 0 (n = 607). We found that age, hypertension and diabetes were significantly associated with an SVD score ≥1, similar to an SVD score ≥2, while there was no statistically significant association between current smoking and an SVD score ≥1. The strength of associations with an SVD score ≥1 was weaker than that of associations with an SVD score ≥2. While the joint effects of hypertension and current smoking and hypertension and diabetes were stronger than the independent effects of each of these factors for an SVD score ≥1, there were no statistically significant synergistic effects (Supplementary Table 1, 2, 3). Therefore, we concluded that a cut-off score of 2 was superior to a cut-off score of 1. A follow-up study is needed to determine whether an SVD score cut-off of 2 is effective for predicting the occurrence of future small strokes.
This study has both strengths and limitations, as described elsewhere [11]. The strengths include the use of a large neurologically healthy population, standardized imaging and a standardized questionnaire. The limitations include the absence of pathological confirmation of SVD severity and the cross-sectional design. Longitudinal studies are required to examine whether the improvement of these risk factors is effective for modifying SVD scores.
In conclusion, we found that moderate to severe SVD scores were associated with age, hypertension, smoking, a higher BMI and diabetes mellitus among a neurologically healthy Japanese population. In addition, this is the first study to show joint effects of hypertension and current smoking and hypertension and diabetes mellitus on SVD severity. These findings suggest that combined strategies for managing blood pressure, smoking, and glycemic control may be effective for preventing the overall cerebral SVD burden.
References
Feigin VL, Roth GA, Naghavi M, Parmar P, Krishnamurthi R, Chugh S, et al. Global burden of stroke and risk factors in 188 countries, during 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet Neurol. 2016;15:913–24.
Ministry of Health, Labour and Welfare. Vital Statistics in JAPAN -Trends up to 2016. https://www.mhlw.go.jp/english/database/db-hw/dl/81-1a2en.pdf. Accessed 4th Jan 2019. (in Japanese)
Hara M, Yakushiji Y, Nannri H, Sasaki S, Noguchi T, Nishiyama M, et al. Joint effect of hypertension and lifestyle-related risk factors on the risk of brain microbleeds in healthy individuals. Hypertens Res. 2013;36:789–94.
Yakushiji Y. Cerebral microbleeds: detection, associations and clinical implications. Front Neurol Neurosci. 2015;37:78–92.
Klarenbeek P, van Oostenbrugge RJ, Rouhl RP, Knottnerus IL, Staals J. Ambulatory blood pressure in patients with lacunar stroke: association with total MRI burden of cerebral small vessel disease. Stroke. 2013;44:2995–9.
Lau KK, Li L, Schulz U, Simoni M, Chan KH, Ho SL, et al. Total small vessel disease score and risk of recurrent stroke: validation in 2 large cohorts. Neurology. 2017;88:2260–7.
Staals J, Makin SD, Doubal FN, Dennis MS, Wardlaw JM. Stroke subtype, vascular risk factors, and total MRI brain small-vessel disease burden. Neurology. 2014;83:1228–34.
Yakushiji Y, Nishiyama M, Yakushiji S, Hirotsu T, Uchino A, Nakajima J, et al. Brain microbleeds and global cognitive function in adults without neurological disorder. Stroke. 2008;39:3323–8.
Yakushiji Y, Noguchi T, Hara M, Nishihara M, Eriguchi M, Nanri Y, et al. Distributional impact of brain microbleeds on global cognitive function in adults without neurological disorder. Stroke. 2012;43:1800–5.
Yakushiji Y, Nanri Y, Hirotsu T, Nishihara M, Hara M, Nakajima J, et al. Marked cerebral atrophy is correlated with kidney dysfunction in nondisabled adults. Hypertens Res. 2010;33:1232–7.
Yakushiji Y, Charidimou A, Noguchi T, Nishihara M, Eriguchi M, Nanri Y, et al. Total small vessel disease score in neurologically healthy Japanese adults in the Kashima scan study. Intern Med. 2018;57:189–96.
Wardlaw JM, Smith EE, Biessels GJ, Cordonnier C, Fazekas F, Frayne R, et al. Neuroimaging STfRVco. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. 2013;12:822–38.
Greenberg SM, Vernooij MW, Cordonnier C, Viswanathan A, Al-Shahi Salman R, Warach S, et al. Cerebral microbleeds: a guide to detection and interpretation. Lancet Neurol. 2009;8:165–74.
Gregoire SM, Chaudhary UJ, Brown MM, Yousry TA, Kallis C, Jager HR, et al. The Microbleed Anatomical Rating Scale (MARS): reliability of a tool to map brain microbleeds. Neurology. 2009;73:1759–66.
Fazekas F, Chawluk JB, Alavi A, Hurtig HI, Zimmerman RA. MR signal abnormalities at 1.5 T in Alzheimer’s dementia and normal aging. Am J Roentgenol. 1987;149:351–6.
Doubal FN, MacLullich AM, Ferguson KJ, Dennis MS, Wardlaw JM. Enlarged perivascular spaces on MRI are a feature of cerebral small vessel disease. Stroke. 2010;41:450–4.
Staals J, Booth T, Morris Z, Bastin ME, Gow AJ, Corley J, et al. Total MRI load of cerebral small vessel disease and cognitive ability in older people. Neurobiol Aging. 2015;36:2806–11.
Andersson T, Alfredsson L, Kallberg H, Zdravkovic S, Ahlbom A. Calculating measures of biological interaction. Eur J Epidemiol. 2005;20:575–9.
Xia C, Chen HS, Wu SW, Xu WH. Etiology of isolated pontine infarctions: a study based on high-resolution MRI and brain small vessel disease scores. BMC Neurol. 2017;17:216.
Mazzone A, Cusa C, Mazzucchelli I, Vezzoli M, Ottini E, Ghio S, et al. Cigarette smoking and hypertension influence nitric oxide release and plasma levels of adhesion molecules. Clin Chem Lab Med. 2001;39:822–6.
Leone A. Smoking and hypertension: independent or additive effects to determining vascular damage? Curr Vasc Pharm. 2011;9:585–93.
Bleakley C, Hamilton PK, Pumb R, Harbinson M, McVeigh GE. Endothelial function in hypertension: victim or culprit? J Clin Hypertens. 2015;17:651–4.
Tabata N, Sueta D, Yamashita T, Utsunomiya D, Arima Y, Yamamoto E, et al. Relationship between asymptomatic intra-cranial lesions and brachial-ankle pulse wave velocity in coronary artery disease patients without stroke. Hypertens Res. 2017;40:392–8.
Karapanayiotides T, Piechowski-Jozwiak B, van Melle G, Bogousslavsky J, Devuyst G. Stroke patterns, etiology, and prognosis in patients with diabetes mellitus. Neurology. 2004;62:1558–62.
Hu G, Sarti C, Jousilahti P, Peltonen M, Qiao Q, Antikainen R, et al. The impact of history of hypertension and type 2 diabetes at baseline on the incidence of stroke and stroke mortality. Stroke. 2005;36:2538–43.
Zhang Y, Jiang X, Bo J, Yin L, Chen H, Wang Y, et al. Investigators PU-C. Risk of stroke and coronary heart disease among various levels of blood pressure in diabetic and nondiabetic Chinese patients. J Hypertens. 2018;36:93–100.
Giacco F, Brownlee M. Oxidative stress and diabetic complications. Circ Res. 2010;107:1058–70.
Song TJ, Kim J, Song D, Yoo J, Lee HS, Kim YJ, et al. Total cerebral small-vessel disease score is associated with mortality during follow-up after acute ischemic stroke. J Clin Neurol. 2017;13:187–95.
Acknowledgements
We wish to thank Ms. Miwako Koga, Ms. Mai Miyamoto, and the medical staff of Yuai-Kai Oda Hospital for their help with medical interviews, the acquisition of imaging data and data entry.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Hara, M., Yakushiji, Y., Suzuyama, K. et al. Synergistic effect of hypertension and smoking on the total small vessel disease score in healthy individuals: the Kashima scan study. Hypertens Res 42, 1738–1744 (2019). https://doi.org/10.1038/s41440-019-0282-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41440-019-0282-y
Keywords
This article is cited by
-
Associations for progression of cerebral small vessel disease burden in healthy adults: the Kashima scan study
Hypertension Research (2023)
-
Health advocacy for reducing smoking rates in Hamamatsu, Japan
Hypertension Research (2020)
-
Clinical outcomes of cerebral infarction in nonagenarians compared among four age groups
Neurological Sciences (2020)