Abstract
Colorectal cancer (CRC) is one of the most common malignant tumors of the digestive tract and a leading cause of cancer-related death worldwide. Since many CRC patients are diagnosed already in the advanced stage, and traditional chemoradiotherapy is prone to drug resistance, it is important to find new therapeutic targets. In this study, the expression levels of ALDOA and p-AKT were detected in cancer tissues and paired normal tissues, and it was found that they were significantly increased in CRC tissues, and their high expression indicated poor prognosis. Moreover, a positive correlation between the expression of ALDOA and p-AKT was found in CRC tissues and paired normal tissues. In addition, the Kaplan–Meier analysis revealed that the group with both negative of ALDOA/p-AKT expression had longer five-year survival rates compared with the other group. Besides, the group with both high expression of ALDOA/p-AKT had a worse prognosis compared with the other group. Based on the expression of ALDOA and p-AKT in tumor tissues, we can effectively distinguish tumor tissues from normal tissues through cluster analysis. Furthermore, we constructed nomograms to predict 3-year and 5-year overall survival, showing that the expression of ALDOA/p-AKT plays a crucial role in predicting the prognosis of CRC patients. Therefore, ALDOA/p-AKT may act as a crucial role in CRC, which may provide new horizons for targeted therapies for CRC.
Similar content being viewed by others

Introduction
Colorectal cancer (CRC) is a common malignant tumors of the digestive tract and one of the leading causes of cancer-related death worldwide1. Despite obvious advances in the systematic treatment of CRC, the 5-year survival rate of patients with advanced stages tumor is still poor because of distant metastases and resistance to chemotherapy1,2. Therefore, there is an urgent need for new therapeutic targets to improve survival and prognosis in CRC patients.
Aldolase A (ALDOA) is mainly expressed in muscle tissue. ALDOA catalyzes the reversible conversion offructose-1,6-bisphosphate to aldehyde 3-phosphate and dihydroxyacetone phosphate by encoding a glycolytic enzyme3,4. Studies have found that gluconeogenesis and glycolysis involved in ALDOA provide energy for the proliferation and migration in a variety of tumors, and are closely related to drug resistance of tumor cells4,5. The progression of tumor is accompanied by metabolic reprogramming, and ALDOA acts as an important role in tumor metabolism6,7. The aberrant expression of ALDOA in various tumor cells promotes the EMT process of tumor cells, enhances proliferation, and accelerates the transition from G1 to G2 phase of cell cycle by regulating tumor metabolism8,9,10.
The increased expression of ALDOA in tissues is related with the occurrence and development of various tumors. The activity of ALDOA protein in lung cancer, gastric cancer and liver cancer were significantly elevated11,12,13,14. Previous literatures indicated that ALDOA promotes cell proliferation and cisplatin resistance via the EGFR-AKT pathway and ALDOA expression was positively correlated with AKT expression in gastric cancer15. However, few study was performed to investigate the relationship between ALDOA and AKT in colorectal cancer. This study focused on the alteration of ALDOA expression in the progression of CRC and its correlation with clinical survival and prognosis. In addition, we established a prediction model, and evaluated the predictive role of ALDOA expression on survival, as well as its correlation with AKT, a target related to tumor metabolism, to provide a novel strategy for CRC targeted therapy.
Results
The expression of ALDOA is aberrantly increased in CRC
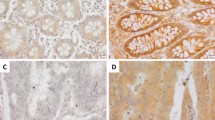
Studies indicates that ALDOA expression is elevated in a variety of tumor tissues, but the alteration of its expression in CRC tissue and adjacent normal tissue, and its potential influence have not been fully investigated. In our study, we investigated the expression of ALDOA in 126 CRC tissues and adjacent normal tissues by IHC (Fig. 1A). The IHC score based on the staining results revealed that ALDOA expression in CRC tissues was significantly higher than that in adjacent normal tissues (Fig. 1B). In subgroup analysis, the IHC score of the T3-4 group was significantly higher than that of the T1-2 group, and the IHC score of the lymph node metastasis group was obviously higher than that of the none lymph node metastasis group (Fig. 1C,D ).
ALDOA expression increased in CRC and indicated a poor prognosis. (A) ALDOA expression detected by IHC staining in 126 CRC tissues and para-cancer tissues (scale bar = 100 μm). (B-D) IHC score of ALDOA in (B) CRC and para-cancer tissues, (C) CRC with T1-2 or T3-4, (D) CRC with or without lymph node metastasis. (E–G) Kaplan–Meier analysis of ALODA positive vs ALDOA negative in (E) 126 CRC patients, (F) the CRC patients with the TMN stage I-II, (G) the CRC patients with the TMN stage III.
According to the IHC score, we divided the expression of ALDOA in CRC tissues and adjacent normal tissues into negative and positive. Subsequently, we evaluated the association between the ALDOA expression in CRC and clinical pathological indexes. The results revealed that the expression of ALDOA was closely related to lymph node metastasis, degree of differentiation, neural invasion and TNM stage. There is no association between ALDOA level and age, gender, tumor size, depth of tumor invasion, venous invasion (Table 1).
Depending on the close connections between ALDOA and clinicopathological indicators, the influence of ALDOA expression level was further assessed in CRC tissues, affecting the overall survival rate of sufferers. Patients were classified according to ALDOApos (ALDOA positive) and ALDOAneg (ALDOA negative) expression. Survival was obviously lower in patients with ALDOApos than in patients with ALDOAneg (P < 0.001, Fig. 1E). Further subgroup analysis indicated that high ALDOA expression was closely related with a lower survival rate in the TNM stage I-II subgroup (P = 0.012, Fig. 1F). However, in the stage III subgroup, ALDOA expression was not statistically associated with survival (P = 0.130, Fig. 1G). It suggested that if the expression of ALDOA in tumor tissue is used to predict the prognosis of CRC patients, the prediction is more accurate for early CRC patients.
In addition, we performed analysis of Cox’s proportional hazard model. The univariate analysis indicated neural invasion, depth of tumor invasion, lymph node metastasis, and ALDOA expression is a prognostic factor affecting the survival of CRC patients (P < 0.05, Table 2). In multivariate analysis, lymph node metastasis, and ALDOA expression were independent risk factors.
High expression of p-AKT indicates a poor prognosis in CRC
Owing to the process of gluconeogenesis and glycolysis ALDOA included in providing energy for the proliferation and migration in cancer cells, we investigated the association between ALDOA and the expression of AKT, a classical target of energy metabolism. We also investigated the p-AKT expression in 126 CRC tissues and adjacent normal colorectal tissues by IHC, and assessed IHC scores based on the staining results (Fig. 2A). The p-AKT expression in CRC tissues was obviously higher than that in paired normal tissues (Fig. 2B). In subgroup analysis, the IHC score of the T3-4 group and lymph node metastasis group was higher than the control groups (Fig. 2C,D). Subsequently, we analyzed the effect of p-AKT expression level on survival of CRC patients. Similar to ALDOA, patients with high expression of p-AKT have a worse prognosis (Fig. 2E). For TNM stage I-II CRC patients, differences in p-AKT expression led to significant differences in prognosis (Fig. 2F). While in the TNM stage III subgroup, there was no significant correlation between p-AKT expression and postoperative survival (Fig. 2G).
The aberrant expression of p-AKT in CRC indicated a poor prognosis. (A) p-AKT expression detected by IHC staining in 126 CRC tissues and para-cancer tissues (scale bar = 100 μm). (B-D) IHC score of p-AKT in (B) CRC and para-cancer tissues, (C) CRC with T1-2 or T3-4, (D) CRC with or without lymph node metastasis. (E–G) Kaplan–Meier analysis of p-AKT positive vs p-AKT negative in (E) 126 CRC patients, (F) the CRC patients with the TMN stage I-II, (G) the CRC patients with the TMN stage III.
Association between ALDOA and p-AKT in CRC tissues
To analyze the potential correlation between ALDOA and p-AKT expression level, we showed the heatmap to exhibit the difference expression between ALDOA and p-AKT in CRC and adjacent normal tissues (Fig. 3A). The results indicated that the expression levels of ALDOA and p-AKT in tumor tissues were remarkably higher than those in normal tissues, and the expression levels of ALDOA and p-AKT were correlated to a certain extent. Subsequently, we calculated the difference of IHC score of ALDOA between CRC and paired normal tissues, and analyzed the expression of ALDOA in tumor tissue and matched normal tissue was analyzed (Fig. 3B,C). More intuitively, the expression of ALDOA in majority tumor tissues was higher than that in paired normal tissues. Then, we also analyzed the expression of p-AKT in CRC tissues and normal tissues in pairs, and the results were similar to ALDOA (Fig. 3D,E). The association between ALDOA/AKT expression in colon cancer tissues and rectal cancer tissues in TCGA datasets was investigated via GEPIA platform (Fig. 3F,G). Based on the IHC staining score, we also conducted a linear analysis of the expression of ALDOA and p-AKT in CRC tissues, and the results showed a positive correlation between them (P < 0.001, Fig. 3H).
Expression of ALDOA and p-AKT in CRC and paired normal tissues. (A) The heatmap exhibiting the difference and correlation of expression between ALDOA and p-AKT in CRC and para-cancer normal tissues. (B-C) The alteration of ALDOA in CRC and para-cancer tissues. (D-E) The alteration of p-AKT in CRC and para-cancer tissues. (F-G) the association between ALDOA/AKT expression in TCGA datasets via GEPIA platform in (F) colon cancer tissues and (G) rectal cancer tissues. (H) The association between ALDOA and p-AKT expression according to IHC score in CRC tissues.
After investigating the association of ALDOA and p-AKT expression in tumor tissue, we also assess the association in normal tissue. Interestingly, in paired normal tissue, ALDOA expression was positively associated with the p-AKT expression (Fig. 4A). The staining results of ALDOA and p-AKT were divided into negative and positive, and constituent ratio displayed the ALDOA and p-AKT expression were also positively correlated in CRC (Fig. 4B). Then the IHC staining was checked to verify the association of ALDOA/p-AKT in subgroup. The outcome suggest that no matter in T1-2 or T3-4 subgroups, the ALDOA expression was significantly correlated with the p-AKT expression in CRC tissues (P < 0.001, Fig. 4C,D ). Similarly, in subgroup of various TNM stages, the result indicated that the positive association of ALDOA/p-AKT expression in both TNM I-II and TNM III staging (P < 0.001, Fig. 4E,F ). Furthermore, we performed a cluster analysis for normal and tumor tissues based on the expression level of ALDOA/p-AKT in CRC and normal tissues (Fig. 4G), with 41.3% tumor tissues and 78.6% normal tissues in Cluster 1, and 58.7% tumor tissues and 21.4% normal tissues in Cluster 2 (Fig. 4H).
Correlation between ALDOA and p-AKT in CRC and paired normal tissues. (A) The association between ALDOA and p-AKT expression according to IHC score in para-cancer normal tissues. (B) Constituent ratio displaying the correlation of ALDOA and p-AKT expression in CRC. (C-F) The association between ALDOA and p-AKT expression according to IHC score in (C) the CRC with the T1-2, (D) the CRC with the T3-4, (E) the CRC with the stage TNM I-II, and (F) the CRC with the stage TNM III. (G) The stratification of CRC and paired normal tissues in Cluster 1 and Cluster 2 based on the ALDOA and p-AKT expression. (H) The percentage of CRC and paired normal tissues in each cluster.
The influence of ALDOA/p-AKT overexpression on prognosis in CRC patients
In this study, we found that both ALDOA and p-AKT are significantly elevated in CRC tissue and represent a poor prognosis. In addition, the expression of ALDOA and p-AKT was positively correlated in CRC tissues. To further explore the efficacy of combined ALDOA and p-AKT detection in the prognosis of CRC patients, we first divided 126 CRC patients into ALDOA/p-AKT negative group and other group. The outcome showed the both negative expression of ALDOA/p-AKT group had a better prognosis compared with the other group (P < 0.001) (Fig. 5A). Besides, CRC patients were divided into ALDOA/p-AKT positive group and other groups. The both positive expression of ALDOA/p-AKT group had a lower 5-year survival rate compared with the other group (P < 0.001) (Fig. 5B).
Nomograms predicting 3 and 5-year overall survival of CRC patients. (A) Kaplan–Meier analysis of ALDOA/p-AKT negative vs other in 126 CRC patients. (B) Kaplan–Meier analysis of ALDOA/p-AKT positive vs other in 126 CRC patients. (C) The 3 and 5-year overall survival rate was predicted by the total points which was calculated by each prognostic factor points on the nomogram point scale.
Furthermore, the nomograms were performed to predict the 3 and 5-year overall survival of CRC patients (Fig. 5C). The survival rate was predicted by the total points which was calculated by each prognostic factor points on the nomogram point scale. The outcome suggested ALDOA/p-AKT expression played crucial roles in predicting the 3 and 5-year overall survival of CRC patients.
Discussion
CRC is the third most common cancer in the world, and its incidence is rising rapidly in developed countries. Although surgical resection, chemotherapy and other treatment strategies significantly prolong the survival of CRC patients, chemotherapy resistance leads to poor prognosis2,16. Therefore, discovering new therapeutic targets and exploring the potential mechanisms are crucial for the treatment of CRC.
ALDOA is mainly expressed in muscle tissue. The ectopic expression of ALDOA plays an important role in the occurrence and development of myocardial hypertrophy, heart failure and many cardiovascular and cerebrovascular diseases17. With the further study of ALDOA in recent years, it has been found ALDOA is involved in gluconeogenesis and glycolysis. Both gluconeogenesis and glycolysis can provide energy for tumor proliferation. Metabolic reprogramming can promote tumor cell growth by producing ATP and provide precursors for macromolecule synthesis3,6,10. Previous studies have indicated that oncogenes enhance glycolysis by increasing the expression of specific glucose transporters and glycolytic enzymes, promoting the proliferation of cancer cells. Studies have shown that ALDOA is included in the pathological processes of various cancers9,12,13,18.
In this study, we found that the expression of ALDOA was significantly elevated in CRC tissues, and ALDOA expression level increased with the progression of the depth of invasion and lymph node metastasis by immunohistochemical evaluation. In survival assessment, high expression of ALDOA predicted a poor prognosis. These findings suggest that evaluating the alteration of ALDOA expression level during the development of CRC has a potential role in predicting disease progression and prognostic outcome. Interestingly, in further analysis, we found that subgroup survival analysis based on TNM stage revealed that increased ALDOA expression level in the TNM I-II staging subgroup represented poor survival, while there was no statistical correlation between ALDOA expression level and survival prognosis in the TNM III staging subgroup. Therefore, if ALDOA expression is used to evaluate the prognosis of CRC patients in clinical applications, the detection of ALDOA expression is more significant in predicting survival for CRC patients with early stage.
ALDOA was found to act as a glycolysis monitor to regulate the NLRP3 inflammasome by sensing changes in the glycolysis process3. In this process, ALDOA maintains mitochondrial damage caused by NLRP3 agonists by controlling the AMPK-mTOR signaling pathway. In addition, ALDOA knockout enhanced the AMPK signaling pathway and inhibited mTORC13,19. According to previous studies, and considering the potential role of ALDOA expression in the process of gluconeogenesis and glycolysis of tumor cells, we explored the potential correlation between ALDOA and AKT, another important upstream target of mTOR20,21,22. PI3K-AKT is an important metabolism-related pathway. Studies have also found that AKT phosphorylation levels are significantly elevated in a variety of tumor cells and are potentially correlated with survival prognosis23,24,25.
We evaluated the gene expression correlation between AKT and ALDOA in the TCGA dataset using the GEPIA platform, and the results showed a positive correlation between the expression of AKT and ALDOA in colon cancer and rectal cancer tissues. We then further evaluated the correlation between the IHC scores of ALDOA and p-AKT in 126 CRC tissues, and the results showed they were positively correlated in tumor tissues. Interestingly, in paired normal colorectal tissues, the expression of ALDOA and p-AKT were also positively correlated. Based on the above findings, we used ALDOA and p-AKT as factors to construct a CRC survival assessment model, and the results showed that the expression of ALDOA and p-AKT in the prediction model acted as a crucial role.
Conclusion
According to IHC staining score analysis, the expressions of ALDOA and p-AKT in CRC tissues were remarkably higher than those in adjacent tissues, and aberrant expressions of ALDOA/p-AKT led to poor prognosis in CRC patients. The expression of ALDOA in CRC was remarkably correlated with the expression of p-AKT. Kaplan–Meier analysis revealed that the 5-year survival rate of the group with negative ALDOA/p-AKT was better than that of the other groups. Besides, the both positive expression of ALDOA/p-AKT group had a lower 5-year survival rate compared with the other group. Moreover, the nomograms to predict the 3 and 5-year overall survival of CRC patients showed ALDOA/p-AKT expression played crucial roles in predicting the 3 and 5-year overall survival of CRC patients. Therefore, ALDOA/p-AKT may act as an important role in CRC, which may provide new horizons for targeted therapies.
Materials and methods
Patients and tissue specimens
This study included 126 cases of CRC tissue and para-cancer tissues obtained from 2015 to 2016 at the Department of General Surgery, the First Affiliated Hospital of Wannan Medical College. These CRC patients did not undergo preoperative chemoradiotherapy before surgery. The Surgical specimens were fixed in 10% formalin and embedded in paraffin. Everyone has complete clinical data and was available to be followed up. This study was approved by the Independent Ethics Committee (IEC) of the First Affiliated Hospital of Wannan Medical College (IRB number: 202248). All patients included were provided written informed consent. The demographic information of patients was listed in Table S1.
Immunohistochemistry (IHC)
These CRC tissues were fixed in 10% formalin and embedded in paraffin. Then the paraffin-embedded tissues were serially cut into 5 μm sections. The ALDOA and p-AKT expression in 126 cases of CRC tissue and para-cancer tissues were investigated using IHC. Sections were incubated with anti-ALDOA (1:100, Abcam, ab252953) and anti-Phospho-AKT (1:100, Abcam, ab81283) at 1:100 dilution at the room temperature for two hours. The whole process was visualized via the IHC staining kit (Zhongshan Biotechnology, Beijing, China).
IHC score assessment
After the IHC staining, five regions were randomly selected for evaluation by two authors. The IHC score was calculated by multiplying staining intensity (0, negative; 1, weak; 2, moderate; and 3, strong) and extent (0, 0–5%; 1, 6–25%; 2, 26–50%; 3, 51–75%; and 4, > 75%). The IHC scores of the five random fields are used to calculate the average, which is the final IHC score for the sample. For the final staining score, 0 was regarded as − ,1 ~ 4 as + , 5 ~ 8 as ++ , 9 ~ 12 as + + + . In this study, ++ or +++ was regarded as high expression, and—or + as low expression.
Statistical analysis
All the data was expressed as the means ± S.D. The statistical analyses were dealt with SPSS 25.0 software (SPSS Inc., Chicago, IL, USA), GraphPad Prism 8 and R programs (version 3.6.1 for Windows, http://cran.r-project.org/). The t-test (unpaired, two-tailed) or Mann–Whitney U test was used to compare means between groups. The Chi-square test was used to assess the IHC results. P < 0.05 was regarded significant difference. The authors have confirmed that all methods were carried out in accordance with relevant guidelines and regulations.
Data availability
Data are stored by the corresponding author of this paper and are available upon request.
References
Siegel, R. L., Wagle, N. S., Cercek, A., Smith, R. A. & Jemal, A. Colorectal cancer statistics, 2023. CA Cancer J. Clin. 73, 233–254. https://doi.org/10.3322/caac.21772 (2023).
Dekker, E., Tanis, P. J., Vleugels, J. L. A., Kasi, P. M. & Wallace, M. B. Colorectal cancer. Lancet 394, 1467–1480. https://doi.org/10.1016/S0140-6736(19)32319-0 (2019).
Bai, D. et al. ALDOA maintains NLRP3 inflammasome activation by controlling AMPK activation. Autophagy 18, 1673–1693. https://doi.org/10.1080/15548627.2021.1997051 (2022).
Lin, J. et al. The POU2F1-ALDOA axis promotes the proliferation and chemoresistance of colon cancer cells by enhancing glycolysis and the pentose phosphate pathway activity. Oncogene 41, 1024–1039. https://doi.org/10.1038/s41388-021-02148-y (2022).
Shen, Y. et al. LncRNA KCNQ1OT1 sponges miR-34c-5p to promote osteosarcoma growth via ALDOA enhanced aerobic glycolysis. Cell Death Dis. 11, 278. https://doi.org/10.1038/s41419-020-2485-1 (2020).
Kuang, Q. et al. The ALDOA metabolism pathway as a potential target for regulation of prostate cancer proliferation. Onco Targets Ther. 14, 3353–3366. https://doi.org/10.2147/OTT.S290284 (2021).
Sun, J. et al. A novel lncRNA ARST represses glioma progression by inhibiting ALDOA-mediated actin cytoskeleton integrity. J. Exp. Clin. Cancer Res. 40, 187. https://doi.org/10.1186/s13046-021-01977-9 (2021).
Zhang, F. et al. Elevated transcriptional levels of aldolase A (ALDOA) associates with cell cycle-related genes in patients with NSCLC and several solid tumors. BioData Min. 10, 6. https://doi.org/10.1186/s13040-016-0122-4 (2017).
Chen, H. et al. ALDOA inhibits cell cycle arrest induced by DNA damage via the ATM-PLK1 pathway in pancreatic cancer cells. Cancer Cell Int. 21, 514. https://doi.org/10.1186/s12935-021-02210-5 (2021).
Fu, H. et al. Aldolase A promotes proliferation and G(1)/S transition via the EGFR/MAPK pathway in non-small cell lung cancer. Cancer Commun. 38, 18. https://doi.org/10.1186/s40880-018-0290-3 (2018).
Wang, C. et al. Exosomes carrying ALDOA and ALDH3A1 from irradiated lung cancer cells enhance migration and invasion of recipients by accelerating glycolysis. Mol. Cell Biochem. 469, 77–87. https://doi.org/10.1007/s11010-020-03729-3 (2020).
Chen, L. et al. Initial clinical and experimental analyses of ALDOA in gastric cancer, as a novel prognostic biomarker and potential therapeutic target. Clin. Exp. Med. https://doi.org/10.1007/s10238-022-00952-8 (2022).
Lu, G., Shi, W. & Zhang, Y. Prognostic implications and immune infiltration analysis of ALDOA in lung adenocarcinoma. Front. Genet. 12, 721021. https://doi.org/10.3389/fgene.2021.721021 (2021).
Tang, Y., Yang, X., Feng, K., Hu, C. & Li, S. High expression of Aldolase A is associated with tumor progression and poor prognosis in hepatocellular carcinoma. J. Gastrointest. Oncol. 12, 174–183. https://doi.org/10.21037/jgo-20-534 (2021).
Gu, M. et al. Aldolase A promotes cell proliferation and Cisplatin resistance via the EGFR pathway in gastric cancer. Am. J. Transl. Res. 14, 6586–6595 (2022).
Fabregas, J. C., Ramnaraign, B. & George, T. J. Clinical updates for colon cancer care in 2022. Clin. Colorectal. Cancer 21, 198–203. https://doi.org/10.1016/j.clcc.2022.05.006 (2022).
Luo, G., Wang, R., Zhou, H. & Liu, X. ALDOA protects cardiomyocytes against H/R-induced apoptosis and oxidative stress by regulating the VEGF/Notch 1/Jagged 1 pathway. Mol. Cell Biochem. 476, 775–783. https://doi.org/10.1007/s11010-020-03943-z (2021).
Ji, S. et al. ALDOA functions as an oncogene in the highly metastatic pancreatic cancer. Cancer Lett. 374, 127–135. https://doi.org/10.1016/j.canlet.2016.01.054 (2016).
Li, M. et al. Aldolase is a sensor for both low and high glucose, linking to AMPK and mTORC1. Cell Res. 31, 478–481. https://doi.org/10.1038/s41422-020-00456-8 (2021).
Shorning, B. Y., Dass, M. S., Smalley, M. J. & Pearson, H. B. The PI3K-AKT-mTOR pathway and prostate cancer: At the crossroads of AR, MAPK, and WNT signaling. Int. J. Mol. Sci. https://doi.org/10.3390/ijms2112450 (2020).
Yao, Y. et al. GLI1 overexpression promotes gastric cancer cell proliferation and migration and induces drug resistance by combining with the AKT-mTOR pathway. Biomed. Pharmacother. 111, 993–1004. https://doi.org/10.1016/j.biopha.2019.01.018 (2019).
Wang, X., Yao, Y. & Zhu, X. The influence of aberrant expression of GLI1/p-S6K on colorectal cancer. Biochem. Biophys. Res. Commun. 503, 3198–3204. https://doi.org/10.1016/j.bbrc.2018.08.124 (2018).
Yao, Y. et al. Fatty acid 2-hydroxylation inhibits tumor growth and increases sensitivity to Cisplatin in gastric cancer. EBioMedicine 41, 256–267. https://doi.org/10.1016/j.ebiom.2019.01.066 (2019).
Paradiso, E. et al. Protein kinase B (Akt) blockade inhibits LH/hCG-mediated 17,20-lyase, but not 17alpha-hydroxylase activity of Cyp17a1 in mouse Leydig cell steroidogenesis. Cell. Signal. https://doi.org/10.1016/j.cellsig.2023.110872 (2023).
Sun, S. et al. Ubiquitinated CD36 sustains insulin-stimulated Akt activation by stabilizing insulin receptor substrate 1 in myotubes. J. Biol. Chem. 293, 2383–2394. https://doi.org/10.1074/jbc.M117.811471 (2018).
Acknowledgements
This study was supported by the Natural Science Research Project of Universities in Anhui Province (2022AH051248, KJ2021A0855), the Health Research Program of Anhui (AHWJ2023BAb20029), and the Nature and Science Fund from Wannan Medical College (WK2022ZF06).
Author information
Authors and Affiliations
Contributions
Menglin Xu, Shihang Xi and Haoran Li conducted the research, analyzed the data, and wrote the manuscript. Yong Xia and Guangliang Mei contributed to data collection and analysis. Zhengwu Cheng designed the study and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Xu, M., Xi, S., Li, H. et al. Prognosis significance and potential association between ALDOA and AKT expression in colorectal cancer. Sci Rep 14, 6488 (2024). https://doi.org/10.1038/s41598-024-57209-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-57209-5
Keywords
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.