Abstract
Colorectal carcinoma (CRC) is one of the most common malignancies with a dismal 5-year survival rate. Our recent study indicated that Rab1A expression was closely related to GLI1 expression. A previous study shows that aberrant overexpression of GLI1 promotes colorectal cancer metastasis via FoxM1 overexpression. However, the potential correlation between Rab1A and FoxM1 in CRC remains elusive. Immunohistochemistry was performed to investigate the association of the expression of Rab1A and FoxM1 and to determine the prognosis in 135 CRC tissue and adjacent normal tissues. Using Oncomine datasets, we found that Rab1A and FoxM1 mRNA were obviously upregulated in CRC tissues compared to normal tissues. Additionally, the expression of Rab1A and FoxM1 was significantly higher in CRC tissues than that in normal tissues. Rab1A expression was positively correlated with FoxM1 expression in CRC, especially in TNM stage III. In addition, Rab1A and FoxM1 overexpression was found to be significantly correlated with poor prognosis in CRC patients. Besides, both high expression of Rab1A and FoxM1 led to a worse prognosis than anyone low group, and both low expression of Rab1A and FoxM1 had a better prognosis than the anyone low group. Therefore, Rab1A and FoxM1 play crucial roles and could be used as clinical biomarkers in CRC.
Similar content being viewed by others
Introduction
Colorectal cancer (CRC) is a common malignant tumor of the digestive tract, which is associated with high morbidity and mortality worldwide1,2. Compared to that in other developed countries, CRC has a high incidence in China3. Recently, comprehensive treatment modalities, such as resection and adjuvant chemotherapy, have been widely used. However, the long-term prognosis in patients remains relatively unsatisfactory mainly due to metastasis and resistance to chemotherapy4,5. Thus, it is necessary to identify novel targets to improve treatment outcomes in patients with CRC.
Rab proteins have been identified as housekeeping proteins that regulate intracellular membrane dynamics6. As a member of the RAB family, Rab1A has been verified to mediate vesicular trafficking7. Recent studies reveal that Rab1A is involved in regulating signal transduction8, cell autophagy9. Previous studies indicated Rab1A acted as an oncogene and Rab1A overexpression promoted a worse prognosis in multiple cancer types such as gastric cancer10 and hepatocellular carcinoma11. In addition, Rab1A overexpression has been positively associated with proliferation, migration, and drug-resistance in various cancers12,13.
Forkhead box M1 (FoxM1) belongs to a large family of Forkhead transcription factors14. Recent studies indicate that FoxM1 plays a significant role in cell-cycle progression, where endogenous FoxM1 expression peaks at the S and G2/M phases15. In addition, FoxM1 overexpression has been linked with cancer progression and poor prognosis in several human cancers, suggesting that FoxM1 might play a crucial role in tumor initiation16,17.
A study shows that aberrant overexpression of GLI1 promotes the proliferation and migration of colon cancer cells through the transcriptional activation of FoxM118. Nowadays, our research revealed Rab1A expression was closely related with GLI1 expression in gastric cancer19. However, the potential correlation of Rab1A and FoxM1 in both expression and prognosis remains largely unknown in CRC. The aim of this study was to explore the clinicopathological features and the potential associations between Rab1A and FoxM1 and to determine how they can be used as novel targets in the individualized treatment of CRC.
Results
Expression levels of Rab1A and FoxM1 in gastrointestinal cancers
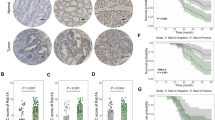
We determined the expression of Rab1A and FoxM1 in gastrointestinal cancers via the GEPIA Platform. These findings suggested that FoxM1 was significantly overexpressed in esophageal carcinoma (ESCA), stomach adenocarcinoma (STAD), colon adenocarcinoma (COAD), and rectal adenocarcinoma (READ) tumor tissues compared to paired normal tissues (P < 0.05) (Fig. 1A). Rab1A expression was moderately higher in ESCA, STAD, COAD, and READ tumor tissues compared to normal tissues; however, this difference was not significant (P > 0.05) (Fig. 1B). Pooled analysis of CRC and normal tissues across 9 Oncomine datasets indicated significant upregulation of FoxM1 mRNA in CRC tissues (P < 0.001) (Fig. 1C). Additionally, using 7 Oncomine datasets, we determined that Rab1A mRNA was obviously upregulated in CRC tissues compared to that in normal tissues (P = 0.006) (Fig. 1D).
Expression of Rab1A and FoxM1 in gastrointestinal cancers in the GEPIA Platform and Oncomine (https://www.oncomine.org). (A) Comparison of FoxM1 levels in gastrointestinal tumor and paired normal tissues. (B) Comparison of Rab1A levels in gastrointestinal tumor and paired normal tissues. (C) Comparison of FoxM1 mRNA expression in CRC and normal tissues across 9 Oncomine datasets. (D) Comparison of Rab1A mRNA expression in CRC and normal tissues across 7 Oncomine datasets. * P < 0.05.
Expression levels of Rab1A and FoxM1 in different TNM stages of CRC
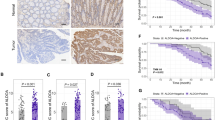
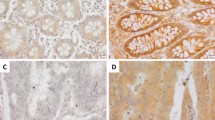
First, immunohistochemistry (IHC) staining was performed in 135 pairs of colorectal cancer and adjacent normal tissues to detect Rab1A and FoxM1 expression (Fig. 2A,B). We found that the expression of Rab1A and FoxM1 was significantly higher in colorectal cancer tissues than in normal tissues (P < 0.001, P < 0.001) (Fig. 2C,D). In addition, a subgroup analysis was performed to detect Rab1A and FoxM1 expression in different TNM stages. Our results revealed that Rab1A expression was remarkably higher in TNM stages II, III, and IV compared to that in TNM stage I (P < 0.01, P < 0.001, P < 0.001) (Fig. 2C). The levels of FoxM1 were significantly higher in TNM stages III and IV compared to that in TNM stage I (P < 0.01, P < 0.001) (Fig. 2D). There was no obvious difference in FoxM1 levels between TNM stages I and II (P > 0.05) (Fig. 2D).
Expression of Rab1A and FoxM1 in 135 pairs of CRC and normal tissues. (A,B) Immunohistochemistry (IHC) staining of Rab1A and FoxM1 in CRC tissues (200 ×) (scale bar = 100 µm). The protein expression of Rab1A was negative (a), weak (b), positive (c), strong positive (d). (C) Staining scores after analysis of Rab1A expression in CRC tissues and adjacent normal tissues (left), and IHC analysis of Rab1A expression in CRC tissues in TNM I stage and TNM II, III, IV stages in patients (right). (D) Staining scores after analysis of FoxM1 expression in CRC tissues and adjacent normal tissues (left), and IHC analysis of FoxM1 expression in CRC tissues in TNM I stage and TNM II, III, IV stages in patients (right). ** P < 0.01, ***P < 0.001.
The association between Rab1A and FoxM1 expression and clinicopathological parameters in patients with CRC
We investigated the association between Rab1A and FoxM1 expression and clinicopathological parameters, and the outcomes are shown in Table 1. The results showed that the expression of Rab1A and FoxM1 was obviously linked with several clinicopathological variables, such as venous invasion (P = 0.029) and TNM staging (P < 0.001). However, no obvious differences existed between Rab1A and FoxM1 expression and the left clinicopathological variables (P > 0.05).
Rab1A expression is positively related to FoxM1 expression in CRC
We first generated a heat map displaying Rab1A and FoxM1 expression detected in 135 CRC patients based on IHC analysis. Then, we compared the levels of Rab1A and FoxM1 detected in 135 CRC tissues and paired normal tissues in a scatter plot of IHC scores (Fig. 3A,B). The outcome suggested that no obvious association existed between Rab1A and FoxM1 expression in normal tissues (P = 0.834) (Fig. 3C). Interestingly, Rab1A expression was closely related to FoxM1 expression in tumor tissues (P < 0.001) (Fig. 3D), which was similar to the results in Table 2 (P < 0.001). Subgroup analysis of associations between Rab1A and FoxM1 expression was performed based on TNM staging. The outcome showed that Rab1A expression was positively associated with FoxM1 expression in TNM stage III (P < 0.001) (Fig. 3G), while no significant associations between Rab1A and FoxM1 expression were obtained in TNM stage I (P = 0.419) (Fig. 3E), stage II (P = 0.113) (Fig. 3F), and stage IV (P = 0.140) (Fig. 3H). According to the Rab1A and FOXM1 IHC score of human CRC and adjacent normal tissues, it was illustrated as a cluster analysis diagram to demonstrate the differences in expression of the two proteins in tumor and normal tissues (Fig. 4A). The tumor and normal samples were distributed in the PC1 and PC2 axes; the location of the dot was significantly different between tumor and normal tissues in the PC1 axis (P < 0.001) (Fig. 4A).
Analysis of the association between Rab1A and FoxM1 expression in 135 CRC tissues and paired normal tissues. (A,B) The association between Rab1A and FoxM1 expression in 135 CRC tissues and paired normal tissues is displayed as a heat map. Scatter plot analysis of the association between Rab1A and FoxM1 expression based on average IHC scores in normal tissues (C) and CRC tissues (D). Scatter plot analysis of the association between Rab1A and FoxM1 expression based on average IHC scores in TNM stage I (E), stage II (F), stage III (G), and stage IV (H).
Association between Rab1A and FoxM1 expression and the influence of Rab1A and FoxM1 expression on prognosis. (A) Cluster analysis of 135 pairs of colorectal cancer (green) and normal tissues (red) based on Rab1A and FoxM1 IHC scores. The x- and y-axes represent the two most informative principal coordinates (PCs) of the PCoA; marginal boxplots describe the distribution of those values for the different groups. Color legends represent the respective variables under analysis. The paired Wilcoxon rank-sum test was used to compare the PC1 or PC2 values between groups; the p values were displayed beside the edge box. Kaplan–Meier survival analysis was used for the analysis of Rab1A (B) and FoxM1 (C) expression in 135 CRC patients. (D) Survival analysis of patients with both high (BH) in Rab1A and FoxM1 expression, any one low (AL) in Rab1A and FoxM1 expression, and both low (BL) in Rab1A and FoxM1 expression. (E) Annual survival rates among the three groups of BH, BL, and AL.
The influence of Rab1A and FoxM1 overexpression in the prognosis of patients with CRC
We first investigated the influence of Rab1A and FoxM1 overexpression on overall survival (OS) using Kaplan–Meier analysis in patients with CRC. Our findings indicated that patients with higher Rab1A and FoxM1 expression had shorter survival time compared to those with lower expression of Rab1A and FoxM1 in CRC (P < 0.001, P < 0.001) (Fig. 4B,C). In addition, both high (BH) expression group of Rab1A and FoxM1 led to a worse prognosis than any one low (AL) expression group (P < 0.001) (Fig. 4D). Conversely, both low (BL) expression group of Rab1A and FoxM1 had a better prognosis than any one low (AL) group (P < 0.001) (Fig. 4D). The annual survival rates among the three groups are shown in Fig. 4E. In addition, clinicopathological parameters and Rab1A and FoxM1 expression were used to estimate the 3- and 5-year OS (Fig. 5). The nomogram shows each prognostic factor as a score via a point scale and to get the total score (Fig. 5). Results revealed that the expression of Rab1A and FOXM1, which played a significant role in predicting the prognosis in patients with CRC, ranked second. The BL expression group had the best prognosis while the BH expression group had the worst 3-year and 5-year survival rates compared to the other two groups.
Nomograms to predict survival of CRC patients. Points of each variable were obtained via a vertical line between each variable and the point scale. The predicted survival rate was correlated with the total points by drawing a vertical line from the total points scale to the overall survival. A, B and C of Rab1A and FOXM1 line represent both low, anyone high, both high.
Discussion
Rab1A is a small GTPase that regulates ER-to-Golgi vesicular transport20. Aberrant Rab1A expression has been linked to a range of human diseases such as Parkinson's disease and cardiomyopathy21,22. Previous studies report that Rab1A acts as an oncogene and is overexpressed in various types of cancers, including tongue squamous carcinoma23 and colorectal cancer24. In our study, we first explored Rab1A expression in CRC and paired normal tissues using 7 Oncomine datasets. Our results indicated that Rab1A mRNA was obviously upregulated in CRC tissues compared to the normal tissues. Besides, using IHC, we determined that Rab1A expression was significantly overexpressed in CRC tissues compared to normal tissues, which was consistent with the findings of a previous study25. Subgroup analysis showed that Rab1A expression was remarkably higher in TNM stages II, III, and IV compared to that in TNM stage I, which indicated that Rab1A expression might play a critical role in tumor initiation and development.
A study reports that Rab1A overexpression is positively related to clinicopathological parameters19,26. Our results indicated that Rab1A expression was obviously linked to the depth of tumor invasion, lymph node invasion, venous invasion, and TNM staging. Rab1A overexpression is positively correlated with poor prognosis in several gastrointestinal cancers, such as CRC6 and GC13. Consistent with these reports, our findings indicated that lower Rab1A expression indicated better prognosis in patients with CRC.
Our previous study indicates that Rab1A expression is closely related to GLI1 expression in gastric cancer 19. A recent study reported that GLI1 expression is closely associated with FoxM1 expression and that GLI1 expression elevation promotes colorectal cancer metastasis via FoxM1 overexpression27. Based on this perspective, the potential correlation of Rab1A and FoxM1 in expression and prognosis was explored in the current study. Pooled analysis of CRC and normal tissues across 9 Oncomine datasets revealed significant upregulation of FoxM1mRNA in the CRC tissues compared to that in normal tissues. In addition, using IHC analysis, the outcomes revealed that FoxM1 expression was significantly higher in CRC tissues than normal tissues, which was consistent with the results from previous studies28. In addition, we observed that FoxM1 expression was significantly higher in TNM stages III and IV compared to that in stage I, which was similar to the results of Rab1A expression level in vary TNM stage. Consistent with previous reports29, we found that increased expression levels of FoxM1 led to significantly worse overall survival in patients with CRC.
To investigate the potential correlation of Rab1A and FoxM1 in both expression and prognosis, first, we compared the levels of Rab1A and FoxM1 in a scatter plot of IHC scores. We found that Rab1A expression was closely related to FoxM1 expression in tumor tissues, whereas no obvious expression association in normal tissues was observed. Then, subgroup analysis of expression associations was performed based on different TNM stages. Results revealed that Rab1A expression was positively associated with FoxM1 expression in TNM stage III, indicating that Rab1A and FoxM1 might have promoted tumor progression. In addition, BH expression of Rab1A and FoxM1 led to a worse prognosis than AL expression, and BL expression had a better prognosis than AL expression. The above outcomes revealed that the overexpression of Rab1A and FoxM1 had a greater impact on OS than negative expression.
However, our study had some limitations. No in vitro or in vivo experiments were performed. To summarize, Rab1A and FoxM1 may be used together in predicting patient prognosis and may be potential novel targets for individualized therapy of CRC.
Conclusion
We used Oncomine datasets and showed that Rab1A and FoxM1mRNA were obviously upregulated in CRC tissues compared to that in normal tissues. Additionally, the expression of Rab1A and FoxM1 was obviously higher in CRC than in normal tissues. Rab1A expression was closely associated with FoxM1 expression in CRC, especially in TNM stage III. In addition, Rab1A and FoxM1 overexpression led to a worse prognosis in CRC patients. Besides, overexpression of both Rab1A and FoxM1 led to a worse prognosis than AL group, and BL expression of Rab1A and FoxM1 had a better prognosis than AL group. Thus, Rab1A and FoxM1 play crucial roles and could be used as clinical biomarkers of CRC.
Methods
Patients and tissue specimens
The Rab1A and FOXM1 expression in gastrointestinal cancers was obtained via the GEPIA Platform (https://gepia.cancer-pku.cn), an online analysis website for The Cancer Genome Atlas (TCGA) and Gene Tissue Expression (GTEX) databases. The expression of Rab1A and FOXM1 was obtained from the Oncomine platform (https://www.oncomine.org/resource/login.html). Data extraction and analysis were performed according to the instructions for authors stated on the website. CRC tissues were collected from 135 patients from the Department of General Surgery, Suzhou Municipal Hospital (Suzhou, China) from 2010–2013. None of these patients had received radiotherapy or chemotherapy before radical surgery. All tissue specimens were verified using histopathological studies. Clinical data were obtained from hospital records and all specimens were obtained after permission. The study was supported by the Independent Ethics Committee (IEC) of Suzhou Municipal Hospital and all patients provided written informed consent. After surgery, each patient was followed-up regularly, as described in our previous research19.
Immunohistochemistry (IHC)
IHC was performed to investigate the expression of Rab1A and FoxM1 in 135 CRC tissue samples and adjacent normal tissues. All analyses were performed in accordance with the relevant guidelines and regulations. Surgical specimens were fixed in 10% formalin, embedded in paraffin, cut into 5-μm sections, dewaxed, and then rehydrated. Next, the processed sections were blocked with 10% goat serum for 30 min and incubated with the polyclonal antibodies of Rab1A and FOXM1 at room temperature overnight. IHC was performed using a tissue staining kit (Zhongshan Biotechnology, Beijing, China) following the manufacturer’s protocol. Immunostaining was independently examined by two clinical pathologists who were blinded to the patient outcome. The staining score was calculated using Yang, K’s method28. The staining scores were as follows: 0 was deemed as (−),1–4 as (+), 5–8 as (++), and 9 –12 as (+++). In our current study, we classified all samples into a high expression group (++ or +++) or a low expression group (− or +) based on protein expression.
Statistical analysis
Data are expressed as the means ± S.E.M. All statistical analyses were performed using SPSS22.0 software (SPSS Inc, Chicago, IL, USA), GraphPad Prism 8 (San Diego, CA) and R programs. Student’s t-test (unpaired, two-tailed) or one-way ANOVA were used to compare means between groups. Survival duration was calculated using the Kaplan–Meier method and compared using the log-rank test. IHC results were analyzed using Chi-squared or Fisher’s exact tests. Self-developed R program (version 3.6.2 for Windows, https://cran.r-project.org/) was used for Cluster analysis and Nomogram analysis. A value of P < 0.05 was considered to indicate a statistically significant difference.
Consent for publication
All the individuals provided written informed consent prior to enrolling in the study, and the study was approved by the Ethics Committees of Suzhou Municipal Hospital.
Data availability
Data are stored by the corresponding author of this paper and are available upon request.
References
Siegel, R. L., Miller, K. D. & Jemal, A. Cancer statistics, 2019. CA Cancer J. Clin. 69, 7–34. https://doi.org/10.3322/caac.21551 (2019).
Brenner, H., Kloor, M. & Pox, C. P. Colorectal cancer. Lancet 383, 1490–1502. https://doi.org/10.1016/S0140-6736(13)61649-9 (2014).
Zhu, J. et al. Epidemiological trends in colorectal cancer in china: an ecological study. Dig. Dis. Sci. 62, 235–243. https://doi.org/10.1007/s10620-016-4362-4 (2017).
Anemone, A. et al. In vivo evaluation of tumour acidosis for assessing the early metabolic response and onset of resistance to dichloroacetate by using magnetic resonance pH imaging. Int. J. Oncol. 51, 498–506. https://doi.org/10.3892/ijo.2017.4029 (2017).
Tiwari, A., Saraf, S., Verma, A., Panda, P. K. & Jain, S. K. Novel targeting approaches and signaling pathways of colorectal cancer: An insight. World J. Gastroenterol. 24, 4428–4435. https://doi.org/10.3748/wjg.v24.i39.4428 (2018).
Thomas, J. D. et al. Rab1A is an mTORC1 activator and a colorectal oncogene. Cancer Cell 26, 754–769. https://doi.org/10.1016/j.ccell.2014.09.008 (2014).
Hutagalung, A. H. & Novick, P. J. Role of Rab GTPases in membrane traffic and cell physiology. Physiol. Rev. 91, 119–149. https://doi.org/10.1152/physrev.00059.2009 (2011).
Charng, W. L. et al. Drosophila Tempura, a novel protein prenyltransferase alpha subunit, regulates notch signaling via Rab1 and Rab11. PLoS Biol. 12, e1001777. https://doi.org/10.1371/journal.pbio.1001777 (2014).
Tanaka, M. et al. Hsc70 contributes to cancer cell survival by preventing Rab1A degradation under stress conditions. PLoS ONE 9, e96785. https://doi.org/10.1371/journal.pone.0096785 (2014).
Xu, B. et al. Significance and prognostic role of human epidermal growth factor receptor 2 and RAB1A expression in gastric cancer. Oncol. Lett. 15, 5185–5192. https://doi.org/10.3892/ol.2018.7992 (2018).
Xu, B. H. et al. Aberrant amino acid signaling promotes growth and metastasis of hepatocellular carcinomas through Rab1A-dependent activation of mTORC1 by Rab1A. Oncotarget 6, 20813–20828. https://doi.org/10.18632/oncotarget.5175 (2015).
Xu, H. et al. Inhibition of RAB1A suppresses epithelial-mesenchymal transition and proliferation of triple-negative breast cancer cells. Oncol. Rep. 37, 1619–1626. https://doi.org/10.3892/or.2017.5404 (2017).
Xu, M. et al. Expression analysis and implication of Rab1A in gastrointestinal relevant tumor. Sci. Rep. 9, 13384. https://doi.org/10.1038/s41598-019-49786-7 (2019).
Laoukili, J., Stahl, M. & Medema, R. H. FoxM1: at the crossroads of ageing and cancer. Biochim. Biophys. Acta 1775, 92–102. https://doi.org/10.1016/j.bbcan.2006.08.006 (2007).
Leung, T. W. et al. Over-expression of FoxM1 stimulates cyclin B1 expression. FEBS Lett. 507, 59–66. https://doi.org/10.1016/s0014-5793(01)02915-5 (2001).
Ahmad, A. et al. FoxM1 down-regulation leads to inhibition of proliferation, migration and invasion of breast cancer cells through the modulation of extra-cellular matrix degrading factors. Breast Cancer Res. Treat. 122, 337–346. https://doi.org/10.1007/s10549-009-0572-1 (2010).
Zhang, H. G. et al. Overexpression of forkhead box protein M1 (FOXM1) plays a critical role in colorectal cancer. Clin. Transl. Oncol. 18, 527–532. https://doi.org/10.1007/s12094-015-1400-1 (2016).
Wang, D. et al. Aberrant activation of hedgehog signaling promotes cell proliferation via the transcriptional activation of forkhead Box M1 in colorectal cancer cells. J. Exp. Clin. Cancer Res. CR 36, 23. https://doi.org/10.1186/s13046-017-0491-7 (2017).
Shao, X. et al. Prognosis, significance and positive correlation of Rab1A and p-S6K/Gli1 expression in gastric cancer. Anticancer Agents Med. Chem. 19, 1359–1367. https://doi.org/10.2174/1871520619666190416110851 (2019).
Zahraoui, A., Touchot, N., Chardin, P. & Tavitian, A. The human Rab genes encode a family of GTP-binding proteins related to yeast YPT1 and SEC4 products involved in secretion. J. Biol. Chem. 264, 12394–12401 (1989).
Coune, P. G., Bensadoun, J. C., Aebischer, P. & Schneider, B. L. Rab1A over-expression prevents Golgi apparatus fragmentation and partially corrects motor deficits in an alpha-synuclein based rat model of Parkinson’s disease. J. Parkinson’s Dis. 1, 373–387. https://doi.org/10.3233/JPD-2011-11058 (2011).
Wu, G. et al. Increased myocardial Rab GTPase expression: a consequence and cause of cardiomyopathy. Circ. Res. 89, 1130–1137. https://doi.org/10.1161/hh2401.100427 (2001).
Shimada, K. et al. Aberrant expression of RAB1A in human tongue cancer. Br. J. Cancer 92, 1915–1921. https://doi.org/10.1038/sj.bjc.6602594 (2005).
Cheng, Z. et al. Rab1A promotes proliferation and migration abilities via regulation of the HER2/AKT-independent mTOR/S6K1 pathway in colorectal cancer. Oncol Rep. 41, 2717–2728. https://doi.org/10.3892/or.2019.7071 (2019).
Wang, Z. K. et al. Aberrant expression of Rab1A and its prognostic significance in human colorectal cancer. Eur. Rev. Med. Pharmacol. Sci. 22, 4509–4517. https://doi.org/10.26355/eurrev_201807_15505 (2018).
Wang, X. et al. Expression of Rab1A is upregulated in human lung cancer and associated with tumor size and T stage. Aging 8, 2790–2798. https://doi.org/10.18632/aging.101087 (2016).
Zhang, C. et al. Gli1 promotes colorectal cancer metastasis in a Foxm1-dependent manner by activating EMT and PI3K-AKT signaling. Oncotarget 7, 86134–86147. https://doi.org/10.18632/oncotarget.13348 (2016).
Yang, K. et al. FOXM1 promotes the growth and metastasis of colorectal cancer via activation of beta-catenin signaling pathway. Cancer Manag. Res. 11, 3779–3790. https://doi.org/10.2147/CMAR.S185438 (2019).
Chu, X. Y. et al. FOXM1 expression correlates with tumor invasion and a poor prognosis of colorectal cancer. Acta Histochem. 114, 755–762. https://doi.org/10.1016/j.acthis.2012.01.002 (2012).
Acknowledgements
This study was supported by the Nature and Science Fund from Wannan Medical College, CN (Grant No. WK2019F07, WK2019F09), Nanjing Medical University Science and Technology development fund (NMUB2018231), Suzhou Special Project of Diagnosis and Treatment for key Clinical Disease (LCZX201814) and Suzhou Science and Technology Development Project (SYSD2018144).
Author information
Authors and Affiliations
Contributions
X.Y.S. and M.L.X. carried out literature research, experimental studies and data acquisition, participated in the study design and drafted the manuscript. H.R.L. and Z.R.Z. performed the clinic cases collection and carried out the data acquisition, participated in the study design and helped to draft the manuscript. M.L.X. participated in the design of the study and performed the statistical analyses. C.L.Z. and Z.W.C. proposed the study and participated in its design and helped to draft, and assisted writing the manuscript. All authors had already read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Xu, M., Shao, X., Li, H. et al. Clinical value and potential association of Rab1A and FoxM1 aberrant expression in colorectal cancer. Sci Rep 10, 20160 (2020). https://doi.org/10.1038/s41598-020-77182-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-77182-z
This article is cited by
-
Prognosis value and positive association of Rab1A/IL4Rα aberrant expression in gastric cancer
Scientific Reports (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.