Abstract
Gastric ulcers are a type of digestive disease that can severely affect a person's quality of life. Our study aimed to investigate the effects of fish oil on ethanol-induced gastric ulcers in rats, with the purpose of providing more comprehensive information on the topic. The study looked at various factors such as gastric ulcer index, and nitric oxide (NO) levels in stomach tissue. To investigate apoptosis, the mRNA levels of Bax, Bcl-2, and Caspase 3 were analyzed. The results showed that fish oil can reduce gastric acidity and the gastric ulcer index in cases of ethanol-induced gastric ulcers. It was found that fish oil can increase NO levels and improve the anti-apoptotic system by increasing the expression of Bcl-2 while decreasing the expression of Bax and Caspase 3. In general, the study demonstrates that fish oil can protect the stomach from ethanol-induced damage by reducing the apoptosis pathway via nitric oxide.
Similar content being viewed by others
Introduction
Fish oil is rich in long-chain n-3 polyunsaturated fatty acids (PUFAs) like eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA)1. PUFAs serve as the building blocks for eicosanoids, a group of signaling molecules that regulate various physiological processes. Among PUFAs, arachidonic acid (20:4, n-6) is the primary precursor for eicosanoid synthesis. Enzymatic transformation of arachidonic acid can lead to the production of prostaglandins or leukotrienes with two or four double bonds, respectively, which are known to have pro-inflammatory effects. On the other hand, eicosanoids synthesized from n-3 fatty acids contain three and five double bonds, respectively, and are less biologically active than those derived from n-6 fatty acids2. The consumption of fish oil supplements can reduce the production of inflammatory cytokines like interleukin-1 (IL-1) and tumor necrosis factor (TNF)3. Leukocyte-produced reactive oxygen species (ROS) can be reduced by n-3 fatty acids4. EPA and DHA are essential for the production of resolvins, which aid in the resolution of inflammation5. Additionally, long-chain n-3 fatty acids have anti-apoptotic properties6.
Fish oil derived from Scomberoides commersonianus reduced the injury severity in the gastric ulcer model induced by cold stress (CRS), aspirin, alcohol, and pyloric ligation. The fish oil has increased the activity of antioxidant enzymes (glutathione peroxidase and catalase) and decreased lipid peroxidation in the gastric mucosa of mice under CRS7. The various chemical drugs used to treat peptic ulcers. However, no life-threatening effects have been reported with antiulcer medications. However, many side effects, including constipation, abdominal pain, indigestion, bloating, diarrhea, nausea, and vomiting have been reported8. Studying compounds of animal and plant origin with fewer side effects is necessary for the treatment of gastrointestinal ulcers9.
Stomach damage is a common disorder in humans and animals. The breakdown of the gastric mucosal barrier is the leading cause of gastritis and gastric ulcers. Stomach foreign bodies and non-observance of diet are common causes of acute gastritis in humans and small animals including, dogs and cats10. One of the most widely used methods for inducing gastric damage in laboratory animals is ethanol, which is similar to acute damage in the stomach11. The ethanol digests the mucous layer and exposes the mucosa to the proteolysis activities of pepsin12.
Nitric oxide (NO) is a molecule with diverse functions in biology, having both positive and negative effects. Its biological effects are complex due to its interactions with other molecules like reactive oxygen species (ROS), metal ions, and proteins. The effects of NO can be affected by multiple factors such as the type of cell and the dosage13. NO can help to protect the gastric ulcer mucosal lesions. The effects of NO may be realized by its anti-angiogenic, anti-inflammatory and anti-apoptotic effects14.
The cell response to an apoptotic stimulus depends on the balance between anti-apoptotic proteins (Bcl-2 and Bcl-xl) and pro-apoptotic proteins (Bax, Bad, and Bac)15.
The NO has anti-apoptotic effects, which it does through various mechanisms, such as inactivation of many caspases, including caspase 3, as well as upregulation of Bcl-2 and Bcl-XL16. So far, there is no known information about the relationship between nitric oxide and apoptosis in the gastric ulcer model induced by ethanol. Our study intends to examine the effect of fish oil on nitric oxide levels and the changes in genes related to the apoptosis pathway in rats with ethanol-induced gastric ulcers.
Material and methods
Animals
Wistar rats (240–260 g) were obtained. The animals were kept in plexiglass cages with a 12-h light and 12-h dark cycle at 23 ± 2 °C. The rats were fed rat chow and provided water ad libitum. In the following reported experiments on live vertebrates and methods used all relevant guidelines and regulations, particularly ARRIVE guidelines27, have been fully considered and noticed17. Furthermore, the protocol of the current study was approved by the Research Ethics Committee of the Faculty of Veterinary Medicine, Shahid Chamran University of Ahvaz, Ahvaz, Iran (EE/1401.2.24.226074/scu.ac.ir).
Experimental design
The rats in this experiment were divided into five groups, each with six rats. In the treatment groups, fish oil was administered orally at either 5 or 10 ml/kg BW18 (Sigma-Aldrich CAS Number: 8002-50-4) for 14 days before the administration of ethanol19. The positive control group received omeprazole at 20 mg/kg19, while the ethanol group was given sunflower oil for 14 days before the administration of ethanol. The control group received only sunflower oil. On the 14th day, 90 min after each ethanol administration, the rats were sacrificed and their gastric tissues were harvested as shown in Fig. 1.
Macroscopic assessment
The stomachs were removed from the greater curvature and rinsed with normal saline. The ulcer size was measured with a vernier caliper and the percentage of inhibition was calculated. Ulcer inhibition% = (GUI in the model − GUI in test) × 100/GUI in model.
Measurement NO levels
The nitric oxide levels in the gastric mucosa were measured following the NO kit instructions (NO, Kiazist, Hamedan, Iran cat#KNO96). Thus, after homogenizing the tissue, the amount of protein was calculated according to the Bradford method. Then, NO was measured according to the instructions of the manufacturer of the kit.
Real-time PCR
Real-time PCR was performed for Bax, Bcl2, and caspase-3. The mRNA was prepared using Total RNA Extraction Kit (Parstous, Iran). The cDNA from total RNA with a cDNA synthesis kit (Parstous, Iran) was synthesized. All primers’ sequences for qPCR listed in Table 1. The quantitative real-time PCR was performed using real-time PCR Master Mix (SYBR® Green-Parstous, Iran). The data was calculated through the 2−△△CT method with GAPDH as an endogenous reference.
Statistical analysis
We analyzed the data using SPSS software version 16. First, we checked the data for normal distribution and homogeneity of variances using the Kolmogorov–Smirnov test in SPSS. Since the data met the normality test, we performed a one-way analysis of variance (ANOVA) to compare the groups. We used Tukey test for post-hoc analyses. We analyzed ulcer index data using the Kruskal–Wallis test. *p < 0.05, **p < 0.01 and ***p < 0.001 were considered statistically significant.
Results
Effect of fish oil pre-treatment on macroscopic inspection of gastric mucosa: Gastric Ulcer Index (GUI)
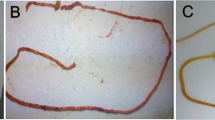
Based on the findings of this study, it was discovered that the average wound size (measured in mm2) was significantly greater in the ethanol, fish oil 5 ml/kg, fish oil 10 ml/kg, and omeprazole 20 mg/kg groups compared to the control group (p < 0.001, p < 0.01, p < 0.001, and p < 0.01). On the other hand, the average wound size in mm2 was significantly lower in the 5 ml/kg fish oil, 10 ml/kg fish oil, and 20 mg/kg omeprazole groups when compared to the ethanol group (p < 0.05) (Figs. 2A and 3).
Effect of fish oil on (A) Gastric ulcer index (B) Ulcer inhibition in ethanol-induced gastric mucosa injury. (1) Control, (2) Ethanol (Eth), (3) Fish oil 5 ml/kg, (4) Fish oil 10 ml/kg, and (5) Omeprazole 20 mg/kg. ##p < 0.05 and ###p < 0.01 when compared with the control group. *p < 0.05 when compared with the Ethanol (Eth) group.
In the study, it was found that the groups given ethanol, fish oil 5 ml/kg, fish oil 10 ml/kg, and omeprazole 20 mg/kg had a significantly lower percentage of ulcer inhibition compared to the control group (p < 0.001, p < 0.001, p < 0.01, p < 0.001). On the other hand, the groups given 5 ml/kg fish oil, 10 ml/kg fish oil, and 20 mg/kg omeprazole had a significantly higher percentage of ulcer inhibition compared to the ethanol group (p < 0/05) (Figs. 2B and 3).
Effect of fish oil pre-treatment on gastric mucosal NO levels
The groups administered with ethanol, fish oil at 5 ml/kg, fish oil at 10 ml/kg, and omeprazole at 20 mg/kg demonstrated significantly lower NO levels compared to the control group (with p-values of less than 0.001, 0.01, 0.01, and 0.01). On the other hand, NO levels were significantly higher in the 5 ml/kg fish oil, 10 ml/kg fish oil, and 20 mg/kg omeprazole groups compared to the ethanol group (with p-values of less than 0.001, less than 0.001, and less than 0.001) (Fig. 4).
Effect of fish oil pre-treatment on gastric mucosal apoptotic genes expressions
The groups treated with ethanol and fish oil 5 ml/kg showed a significant increase in the mRNA expression of Bax compared to the control group (p < 0.001 and p < 0.001). The mRNA expression level of Bax in the groups treated with fish oil 10 ml/kg and omeprazole 20 mg/kg did not differ significantly from the control group. However, the groups treated with 5 ml/kg fish oil, 10 ml/kg fish oil, and 20 mg/kg omeprazole showed a significant decrease in the mRNA expression of Bax compared to the group treated with ethanol (p < 0.01, p < 0.001, and p < 0.001) (Fig. 5A).
The ethanol group showed significantly lower Bcl-2 mRNA expression than the control group (p < 0.001). The 5 and 10 ml/kg fish oil and 20 mg/kg omeprazole groups did not exhibit any significant differences in the level of Bcl-2 mRNA expression compared to the control group. However, the mRNA expression of Bcl-2 in the 5 ml/kg fish oil, 10 ml/kg fish oil, and 20 mg/kg omeprazole groups was significantly higher than that of the ethanol group (p < 0.01, p < 0.001, and p < 0.001) as shown in Fig. 5B.
The amount of Caspase 3 mRNA expression was found to be significantly higher in the groups treated with ethanol and fish oil at doses of 5 and 10 ml/kg, compared to the control group (p < 0.001, p < 0.01 and p < 0.05). However, in the group treated with omeprazole at a dose of 20 mg/kg, there was no significant difference in the level of Caspase 3 mRNA expression when compared to the control group. Interestingly, the groups treated with 5 and 10 ml/kg of fish oil and 20 mg/kg of omeprazole had significantly lower levels of Caspase 3 mRNA expression when compared to the ethanol group (p < 0.05, p < 0.01 and p < 0.001) (Fig. 5C).
Discussion
In recent decades, numerous epidemiological studies have investigated the abundant health benefits of omega-3 PUFAs. Numerous clinical studies have demonstrated the advantages of fish oil20. Research has found that fish oil can help prevent damage to the stomach lining caused by stress, certain drugs, and harmful substances. When given at a dosage of 5 or 10 ml/kg of body weight, fish oil has been demonstrated to provide substantial protection in various experimental models. It can reduce damage caused by ulcers from pyloric ligation, indomethacin, aspirin, reserpine, or hypothermic restraint. Additionally, fish oil has been found to have a significant inhibitory effect on gastric mucosal lesions caused by different necrotizing agents7,21.
The ethanol-stimulated gastric ulcer model has been widely used to investigate the gastroprotective effect of various drugs and natural products19,22,23,24. In the current study, we observed that the damage caused by ethanol was severe in the group treated with ethanol.
NO mediates critical physiological functions in the body, including the digestive system. The NO plays a role in the digestive system in regulating mucosal blood flow and maintaining the integrity of the mucosa. The NO also stimulates mucus secretion in gastric mucosal cells25. The NO plays a vital role in the defense of the gastrointestinal tract in pathological conditions26. The studies have shown that NO has protects against NSAID-induced gastric ulcers27. Ethanol induces hypersecretion of gastric acid, proinflammatory cytokines, and ROS. These factors function together to induce apoptosis and reduce the levels of NO28,29,30. Consistent with previous studies in our study, ethanol decreased NO content. The process by which different doses of ethanol reduces nitric oxide levels in the gastric tissue has not been fully understood. However, the reduction of NO is likely not caused by ethanol's effect on NO biosynthesis, but rather by its impact on the nitric oxide derived from the gastric tissue, ultimately leading to a decrease in NO31. However, like to omeprazole, fish oil 5 and 10 ml/kg significantly increased NO levels compared to the control group.
Evidence has shown that mitochondrial damage during apoptosis is closely related to Bax and Caspase-3 genes, which disrupt the integrity of gastric mucosa after ethanol administration32,33,34. The oxidative stress caused by ethanol causes the accumulation of free radicals derived from oxygen in the wound areas, the resistance of the antioxidant defense system decreases, and the intrinsic pathway of apoptosis is activated. In the intrinsic pathway of apoptosis, the activation of Bax causes the release of other pro-apoptotic factors and the formation of apoptosomes stimulated, and finally, caspase-3 is activated35,36. The current study showed that fish oil 5 and 10 ml/kg pretreatment decreased the expression of Bax and Caspase-3 in ethanol-induced rats. Also, fish oil 5 and 10 ml/kg pretreatment increases the expression of Bcl-2 in ethanol-induced rats. Similar effects observed for omeprazole.
The beneficial role of fish oil in gastric ulcers has been shown by inhibiting the invading mucosal factors and strengthening the defensive mucosal factors7,37. Fish oil increase the activity of the l-arginine-NO pathway and NO synthase (NOS) expression38. Fish oil supplementation increases NO and reduce inflammation, and, thus that can improve antioxidant capacity39. NO has a crucial role in the mechanism of gastric mucosal protection and injury induced by ethanol40. Its apoptotic and anti-apoptotic response depends on the cell type. NO activates apoptosis in many types of cells, including pancreatic islets, macrophages, thymocytes, and neurons. However, NO shows anti-apoptotic properties in gastric mucosa. Several anti-apoptotic mechanisms have demonstrated for NO13. Once apoptosis is triggered, proenzymes are cleaved into subunits that rearrange to form active cysteine proteases, activating caspases. Initiator caspases enhance the apoptotic signal by activating other executor caspases. In this work, the substrate used primarily measures the activity of executor caspases, such as caspase 3. A significant aspect of apoptosis is the condensation of chromatin and the specific cleavage of double-stranded DNA. This process involves the caspase-dependent removal of an inhibitory subunit from a DNAase, which is then transferred to the nucleus41. The NO inhibits caspase activation in both death-ligand-dependent and apoptosis-ligand-dependent ways. It has stated that the regulation of caspase activation by NO causes changes in the downstream genes, namely Bcl-2 and Bax. The NO prevented the reduction of Bcl-2 protein expression and its mRNA levels and also prevented the increase of Bax13.
Conclusion
Our study showed that the administration of fish oil has a protective role against gastric damage caused by oral ethanol administration. The observed effects are probably due to the change in NO levels in the gastric tissue. As a result, the expression of genes related to apoptosis decreases, and the expression of anti-apoptotic genes increases.
Data availability
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.
References
Harris, W. S., Pottala, J. V., Sands, S. A. & Jones, P. G. Comparison of the effects of fish and fish-oil capsules on the n 3 fatty acid content of blood cells and plasma phospholipids. Am. J. Clin. Nutr. 86, 1621–1625 (2007).
Swanson, D., Block, R. & Mousa, S. A. Omega-3 fatty acids EPA and DHA: Health benefits throughout life. Adv. Nutr. (Bethesda, Md) 3, 1–7 (2012).
Ramirez-Ramirez, V. et al. Efficacy of fish oil on serum of TNF α, IL-1 β, and IL-6 oxidative stress markers in multiple sclerosis treated with interferon beta-1b. Oxid. Med. Cell. Longev. 2013, 709493 (2013).
Yang, J. et al. Oxidative stress and non-alcoholic fatty liver disease: Effects of omega-3 fatty acid supplementation. Nutrients. 11, 872–920 (2019).
Troesch, B. et al. Expert opinion on benefits of long-chain omega-3 fatty acids (DHA and EPA) in aging and clinical nutrition. Nutrients 12, 2255–2280 (2020).
Li, K. et al. N-3 polyunsaturated fatty acids effectively protect against neural tube defects in diabetic mice induced by streptozotocin. Food Funct. 12, 9188–9196 (2021).
Bhattacharya, A., Ghosal, S. & Bhattacharya, S. K. Effect of fish oil on offensive and defensive factors in gastric ulceration in rats. Prostaglandins Leukot Essent Fatty Acids 74, 109–116 (2006).
Henry, D. A. & Langman, M. J. S. Adverse effects of anti-ulcer drugs. Drugs 21, 444–459 (1981).
Kuna, L. et al. Peptic ulcer disease: A brief review of conventional therapy and herbal treatment options. J. Clin. Med. 8, 179–198 (2019).
Webb, C. & Twedt, D. C. Canine gastritis. Vet. Clin. N. Small Anim. Pract. 33(969–985), v–vi (2003).
Lahiri, S. & Palit, G. An overview of the current methodologies used for evaluation of gastric and duodenal anti-ulcer agents. Pharmacologia 3, 249–257 (2012).
Adinortey, M., Ansah, C., Galyuon, I., and Nyarko, A. (2013). In vivo models used for evaluation of potential antigastroduodenal ulcer agents. Ulcers 2013.
Kim, P. K., Zamora, R., Petrosko, P. & Billiar, T. R. The regulatory role of nitric oxide in apoptosis. Int. Immunopharmacol. 1, 1421–1441 (2001).
Liang, T. Y., Deng, R. M., Li, X., Xu, X. & Chen, G. The role of nitric oxide in peptic ulcer: A narrative review. Med. Gas Res. 11, 42–45 (2021).
Li, Y. et al. Tert-butylhydroquinone attenuates scrotal heat-induced damage by regulating Nrf2-antioxidant system in the mouse testis. Gen. Comp. Endocrinol. 208, 12–20 (2014).
Snyder, C. M., Shroff, E. H., Liu, J. & Chandel, N. S. Nitric oxide induces cell death by regulating anti-apoptotic BCL-2 family members. PLOS ONE 4, e7059 (2009).
Percie du Sert, N. et al. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. J. Cereb. Blood Flow Metab. 40, 1769–1777 (2020).
Al-Harbi, M. M., Islam, M. W., Al-Shabanah, O. A. & Al-Gharably, N. M. Effect of acute administration of fish oil (omega-3 marine triglyceride) on gastric ulceration and secretion induced by various ulcerogenic and necrotizing agents in rats. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 33, 553–558 (1995).
Rahimi, K., Shirvani, N., Sanaie, P., Javadi, A. & Khademi, M. The effects of alpha-pinene on the Nrf2-HO1 signaling pathway in gastric damage in rats. Mol. Biol. Rep. 50, 8615–8622 (2023).
Shahidi, F. & Ambigaipalan, P. Omega-3 polyunsaturated fatty acids and their health benefits. Annu. Rev. Food Sci. Technol. 9, 345–381 (2018).
Al-Harbi, M. M., Islam, M. W., Al-Shabanah, O. A. & Al-Gharably, N. M. Effect of acute administration of fish oil (omega-3 marine triglyceride) on gastric ulceration and secretion induced by various ulcerogenic and necrotizing agents in rats. Food Chem. Toxicol. 33, 553–558 (1995).
Simões, S. et al. Animal models of acute gastric mucosal injury: Macroscopic and microscopic evaluation. Anim. Models Exp. Med. 2, 121–126 (2019).
Aziz, R. S., Siddiqua, A., Shahzad, M., Shabbir, A. & Naseem, N. Oxyresveratrol ameliorates ethanol-induced gastric ulcer via downregulation of IL-6, TNF-α, NF-ĸB, and COX-2 levels, and upregulation of TFF-2 levels. Biomed. Pharmacother. 110, 554–560 (2019).
Sanpinit, S., Chonsut, P., Punsawad, C. & Wetchakul, P. Gastroprotective and antioxidative effects of the traditional Thai polyherbal formula Phy-Blica-D against ethanol-induced gastric ulcers in rats. Nutrients 14, 172–188 (2021).
Moncada, S. & Higgs, A. The l-arginine-nitric oxide pathway. N. Engl. J. Med. 329, 2002–2012 (1993).
Wallace, J. L. & Miller, M. J. Nitric oxide in mucosal defense: A little goes a long way. Gastroenterology 119, 512–520 (2000).
Santos, C. L. et al. Sildenafil prevents indomethacin-induced gastropathy in rats: Role of leukocyte adherence and gastric blood flow. Br. J. Pharmacol. 146, 481–486 (2005).
Laloo, D., Prasad, S. K., Krishnamurthy, S. & Hemalatha, S. Gastroprotective activity of ethanolic root extract of Potentilla fulgens Wall. ex Hook. J. Ethnopharmacol. 146, 505–514 (2013).
Antonisamy, P. et al. Gastroprotective effect of nymphayol isolated from Nymphaea stellata (Willd.) flowers: Contribution of antioxidant, anti-inflammatory and anti-apoptotic activities. Chem.-Biol. Interact. 224, 157–163 (2014).
Albaayit, S.F.A., Abba, Y., Abdullah, R. & Abdullah, N. Prophylactic effects of Clausena excavata Burum. f. leaf extract in ethanol-induced gastric ulcers. Drug Design Develop. Ther. 10, 1973–1986 (2016).
Yoshimura, T. & Sugata, H. Alcohol scavenges nitric oxide in gastric lumen. Nitric Oxide Biol. Chem. 6, 347–352 (2002).
Haibo, H. et al. Gastroprotective effect of araloside A on ethanol-and aspirin-induced gastric ulcer in mice: Involvement of H+/K+-ATPase and mitochondrial-mediated signaling pathway. J. Nat. Med./Japan. Soc. Pharmacognosy 73, 339–352 (2019).
Chen, P. et al. Gastroprotective effects of Kangfuxin-against ethanol-induced gastric ulcer via attenuating oxidative stress and ER stress in mice. Chem.-Biol. Interact. 260, 75–83 (2016).
Liu, W., Yang, M., Chen, X., Li, L., Zhou, A., Chen, S., You, P., & Liu, Y. (2018). Mechanisms of antiulcer effect of an active ingredient group of modified Xiao Chaihu decoction. Evid.-Based Complement. Alternat. Med. 2018.
Li, H. et al. Paeonol and danshensu combination attenuates apoptosis in myocardial infarcted rats by inhibiting oxidative stress: Roles of Nrf2/HO-1 and PI3K/Akt pathway. Sci. Rep. 6, 23693 (2016).
Li, H. et al. Cardioprotective effect of paeonol and danshensu combination on isoproterenol-induced myocardial injury in rats. PloS One 7, e48872 (2012).
Park, J. M. et al. Omega-3 polyunsaturated fatty acids as an angelus custos to rescue patients from NSAID-induced gastroduodenal damage. J. Gastroenterol. 50, 614–625 (2015).
Martins, M. A. et al. Role of dietary fish oil on nitric oxide synthase activity and oxidative status in mice red blood cells. Food Funct. 5, 3208–3215 (2014).
Zekri Kondalaji, R., Sarisarraf, V. & Nourshahi, M. Investigating the effect of 4-week fish oil supplementation on inflammation and plasma nitric oxide and reactive oxygen species in response to exhaustive exercise. J. Sport Exercise Physiol. 13, 16–26 (2020).
Tepperman, B. L. & Soper, B. D. Nitric oxide synthase induction and cytoprotection of rat gastric mucosa from injury by ethanol. Can. J. Physiol. Pharmacol. 72, 1308–1312 (1994).
Wilson, M. R. Apoptotic signal transduction: emerging pathways. Biochem. Cell Biol. Biochimie et Biologie Cellulaire. 76, 573–582 (1998).
Acknowledgements
We are grateful to the Research Council of Shahid Chamran University of Ahvaz for financial support (GN: SCU.VB1401.50857).
Author information
Authors and Affiliations
Contributions
K.R was the supervisor of this study. "N.P, K.R, Z.GH and MR.T collaborated in designing the study, conducting experiments, statistical analysis and writing the article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Parham, N., Rahimi, K., Ghotbeddin, Z. et al. Fish oil ameliorates ethanol-induced gastric injury in rat by modulating gene related to apoptosis. Sci Rep 14, 6193 (2024). https://doi.org/10.1038/s41598-024-56647-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-56647-5
Keywords
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.