Abstract
Cardiovascular disease (CVD) is a collective term for disorders of the heart and blood vessels. The molecular events and biochemical pathways associated with CVD are difficult to study in clinical settings on patients and in vitro conditions. Animal models play a pivotal and indispensable role in CVD research. Caenorhabditis elegans, a nematode species, has emerged as a prominent experimental organism widely utilized in various biomedical research fields. However, the specific number of CVD-related genes and pathways within the C. elegans genome remains undisclosed to date, limiting its in-depth utilization for investigations. In the present study, we conducted a comprehensive analysis of genes and pathways related to CVD within the genomes of humans and C. elegans through a systematic bioinformatic approach. A total of 1113 genes in C. elegans orthologous to the most significant CVD-related genes in humans were identified, and the GO terms and pathways were compared to study the pathways that are conserved between the two species. In order to infer the functions of CVD-related orthologous genes in C. elegans, a PPI network was constructed. Orthologous gene PPI network analysis results reveal the hubs and important KRs: pmk-1, daf-21, gpb-1, crh-1, enpl-1, eef-1G, acdh-8, hif-1, pmk-2, and aha-1 in C. elegans. Modules were identified for determining the role of the orthologous genes at various levels in the created network. We also identified 9 commonly enriched pathways between humans and C. elegans linked with CVDs that include autophagy (animal), the ErbB signaling pathway, the FoxO signaling pathway, the MAPK signaling pathway, ABC transporters, the biosynthesis of unsaturated fatty acids, fatty acid metabolism, glutathione metabolism, and metabolic pathways. This study provides the first systematic genomic approach to explore the CVD-associated genes and pathways that are present in C. elegans, supporting the use of C. elegans as a prominent animal model organism for cardiovascular diseases.
Similar content being viewed by others
Introduction
Cardiovascular Disease (CVD) is a collective term for disorders of heart and blood vessels that include coronary heart disease, hypertension, and heart failure along with other heart and vascular system-related diseases such as atherosclerosis and deep venous thrombosis. CVDs are the leading cause of global mortality1 with 17.9 million deaths each year2 which is expected to rise to 23.6 million by 20303, according to the reports of WHO four out of five CVD patients die because of heart attack or myocardial infarction every year4. CVD is influenced by a multitude of risk factors, prominent among these are lifestyle factors such as being overweight, having high blood sugar, increased blood pressure, smoking, and unhealthy lifestyles along with genetic risk factors such as a family history of CVD that contribute to the development of CVD5. A thorough understanding of the pathophysiology and pathological manifestations of CVD is required for developing efficient treatment strategies or preventative measures and lowering the rate of incidence and morbidity.
The molecular events and biochemical pathways associated with CVD are difficult to study in clinical settings on patients and in vitro conditions. In vitro, studies don’t give such complete information on the pathogenesis of organ system level6. Thus, many aspects of human CVD remain poorly understood. Since cellular metabolism consists of an integrated network of biochemical pathways, the precise understanding of the disease-related genes within those pathways is crucial for understanding the disease. A better understanding of interacting partners is a key aspect of translational research to develop a therapeutic strategy7. Therefore, to better understand the molecular and biochemical pathways it is crucial to understand changes in the molecular events causing CVD by employing animal models.
Animal models play a very essential role in the research area of CVD. For example, Neuberger et al. studied chronic atrial dilation and atrial fibrillation using a goat model8. Human atherosclerosis is studied using rabbits and pigs9,10. Similarly, Canine, primate, and rodent models have also been employed to study CVD11. However, mammalian animal model systems have their limitations of high experimental cost, ethical issues, limited genomic tools available, complicated operation, and high reproductive environment demands despite having genomic relatedness12.
The nematode Caenorhabditis elegans (C. elegans)13 has been widely employed in various fields of developmental and evolutionary biology, genetics, and complicated disease studies14,15,16,17. The genomic comparison of human and C elegans describes that the majority of human disease genes and pathways are found in C. elegans18 The percentage of human disease-related genes that have at least minor similarity (E < 10 − 10 on BLASTP searches) with C. elegans genes ranges between 40 and 75%19,20,21,22,23,24. Comparative proteomic analysis of 18,452 C. elegans protein sequences revealed that human gene homologs exist for ~ 83% of the C. elegans proteome25. Moreover, C. elegans homologs were found to exist for ~ 60–80% of human protein-coding genes23,25,26,27. Furthermore, even if a human disease-related gene lacks an orthologue in C. elegans, there is a high probability that a homologous gene, a protein domain, or the components of a related biological pathway are the constituents of an (especially, if it encompasses a core cellular function like signal transduction, synaptic transmission, or membrane trafficking) are conserved to the extent that this model system can be used to gain insight into human pathobiology28,29.
C. elegans is the first multicellular organism whose genome has been completely sequenced and thoroughly analyzed18,30. It possesses many lipid-binding proteins and transporters31 along with biosynthesis of polyunsaturated fatty acids32, ceramides33, and phospholipids34,35,36,37,38. The body wall muscle cells of C. elegans are useful for the study of human cardiomyocytes and their homologous structures and proteins39. The nematode C elegans continues to be an excellent model for studying the organization, assembly, and maintenance of the sarcomere39,40. The major striated muscle of C elegans is found in the body wall and is required for the animal’s locomotion. Nevertheless, previous studies have proven that the close homology of proteins and structures of interest justify the study of nematode body wall muscle as a way to understand human heart muscle39. It was pertinent, therefore, to understand the possibilities of using C elegans as a model for studying cardiovascular disease. However, how many CVD-related genes and pathways are present in the C. elegans genome is not known to date. Similarly, for its utilization as an animal model for CVD, the genomic and functional conservation of pathways and genes from C. elegans to humans is unknown thus, limiting its in-depth utilization for investigations.
In the present study, we analyzed the datasets related to human CVD to obtain DEGs which were used to identify essential key regulators by integrating the protein–protein interaction (PPI) network and their topological properties in humans. The key regulators and their first neighbour genes were used for the identification of orthologous genes present in C. elegans. The orthologous genes were used to construct the PPI Network, key gene identification and module analysis, gene ontology, and pathways analysis. Comparison of human and C elegans cardiovascular disease gene network, pathways, and functional similarity could provide vital support to use C elegans as a model.
Methodology
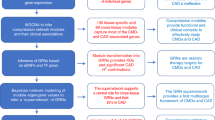
A schematic representation of the methodology (Fig. 1) adopted for obtaining differentially expressed genes related to CVD, identifying orthologous genes in C. elegans, comparing the pathways, developing the PPI network, topological analysis, and identification of novel key regulators are discussed in detail section-wise.
Selection of cardiovascular disease genes in human
There are several disorders that fall under the category of CVD. We selected the five most prevalent cardiac pathophysiologies for our analysis based on their significant contributions to CVD-related mortality which are as follows: cardiomyopathy, heart failure (HF), coronary artery disease (CAD), myocardial infarction (MI) and acute coronary syndrome (ACS)11,41. For obtaining CVD-associated genes in humans, we studied the GEO microarray datasets from the National Centre for Biotechnology Information (NCBI). Six datasets GSE42955, GSE57338, GSE18612, GSE64554, GSE66360, and GSE60993 belonging to cardiomyopathy, HF, CAD, MI, and ACS were selected for the study. Basic information on GEO Datasets used in the study is given in Table 1. The gene expression scores for the selected datasets were obtained by comparing data from patients with diseases and healthy individuals using the GEO2R tool which is an online analytical tool with an inbuilt R programme (https://www.ncbi.nlm.nih.gov/geo/geo2r/)42. All the datasets were normalized and the statistical threshold of the p value was set as ≤ 0.05. Lastly, Log fold change was used as the primary index for DEG screening. In this investigation, Log fold change |0.25–1.5| was used as a DEG screening condition. The obtained up and downregulated genes of CVD were further used for pathway analysis and construction of the PPI Network.
Human CVD PPI network construction and hub gene identification
To understand the regulatory function of the genes, the protein–protein interactions (PPI) network has been constructed. The human CVD PPI network was constructed using the STRING database (https://string-db.org/)43. The physical interaction data of STRING was then used for network construction, visualisation, and analysis through Cytoscape 3.6.0 platform44. Finally, a PPI network of 1099 upregulated and 815 downregulated genes was constructed on the Cytoscape after the removal of isolated nodes and duplicate edges. The network’s topological properties were analyzed using Network Analyser45 and Cytohubba46 plugin of Cytoscape.
The most influential nodes of the network are considered hubs of the network. The highest degree nodes also having higher centrality values were identified as the hubs of the CVD PPI network. For identification of the key regulators (KRs) of the PPI network, first the network’s most important genes according to their degrees (k) and the centrality measures (Cc, CB) were identified. The top 20 genes of different topological properties were compared to identify KR genes47,48.
Identification of CVD-related orthologous genes in C. elegans
In order to investigate how many potential CVD-related genes and pathways are present in the C. elegans genome, the orthologous genes of most significant CVD-associated genes were identified. This was achieved by identifying the first Neighbours of KRs of the human CVD PPI network and finding its orthologous in C. elegans. The first neighbour nodes of KR genes in the human interactome are defined as proteins directly and physically interacting with KR proteins49. First neighbours play the same important roles in numerous PPI networks and signalling pathways as the associated KR proteins49. Therefore, for identifying orthologous genes of the most significant human CVD-related genes, the first neighbours of the essential key regulator genes were identified and extracted from the human CVD PPI Network. The identified 648 first neighbour genes that were highly significant were used for the identification of orthologous genes present in C. elegans. g: Profiler that allows mapping of orthologous genes across species has been a popular tool for identification of C. elegans-human orthologs50. In addition to g: Profiler, we utilized the OrthoList 2 database for identification of orthologous genes. OrthoList 2 (OL2) is a compendium of C. elegans-human orthologs compiled by a meta-analysis in 201851. The HGNC-approved symbols of CVD genes of human is queried in g: Orth tool of g: Profiler (https://biit.cs.ut.ee/gprofiler/orth) and OrthoList 2 to obtain the C. elegans genes.
Orthologous gene PPI network construction and analysis
Animal models are employed to study the roles of specific genes or proteins associated with a disease with the speculation that the roles may be similar in humans. Comparison of human and model organism interactome can suggest that the proteins under study might play similar roles in human disease if their interaction is highly conserved52. Therefore, we constructed the PPI network of CVD-related C. elegans orthologous genes. For this, we utilized the STRING database (https://string-db.org/)43. First, an interactome network of orthologous genes was constructed on STRING. The physical interaction data of STRING was then used for network construction, visualization, and analysis through the Cytoscape 3.6.0 platform44. Finally, a PPI network of 581 nodes with 2519 connections (edges) was constructed on the Cytoscape after the removal of isolated nodes and duplicate edges.
Defining the fundamental topology of a network is generally done by analyzing its topological parameters, especially P(k), C(k), and CN (k)53. So, first, the fundamental topology of the orthologous gene network was defined by analyzing the above three parameters using the Network Analyzer app45 in Cytoscape 3.6.0 taking the network as an undirected network. The three parameters P(k), C(k), and CN (k) of the network were analyzed by plotting against the degree k, and their distribution behaviour was analyzed to define the topologies of the orthologous gene network. The centrality measures which include Closeness centrality (Cc) and betweenness centrality (CB) of every node in the network were analyzed, using the Network Analyzer app and CytoHubba app46 in Cytoscape 3.6.0 to study the influence and control capabilities of the nodes in signal processing and their dominance in network integration.
Identification of essential key regulators of orthologous gene network
For identification of the key regulators of the PPI network, first, the network’s most important genes according to their degrees (k) and the centrality measures (Cc, CB) were identified. Accordingly, the highest degree nodes also had higher centrality values, so, they were identified as the most influential nodes and considered as hubs of the network. Therefore, the key regulators having the most significant roles in PPI network integration and stability would be among these most influential nodes and hubs in the network.
Hence, a group of top highest degree nodes, or hubs, that had the largest impact on the network from the level of the core network up to the level of motifs, were used to identify the network’s important regulators. The hubs functioning as the backbone of the system-level structure and represented at every hierarchical level were found by manual hub tracing and were regarded to be significant regulators.
Analysis of modules
Modules of large PPI networks are defined as the set of statistics and functionally significant interacting genes. We constructed modules, using MCODE (Molecular Complex Detection) version 1.5.1 which follows the principle that highly connected regions (or clusters) of interaction networks are often complexes54. MCODE, identifies the clusters that are highly interconnected regions in a network. The default MCODE parameters i.e., “Degree cut-off = 2,” “node score cut-off = 0.2,” “k-score = 2,” and “max. depth = 100.” were used for network scoring and cluster finding. Significant modules were identified and filtered out on the basis of MCODE score.
Comparative pathway analysis of human and C. elegans
Pathway-based analysis is critical for understanding the interaction of diseases at the molecular level. DAVID (https://david.ncifcrf.gov/) which is a comprehensive set of functional annotation tool was used to identify GO terms and Pathways55. CVD-related DEG in case of human and C. elegans orthologous genes were used to perform gene enrichment analysis. Comparison of pathways and GO terms of human CVD-related genes and C. elegans orthologous genes was done to identify common pathways.
Results
Identification and selection of CVD-related genes in humans
For gaining insights into the genes and pathways of CVD in model organism C elegans , six datasets belonging to cardiomyopathy, HF, CAD, MI and ACS were selected on the basis of high prevalence (Prevalence—CAD and ACS: 30%, MI: 5–30% for different age group, HF: 15–25% relative to age, cardiomyopathy: 0.2–0.5%) and its contribution to CVD-related mortality (Annual mortality rate—CAD and ACS: 5–6% per 1,00,000 population, MI: 34–42%, HF: 15%, Cardiomyopathy—6 to 8%)11,41. The details of all datasets used in the study are given in Table 1. The selected datasets were analyzed using GEO2R. For the screening of DEGs, the statistical threshold of p value was set as ≤ 0.05 and Log fold change was used as the primary index for filtering out the genes. Common DEGs shared by two datasets were identified and considered only once in the final gene list by removing duplicates. Finally, a total of 1099 upregulated and 815 downregulated genes associated with human CVD were retrieved by analyzing the selected datasets (Supplementary file 1). The obtained DEGs were used for further study.
Human CVD-related gene network construction and hub gene identification
PPI network refers to the biochemical and biological activities of proteins in cells. It adds considerably to our understanding of cell physiology and disease association in the fields of biology and bioinformatics4. Therefore, a PPI network was constructed using 1099 upregulated and 815 downregulated genes identified to be associated with human CVD. In the PPI network, proteins are denoted by nodes and the association between the proteins is given by undirected edges. The results of network analysis reveal that the CVD PPI network comprises 1626 interacting nodes and 16,903 edges (Supplementary Fig. 1).
Topological properties define the structural properties of a complex biological network56. Therefore, to understand the essential behaviour of the CVD PPI network we studied the topological properties using the Network Analyzer app and CytoHubba app in Cytoscape. The “hubs” of the network are defined as the nodes with the highest degree in the system and tend to have an essential role in the PPI network. After analysis, we identified the top 10 most important hub genes (IL6, IL1B, PTPRC, MYC, FN1, ITGAM, TLR4, STAT3, JUN, and CXCL8) from the CVD PPI network as per the decreasing value. Similarly, analysis of topological properties of the CVD PPI Network resulted in the top 10 centrality measures (Betweenness and Closeness centrality) as listed in Table 2.
KR genes in a PPI network refer to a subset of genes that play pivotal roles in orchestrating and controlling various cellular processes through their interactions with other proteins. These genes exert significant influence over the network’s dynamics and functionality, often acting as central hubs or master regulators57. Therefore, for the identification of essential KRs serving as the backbone of the CVD PPI network, the top 20 genes from three distinct topological features (degree, betweenness, and closeness centrality) were compared (Fig. 2A–C). Eleven key regulators of the Human CVD that were common for all three attributes (Fig. 2D) were identified as follows: JUN, HSP90AA1, TLR4, CXCL8, MAPK3, PTPRC, IL6, IL1B, STAT3, MYC, and FN1 indicating that these genes might play important regulatory functions in CVD development and progression in human. Seven out of eleven KRs were found to be upregulated with IL1B having maximum fold change (2.87) while four genes were downregulated with HSP90AA1 having fold change of − 1.21 (Fig. 2E). The expression of KRs in control and disease groups is presented by a box plot in Fig. 3. The expression of IL1B, JUN, FN1, CXCL8, TLR4, PTPRC, and MAPK3 was significantly higher in diseased condition than in control while the expression of HSP90AA1, IL6, STAT3, and MYC was found to be lower in diseased than control.
Identification of key regulators using three attributes of CVD PPI Network. (A–C) Topological properties of the top 20 DEGs in terms of centrality measurements of degree, betweenness, and closeness. (D) Intersections among top ten genes having the highest centrality values of closeness, betweenness and degree. 11 common genes among the top 20 genes of each of topological parameter. (E) Bar plot showing the fold change for expression of 11 key regulators in individual healthy control and CVD patients. The red bar shows log2 foldchange of upregulated genes and green bars shows log2 foldchange of downregulated genes.
Relative expression of 11 key regulators normalized (norm) signal values between healthy controls and CVD patients. IL1B: interleukin 1 beta; JUN: Jun proto-oncogene, AP-1 transcription factor subunit; FN1: fibronectin 1; CXCL8: C-X-C motif chemokine ligand 8; TLR4: toll-like receptor 4; PTPRC: protein tyrosine phosphatase, receptor type C; MAPK3: mitogen-activated protein kinase 3; HSP90AA1: heat shock protein 90 alpha family class A member 1; IL6: interleukin 6; STAT3: signal transducer and activator of transcription 3; MYC: v-myc avian myelocytomatosis viral oncogene homolog.
Orthologous genes of human CVD in C. elegans
Completion of the C. elegans genome sequence in 199818 demonstrated that roughly 38% of worm genes have a human ortholog58. The percentage of human disease-related genes that have at least minor similarity (E < 10−10 on BLASTP searches) with C. elegans genes ranges between 40 and 75%19,20,21,22,23,24. For identifying orthologous genes of most significant human CVD-related genes, the first neighbours of the essential key regulator genes were identified and extracted from the human CVD PPI Network. The first neighbour nodes of KR genes in the PPI network are the proteins that are directly and physically interacting with KR proteins49. Therefore, we selected the first neighbour genes for C. elegans orthologue identification as they have similarly important roles in numerous PPI networks and signaling pathways as the associated KR proteins. A total of 637 first neighbour genes were found to be associated with the 11 KR proteins in the human CVD PPI network. Finally, 1113 C. elegans genes that were orthologous to 648 human CVD-related genes were identified (Supplementary file 2). The identified orthologous genes were used for the construction PPI network and comparative pathway study.
Orthologous gene network construction and analysis:
Many genes of different diseases in the genome of C. elegans have been extensively studied and their functions are well characterized. By studying the PPI network of CVD-related orthologous genes in C. elegans, we can infer the functions. Therefore, a PPI Network was constructed through STRING using the total C. elegans orthologous genes (Supplementary Fig. 2). In the PPI network, proteins are represented by nodes, and the relationship between proteins is shown by undirected edges.
Further, to understand the structural properties of the PPI network based on network topology and connectivity patterns, Orthologous gene network analysis was done using the Network Analyzer app and the Cytohubba plugin of Cytoscape. The “hubs” of the network are defined as the nodes with the highest degree in the system. After analysis, we identified the top 10 most important hub genes (aha-1, daf-21, pmk-1, gpb-1, pmk-2, cyb-2.1, cyb-2.2, hif-1, eef-1G and cyb-1) from the PPI network. Similarly, analysis of topological properties of the CVD PPI Network resulted in the top 10 centrality measures (Betweenness and Closeness centrality) as listed in Table 3. Figure 4A–D shows the interaction of top 10 different Topological properties of orthologous gene network.
C. elegans orthologous gene PPI regulatory network of the top ten hub genes of CVD. Regulatory network of the top ten (A) Degree genes (B) Betweenness centrality genes (C) Closeness centrality genes (D) Bottleneck genes. (E) Intersections among top 20 genes having the highest degree and centrality values of closeness, betweenness. 10 common genes among the top twenty genes of each of topological parameter.
For the identification of essential key regulators of the CVD orthologous gene PPI network, the top 20 genes from four distinct topological features (degree, bottleneck, betweenness and closeness centrality) were compared (Fig. 4E). Ten genes: pmk-1, daf-21, gpb-1, crh-1, enpl-1, eef-1G, acdh-8, hif-1, pmk-2 and aha-1 were found to be common for all the four attributes. When comparison of the identified key regulator nodes of human and C. elegans was done, it was found that two orthologous genes (pmk-1 and pmk-2) of MAPK3 which is an important key regulator of human CVD is serving as key regulators in C. elegans PPI network as well. sta-1 which is orthologous to human gene STAT3 is also among the top 10 topological properties of C. elegans orthologous PPI Network. All these results indicate that identified orthologous genes may play important role in elucidating the mechanisms of cardiovascular disease, which are currently poorly understood and C. elegans may serve as a good animal model.
Analysis of modules in orthologous network
Modules represent groups of proteins that tend to interact with each other more frequently than with proteins outside the module59. Modules can help us understand the dynamics of PPI networks. They may be transiently active in response to specific cellular conditions or environmental cues. Investigating module dynamics can reveal how the network adapts to changing circumstances. Hence, for determining the role of the orthologous genes in C elegans at various levels in the created network, the native or parent network was then divided into subnetworks or modules using the MCODE plug-in of the Cytoscape programme. The default MCODE parameters i.e., “Degree cut-off = 2,” “node score cut-off = 0.2,” “k-score = 2,” and “max. depth = 100.” were used for network scoring and cluster finding. A total of 25 modules were identified, out of which 9 modules having MCODE score ≥ 5 were considered significant and filtered from the PPI network. Interestingly, the identified key regulators were found to be part of these modules (Fig. 5). Among the top 4 modules of Cardiovascular disease network, Module 1 has 24 nodes and 276 edges with a score of 24 (Fig. 5A) ; Module 2 has 49 nodes and 361 edges with a score of 15.042 and contains four regulatory gene (pmk-1,pmk-2,gpb-1,eef-1G) (Fig. 5B); Module 3 has 17 nodes and 114 edges with a score of 14.250 and has one key regulator genes acdh-8 (Fig. 5C) and Module 4 has 8 nodes and 25 edges with a score of 7.143 (Fig. 5D).
Top 4 modules of C. elegans orthologous gene PPI Network: (A) Module 1 has 24 nodes and 276 edges with a score of 24.000; (B) Module 2 has 49 nodes and 361 edges with a score of 15.042; (C) Module 3 has 17 nodes and 114 edges with a score of 14.250 and (D) Module 4 has 8 nodes and 25 edges with a score of 7.143.
Comparative pathway analysis of human and C. elegans
In order to dissect the molecular mechanism of CVD in humans using C. elegans as an animal model, it is imperative to study the pathways that are conserved between the two species. In this study, we used the DAVID database to identify GO terms and KEGG pathways of the human CVD-related genes (Supplementary files 3 and 4) and its orthologous genes in C. elegans (Supplementary file 5). A comparison of pathways and GO terms of humans and C. elegans was done to identify CVD-related pathways that are present in C. elegans. The pathway and GO analysis of 1099 upregulated and 815 downregulated human genes and 1113 C. elegans orthologous genes showed that they are highly enriched in similar pathways. There are 9 commonly enriched pathways between humans and C. elegans that include Autophagy—animal, ErbB signaling pathway, FoxO signaling pathway, MAPK signalling pathway, ABC transporters, Biosynthesis of unsaturated fatty acids, Fatty acid metabolism, Glutathione metabolism, and Metabolic pathways. The list of pathways along with corresponding P-values in C. elegans and Humans are given in Fig. 6A. The total number of CVD-related genes and orthologous genes that are involved in these pathways are given by bar plot in Fig. 6B. The graph demonstrates that CVD-related genes and their orthologous are involved in metabolic pathways.
Functional comparison of the protein associated with CVD in humans and C. elegans. (A) Biological pathways of CVD proteins in humans and C. elegans using KEGG pathways with p-values. (B) Bar plot shows pathways comparison of the protein associated with CVD in humans and C. elegans. Y-axes represent no. of proteins and X-axis represents pathways.
Further, GO functional enrichment analysis also predicted similar functions in both the protein sets of human and C elegans. GO functions were classified into three categories. In each of these categories, several common functions were identified. We found 20 common Biological Processes describing the biological purpose of the gene or a gene product for carrying out a molecular function. Similarly, we also discovered 9 common Cellular Component and 13 common Molecular Functions through the comparative study of GO terms. Several important BP such as MAPK cascade, carnitine metabolic process, cell differentiation, chaperone-mediated protein folding requiring cofactor, fatty acid metabolic pathway, glutathione metabolic process, etc. are common among C. elegans and humans. The bubble plot in Fig. 7 illustrates the GO terms and KEGG pathways for C. elegans orthologous genes. The red colour highlights the enrichment of similar GO terms and Pathways in humans and C. elegans. As a result of common pathways and GO terms present between humans and C. elegans we speculate that C. elegans could serve as an animal model for studying cardiovascular diseases.
Functional enrichment analysis of C. elegans orthologous genes. (A) The significant enriched biological process of targeted C. elegans orthologous genes. (B) The significantly enriched cellular component, (C) The significant enriched molecular function and (D) KEGG Pathway. Dot size indicate count. The count represents the number of genes associated with each process. The dot colour denotes the p values of process, and the x-axis represents the fold enrichment score. The red colour text represents the similar gene enrichment and Pathways in humans and C. elegans.
Discussion
With the rise in global incidences of CVD, it is becoming important to understand the CVD-related genes and pathways for gaining insights into the molecular and biochemical mechanism of the disease in order to develop efficient treatment strategies or preventative measures and lower the rate of incidence and morbidity. Various mammalian animal models are often employed to study CVD. In spite of having genomic relatedness, mammalian animal model systems have their own limitation such as high experimental cost, ethical issues, limited genomic tools available, complicated operation, and high reproductive environment demands12. A simple well-known model organism such as C. elegans whose genome has been completely sequenced and thoroughly studied18 can give a comprehensive genomic evaluation of a wide range of human diseases including CVD39,60,61,62. However, to date, we lack information regarding the exact number of CVD-related genes and pathways within the C. elegans genome. Likewise, the extent of genomic and functional similarities between C. elegans and humans in relation to CVD remains unknown, which restricts its comprehensive use as an animal model for cardiovascular disease research.
Since it is crucial to recognize the potential of the model organism as a valuable candidate for studying CVD, we have used a bioinformatics approach to compare the genes and pathways related to CVD of humans with those in C. elegans. We analyzed microarray datasets of patients to obtain CVD-related genes. A total of 1099 upregulated and 815 downregulated human CVD-associated genes were identified from gene expression analysis. In order to understand the cell physiology and disease connection of these DEGs, we constructed a PPI network. The network constructed from DEG shows hierarchical features, which means that the network has a system-level organisation involving modules which are interrelated. Since the network is hierarchical, their synchronisation exhibits various important functional regulations of the network. Significant genes (leading hubs) were recognized as key regulators of the network by influencing motifs and module regulation, indicating their biological significance. The leading hubs have significantly important functions. They integrate the lower degree nodes for organizing and regulating activities like inter and intra-cross-talk among other essential genes, maintaining network properties and stability, and optimizing the network signal processing63,64.
After comprehensive network analysis using the Network Analyzer app and Cytohubba plugin of Cytoscape, we identified the eleven proteins as central or key regulators within the human CVD network which are as follows: JUN, HSP90AA1, TLR4, CXCL8, MAPK3, PTPRC, IL6, IL1B, STAT3, MYC and FN1. Our results demonstrate that the expression of IL1B, JUN, FN1, CXCL8, TLR4, PTPRC, and MAPK3 was significantly higher in the diseased condition than control while the expression of HSP90AA1, IL6, STAT3, and MYC was found to be lower in diseased than control (Figs. 2B and 3). There is increasing evidence suggesting that inflammation plays a role in the development of CVD. IL1B is a pro-inflammatory cytokine65. Many research investigations have shown that individuals with heart failure display elevated levels of inflammatory cytokines in their bloodstream, including IL-1B65. The product of the CXCL8 gene (commonly known as IL8) belongs to the CXC chemokine family and serves as a primary mediator of the inflammatory response66. Several research investigations have detected the presence of IL-8 at locations of vascular damage, while separate studies have suggested that IL-8 may be involved in different phases of atherosclerosis66. In the findings by Rus et al. elevated concentrations of IL-8 were reported within the atherosclerotic wall of human arteries67. In another study by Liu et al., it was identified that foam cells originating from human atherosclerotic tissues had elevated concentrations of IL-868. Toll-like receptors (TLRs) are present in a variety of heart cell types, including cardiac myocytes, smooth muscle cells, and endothelial cells69. They have been reported to be involved in a variety of functions in diseased conditions such as CVD, apoptosis, allergic diseases etc70. Several research investigations have provided evidence that TLR4 activation leads to the upregulation of multiple genes associated with pro-inflammatory cytokines71,72, which have significant roles in promoting myocardial inflammation, especially in conditions such as myocarditis73, myocardial infarction, ischemia-reperfusion injury74, and heart failure75. Our result shows the upregulation of these inflammation-related genes except IL6 which is downregulated. The fact that these play a significant role as key regulators emphasizes the critical role of inflammation in CVD is an important finding of our study.
In addition to the identification of inflammation-related genes in the study, we have also uncovered non-inflammatory genes that bear significance in the context of CVD such as JUN is found to be a key player implicated in the prevention of stress-induced maladaptive remodelling of the heart76. Additionally, MAPK3, a participant in intracellular mitogen-activated protein kinase (MAPK) signaling cascades, is believed to play an important role in the pathogenesis of cardiac and vascular diseases77. HSP90AA1, an isoform of heat shock protein 90, has been associated with its role in cardiac remodelling78. The presence of PTPRC has been noted in atherosclerotic plaque development79, while MYC has exhibited involvement in cardiac myotropy and angiogenesis80. FN1 has been linked to coronary artery disease81, and STAT3 has been recognized for its cardioprotective functions82. These genes, integral to cellular physiology, contribute significantly to diverse aspects of cardiovascular health and disease. Within the PPI network, key regulators (KRs) identified in this study offer valuable insights into the molecular mechanisms underpinning cardiovascular conditions. Consequently, these proteins emerge as promising targets for future research and therapeutic interventions in the development and progression of CVD. It is imperative to underscore the necessity for further experimental studies to validate these findings and elucidate the precise roles of these key regulators in cardiovascular health and disease.
For a better understanding of cell physiology and disease connection of orthologous genes in C. elegans we constructed the PPI network of orthologous genes. In the PPI network, proteins are represented by nodes, and the relationship between proteins is shown by undirected edges. Orthologous gene network analysis results identified the top 10 most important hub genes (aha-1, daf-21, pmk-1, gpb-1, pmk-2, cyb-2.1, cyb-2.2, hif-1, eef-1G and cyb-1) from the PPI network. Ten essential key regulator genes which are as follows: pmk-1, daf-21, gpb-1, crh-1, enpl-1, eef-1G, acdh-8, hif-1, pmk-2 and aha-1 were identified from the PPI network suggesting its crucial role. In diseased conditions such as cancer and various heart and lung diseases, cells and tissue suffer from low oxygen conditions (pathological hypoxia)83. Studies have reported that hypoxia activates hif-1 gene in C. elegans84,85. Similarly, in case of humans, HIF (ortholog of hif-1) is activated in hypoxic environment. This activation plays a central role in tissue repair, ischemia and cancer86. Additionally, results also show that ortholog of important CVD-related genes such as MAPK3, HSP90AA1, HSP90AB1, HSP90B1 (pmk-1, pmk-2, daf-21, enpl-1) playing important role in ortholog gene network. HSP90 has been found to be associated to several CVD. Its client proteins have been found to be involved in cardiac disease pathways such as MAPK signaling, TNF-α signaling, etc.87. A study of these proteins and signaling pathways using C. elegans ortholog can provide better insights into the molecular mechanism associated with the disease.
Results of comparative pathway analysis showed that there are 9 commonly enriched pathways that are present between humans and C. elegans. Among the common pathways, two belonged to lipid metabolic pathways “Biosynthesis of unsaturated fatty acid” and “Fatty acid metabolism”. Pathways related to lipid metabolism play a very crucial role in CVD as dysregulation in any step can cause disorders such as diabetes, obesity, atherosclerosis, etc. A study by Zhang et al. clearly establishes C. elegans as a promising model for the study of lipid metabolism and lipid metabolic diseases88 which is consistent with our result as dysregulation in lipid metabolism pathways is a risk factor for CVD. C. elegans and humans also have Autophagy—animal, ErbB signalling pathway, FoxO signalling pathway, MAPK signalling pathway, ABC transporters, Glutathione metabolism and Metabolic pathways that are commonly enriched that might play a role in human CVD development and progression thus making C. elegans as a suitable model organism to study the molecular events and pathways related to CVD.
Autophagy can also play a role in cardiovascular disease through several key signaling pathways including PI3K/Akt/mTOR, IGF/EGF, AMPK/mTOR, MAPKs, p53, Nrf2/p62, Wnt/β-catenin and NF-κB pathways89. Recent studies have revealed the mechanism underlying the therapeutic effects of NRG-1/ErbB signaling in the treatment of heart failure. Through activation of upstream signaling molecules such as phosphoinositide 3-kinase, mitogen-activated protein kinase, and focal adhesion kinase, NRG-1/ErbB pathway activation results in increased cMLCK expression and enhanced intracellular calcium cycling. The former is a regulator of the contractile machinery, and the latter triggers cell contraction and relaxation90.
FoxOs have unexpected and diverse roles in countering stress, determining cell fate, and regulating energy availability. Because the heart is constantly adapting to different stresses and metabolic conditions, FoxOs appear to play an extremely important role in cardiac physiology91. The role of the MAPKs extracellular signal-regulated kinase (ERK), C-jun N-terminal kinase (JNK), and p38 MAPK in cardiac hypertrophy, cardiac remodelling after myocardial infarction, atherosclerosis and vascular restenosis77 Adenosine triphosphate (ATP)-binding cassette (ABC) transporters may play an important role in the pathogenesis of atherosclerotic vascular diseases due to their involvement in cholesterol homeostasis, blood pressure regulation, endothelial function, vascular inflammation, as well as platelet production and aggregation92. A molecule that actively participates in counteracting the oxidizing effect of reactive species is reduced glutathione (GSH), a tripeptide that is present in all tissues, and its synthesis and/or regeneration is very important to be able to respond to the increase in oxidizing agents93.
Further, GO functional enrichment analysis also predicted similar functions in both the protein sets of human and C elegans. In each of the GO categories, several common functions were identified. We found 20 common Biological Processes describing the biological purpose of the gene or a gene product for carrying out a molecular function. Similarly, we also discovered 9 common Cellular Component and 13 common Molecular Functions through the comparative study of GO terms. As a result of common pathways and GO terms present between humans and C. elegans that are related to CVD All these results indicate that C. elegans can be a potential animal model to study various pathways and genes that are associated with CVD. Consequently, the CVD-related genes present in C. elegans should be considered as a starting point, and further experimental investigations are required to fully explore C. elegans as a model organism for CVD.
Conclusion
In summary, we explored the applicability of C. elegans as an animal model to study CVD. We identified 1113 orthologous genes in C. elegans that are related to CVD in humans. We also explored the pathways and GO terms related to CVD that are common between humans and C. elegans. This study provides the first genomic view on CVD-related genes and pathways that are present in C. elegans, supporting its use as a prominent animal model for the study of CVD.
Data availability
The datasets analysed during the current study are available in the GEO repository. It is a public free repository database, which stores a large number of gene functions and expressions. The working link are as follows: GSE42955: https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE42955, GSE57338: https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE57338, GSE18612: https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE18612, GSE64554: https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE64554, GSE66360: https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE66360, GSE60993: https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE60993
References
Joseph, P. et al. Reducing the global burden of cardiovascular disease, Part 1. Circ. Res. 121, 677–694 (2017).
Jia, T. et al. Experimental rodent models of cardiovascular diseases. Front. Cardiovasc. Med. 7, 588075 (2020).
Alissa, E. M. & Ferns, G. A. Heavy metal poisoning and cardiovascular disease. J. Toxicol. 2011, 1–21 (2011).
Barua, J. D. et al. Bioinformatics and system biological approaches for the identification of genetic risk factors in the progression of cardiovascular disease. Cardiovasc. Ther. 2022, 1–14 (2022).
Lelieveld, J. et al. Cardiovascular disease burden from ambient air pollution in Europe reassessed using novel hazard ratio functions. Eur. Heart J. 40, 1590–1596 (2019).
Barré-Sinoussi, F. & Montagutelli, X. Animal models are essential to biological research: Issues and perspectives. Future Sci. OA https://doi.org/10.4155/fso.15.63 (2015).
Silverman, G. A. et al. Modeling molecular and cellular aspects of human disease using the nematode caenorhabditis elegans. Pediatr. Res. 65, 10–18 (2009).
Neuberger, H.-R. et al. Chronic atrial dilation, electrical remodeling, and atrial fibrillation in the goat. J. Am. Coll. Cardiol. 47, 644–653 (2006).
Fan, J. et al. Rabbit models for the study of human atherosclerosis: From pathophysiological mechanisms to translational medicine. Pharmacol. Ther. 146, 104–119 (2015).
Getz, G. S. & Reardon, C. A. Animal models of atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 32, 1104–1115 (2012).
Savoji, H. et al. Cardiovascular disease models: A game changing paradigm in drug discovery and screening. Biomaterials 198, 3–26 (2019).
Zaragoza, C. et al. Animal models of cardiovascular diseases. J. Biomed. Biotechnol. 2011, 1–13 (2011).
Brenner, S. The genetics of caenorhabditis elegans. Genetics 77, 71–94 (1974).
Snoek, L. B. et al. WormQTL—Public archive and analysis web portal for natural variation data in Caenorhabditis spp.. Nucleic Acids Res. 41, D738–D743 (2012).
Félix, M.-A. & Barkoulas, M. Robustness and flexibility in nematode vulva development. Trends Genet. 28, 185–195 (2012).
Kaletta, T. & Hengartner, M. O. Finding function in novel targets: C. elegans as a model organism. Nat. Rev. Drug Discov. 5, 387–399 (2006).
Markaki, M. & Tavernarakis, N. Modeling human diseases in Caenorhabditis elegans. Biotechnol. J. 5, 1261–1276 (2010).
C. elegans Sequencing Consortium*. Genome sequence of the nematode C. elegans: A platform for investigating biology. Science 282, 2012–2018 (1998).
Aboobaker, A. A. & Blaxter, M. L. Medical significance of Caenorhabditis elegans. Ann. Med. 32, 23–30 (2000).
Ahringer, J. Turn to the worm!. Curr. Opin. Genet. Dev. 7, 410–415 (1997).
Culetto, E. A role for Caenorhabditis elegans in understanding the function and interactions of human disease genes. Hum. Mol. Genet. 9, 869–877 (2000).
O’Kane, C. J. Modelling human diseases in Drosophila and Caenorhabditis. Semin. Cell Dev. Biol. 14, 3–10 (2003).
Sonnhammer, E. L. L. & Durbin, R. Analysis of protein domain families in Caenorhabditis elegans. Genomics 46, 200–216 (1997).
Wheelan, S. J., Boguski, M. S., Duret, L. & Makałowski, W. Human and nematode orthologs — Lessons from the analysis of 1800 human genes and the proteome of Caenorhabditis elegans. Gene 238, 163–170 (1999).
Lai, C.-H., Chou, C.-Y., Ch’ang, L.-Y., Liu, C.-S. & Lin, W. Identification of novel human genes evolutionarily conserved in Caenorhabditis elegans by comparative proteomics. Genome Res. 10, 703–713 (2000).
Chen, N. WormBase: A comprehensive data resource for Caenorhabditis biology and genomics. Nucleic Acids Res. 33, D383–D389 (2004).
Kuwabara, P. E. & O’Neil, N. The use of functional genomics in C. elegans for studying human development and disease. J. Inherit. Metab. Dis. 24, 127–138 (2001).
Mushegian, A. R., Bassett, D. E., Boguski, M. S., Bork, P. & Koonin, E. V. Positionally cloned human disease genes: Patterns of evolutionary conservation and functional motifs. Proc. Natl. Acad. Sci. 94, 5831–5836 (1997).
Rubin, G. M. et al. comparative genomics of the Eukaryotes. Science 1979(287), 2204–2215 (2000).
Gerstein, M. B. et al. Integrative analysis of the Caenorhabditis elegans genome by the modENCODE project. Science 1979(330), 1775–1787 (2010).
Van Gilst, M. R., Hadjivassiliou, H., Jolly, A. & Yamamoto, K. R. Nuclear hormone receptor NHR-49 controls fat consumption and fatty acid composition in C. elegans. PLoS Biol. 3, e53 (2005).
Watts, J. L. & Browse, J. Genetic dissection of polyunsaturated fatty acid synthesis in Caenorhabditis elegans. Proc. Natl. Acad. Sci. 99, 5854–5859 (2002).
Deng, X. et al. Ceramide biogenesis is required for radiation-induced apoptosis in the germ line of C. elegans. Science 322, 110–115 (2008).
Matsuda, S. et al. Member of the membrane-bound O -acyltransferase (MBOAT) family encodes a lysophospholipid acyltransferase with broad substrate specificity. Genes Cells 13, 879–888 (2008).
Lee, H.-C. et al. Caenorhabditis elegans mboa-7, a member of the MBOAT family, is required for selective incorporation of polyunsaturated fatty acids into phosphatidylinositol. Mol. Biol. Cell 19, 1174–1184 (2008).
Satouchi, K., Hirano, K., Sakaguchi, M., Takehara, H. & Matsuura, F. Phospholipids from the free-living nematode Caenorhabditis elegans. Lipids 28, 837–840 (1993).
Walker, A. K. et al. A conserved SREBP-1/phosphatidylcholine feedback circuit regulates lipogenesis in metazoans. Cell 147, 840–852 (2011).
Li, Y., Na, K., Lee, H.-J., Lee, E.-Y. & Paik, Y.-K. Contribution of sams-1 and pmt-1 to lipid homoeostasis in adult Caenorhabditis elegans. J. Biochem. 149, 529–538 (2011).
Benian, G. M. & Epstein, H. F. Caenorhabditis elegans muscle. Circ. Res. 109, 1082–1095 (2011).
Ono, S. Regulation of structure and function of sarcomeric actin filaments in striated muscle of the nematode Caenorhabditis elegans. Anat. Rec. 297, 1548–1559 (2014).
Roth, G. A. et al. Global burden of cardiovascular diseases and risk factors, 1990–2019. J. Am. Coll. Cardiol. 76, 2982–3021 (2020).
Barrett, T. et al. NCBI GEO: Archive for functional genomics data sets—Update. Nucleic Acids Res. 41, D991–D995 (2012).
Szklarczyk, D. et al. The STRING database in 2023: Protein–protein association networks and functional enrichment analyses for any sequenced genome of interest. Nucleic Acids Res. 51, D638–D646 (2023).
Shannon, P. et al. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 13, 2498–2504 (2003).
Assenov, Y., Ramírez, F., Schelhorn, S.-E., Lengauer, T. & Albrecht, M. Computing topological parameters of biological networks. Bioinformatics 24, 282–284 (2008).
Chin, C.-H. et al. cytoHubba: Identifying hub objects and sub-networks from complex interactome. BMC Syst. Biol. 8, S11 (2014).
Bhattacharyya, N. et al. CDK1 and HSP90AA1 appear as the novel regulatory genes in non-small cell lung cancer: A bioinformatics approach. J. Pers. Med. 12, 393 (2022).
Chirom, K., Malik, M. Z., Mangangcha, I. R., Somvanshi, P. & Singh, R. K. B. Network medicine in ovarian cancer: Topological properties to drug discovery. Brief Bioinform 23, bbac085 (2022).
Módos, D. et al. Neighbours of cancer-related proteins have key influence on pathogenesis and could increase the drug target space for anticancer therapies. NPJ Syst. Biol. Appl. 3, 2 (2017).
Raudvere, U. et al. g:Profiler: A web server for functional enrichment analysis and conversions of gene lists (2019 update). Nucleic Acids Res. 47, W191–W198 (2019).
Kim, W., Underwood, R. S., Greenwald, I. & Shaye, D. D. OrthoList 2: A new comparative genomic analysis of human and Caenorhabditis elegans genes. Genetics 210, 445–461 (2018).
Kotlyar, M., Pastrello, C., Sheahan, N. & Jurisica, I. Integrated interactions database: Tissue-specific view of the human and model organism interactomes. Nucleic Acids Res. 44, D536–D541 (2016).
Pavlopoulos, G. A. et al. Using graph theory to analyze biological networks. BioData Min. 4, 10 (2011).
Bader, G. D. & Hogue, C. W. An automated method for finding molecular complexes in large protein interaction networks. BMC Bioinform. 4, 2 (2003).
Huang, D. et al. The DAVID gene functional classification tool: A novel biological module-centric algorithm to functionally analyze large gene lists. Genome Biol. 8, R183 (2007).
Cannistraci, C. V., Alanis-Lobato, G. & Ravasi, T. Minimum curvilinearity to enhance topological prediction of protein interactions by network embedding. Bioinformatics 29, i199–i209 (2013).
Mangangcha, I. R., Malik, Md. Z., Küçük, Ö., Ali, S. & Singh, R. K. B. Identification of key regulators in prostate cancer from gene expression datasets of patients. Sci. Rep. 9, 16420 (2019).
Shaye, D. D. & Greenwald, I. OrthoList: A compendium of C. elegans genes with human orthologs. PLoS One 6, e20085 (2011).
Guimarães, P. R., Pires, M. M., Cantor, M. & Coltri, P. P. Interaction paths promote module integration and network-level robustness of spliceosome to cascading effects. Sci. Rep. 8, 17441 (2018).
Moreno-Arriola, E. et al. Caenorhabditis elegans: A useful model for studying metabolic disorders in which oxidative stress is a contributing factor. Oxid. Med. Cell Longev. 2014, 1–9 (2014).
Zhang, S., Li, F., Zhou, T., Wang, G. & Li, Z. Caenorhabditis elegans as a useful model for studying aging mutations. Front. Endocrinol. (Lausanne) 11, 554994 (2020).
Nas, J. S. Caenorhabditis elegans as a model in studying physiological changes following heart failure. Asian J. Biol. Life Sci. https://doi.org/10.5530/ajbls.2021.10.69 (2022).
Alcalá-Corona, S. A., Sandoval-Motta, S., Espinal-Enríquez, J. & Hernández-Lemus, E. Modularity in biological networks. Front. Genet. 12, 701331 (2021).
Tazyeen, S. et al. Identification of key regulators in Sarcoidosis through multidimensional systems biological approach. Sci. Rep. 12, 1236 (2022).
Segiet, O. A. The role of interleukins in heart failure with reduced ejection fraction. Anatol. J. Cardiol. https://doi.org/10.14744/AnatolJCardiol.2019.32748 (2019).
Apostolakis, S., Vogiatzi, K., Amanatidou, V. & Spandidos, D. A. Interleukin 8 and cardiovascular disease. Cardiovasc. Res. 84, 353–360 (2009).
Rus, H. G., Vlaicu, R. & Niculescu, F. Interleukin-6 and interleukin-8 protein and gene expression in human arterial atherosclerotic wall. Atherosclerosis 127, 263–271 (1996).
Liu, Y., Hultén, L. M. & Wiklund, O. Macrophages isolated from human atherosclerotic plaques produce IL-8, and oxysterols may have a regulatory function for IL-8 production. Arterioscler. Thromb. Vasc. Biol. 17, 317–323 (1997).
Mitchell, J. A., Ryffel, B., Quesniaux, V. F. J., Cartwright, N. & Paul-Clark, M. Role of pattern-recognition receptors in cardiovascular health and disease. Biochem. Soc. Trans. 35, 1449–1452 (2007).
Yu, L. & Feng, Z. The role of toll-like receptor signaling in the progression of heart failure. Mediat. Inflamm. 2018, 1–11 (2018).
Yang, Y. et al. The emerging role of Toll-like receptor 4 in myocardial inflammation. Cell Death Dis. 7, e2234–e2234 (2016).
Wu, B. et al. TLR4 activation promotes the progression of experimental autoimmune myocarditis to dilated cardiomyopathy by inducing mitochondrial dynamic imbalance. Oxid. Med. Cell Longev. 2018, 1–15 (2018).
Fairweather, D. et al. IL-12 receptor β1 and toll-like receptor 4 increase IL-1β- and IL-18-associated myocarditis and coxsackievirus replication. J. Immunol. 170, 4731–4737 (2003).
Ding, H.-S. et al. The HMGB1–TLR4 axis contributes to myocardial ischemia/reperfusion injury via regulation of cardiomyocyte apoptosis. Gene 527, 389–393 (2013).
Liu, L. et al. Up-regulated TLR 4 in cardiomyocytes exacerbates heart failure after long-term myocardial infarction. J. Cell Mol. Med. 19, 2728–2740 (2015).
Windak, R. et al. The AP-1 transcription factor c-jun prevents stress-imposed maladaptive remodeling of the heart. PLoS One 8, e73294 (2013).
Muslin, A. J. MAPK signalling in cardiovascular health and disease: Molecular mechanisms and therapeutic targets. Clin. Sci. 115, 203–218 (2008).
García, R. et al. Extracellular heat shock protein 90 binding to TGFβ receptor I participates in TGFβ-mediated collagen production in myocardial fibroblasts. Cell Signal 28, 1563–1579 (2016).
Xiao, L., Yang, Z. & Lin, S. Identification of hub genes and transcription factors in patients with rheumatoid arthritis complicated with atherosclerosis. Sci. Rep. 12, 4677 (2022).
Souders, C. A. et al. c-Myc is required for proper coronary vascular formation via cell- and gene-specific signaling. Arterioscler. Thromb. Vasc. Biol. 32, 1308–1319 (2012).
Page, M. M. et al. A variant in the fibronectin (FN1) gene, rs1250229-T, is associated with decreased risk of coronary artery disease in familial hypercholesterolaemia. J. Clin. Lipidol. 16, 525–529 (2022).
Harhous, Z., Booz, G. W., Ovize, M., Bidaux, G. & Kurdi, M. An update on the multifaceted roles of STAT3 in the heart. Front. Cardiovasc. Med. 6, 150 (2019).
Helmlinger, G., Yuan, F., Dellian, M. & Jain, R. K. Interstitial pH and pO2 gradients in solid tumors in vivo: High-resolution measurements reveal a lack of correlation. Nat. Med. 3, 177–182 (1997).
Jiang, H., Guo, R. & Powell-Coffman, J. A. The Caenorhabditis elegans hif-1 gene encodes a bHLH-PAS protein that is required for adaptation to hypoxia. Proc. Natl. Acad. Sci. 98, 7916–7921 (2001).
Yu, R., Gao, L., Jiang, S., Guan, P. & Mao, B. Association of HIF-1alpha expression and cell apoptosis after traumatic brain injury in the rat. Chin. J. Traumatol. 4, 218–221 (2001).
Benizri, E., Ginouvès, A. & Berra, E. The magic of the hypoxia-signaling cascade. Cell. Mol. Life Sci. 65, 1133–1149 (2008).
Qi, S., Yi, G., Yu, K., Feng, C. & Deng, S. The role of HSP90 inhibitors in the treatment of cardiovascular diseases. Cells 11, 3444 (2022).
Zhang, Y. et al. Comparative genomics and functional study of lipid metabolic genes in Caenorhabditis elegans. BMC Genom. 14, 164 (2013).
Jiang, B. et al. The role of autophagy in cardiovascular disease: Cross-interference of signaling pathways and underlying therapeutic targets. Front. Cardiovasc. Med. 10, 1088575 (2023).
Yin, H.-K. Progress in neuregulin/ErbB signaling and chronic heart failure. World J. Hypertens. 5, 63 (2015).
Ronnebaum, S. M. & Patterson, C. The FoxO family in cardiac function and dysfunction. Annu. Rev. Physiol. 72, 81–94 (2010).
Schumacher, T. & Benndorf, R. A. ABC transport proteins in cardiovascular disease—A brief summary. Molecules 22, 589 (2017).
Matuz-Mares, D., Riveros-Rosas, H., Vilchis-Landeros, M. M. & Vázquez-Meza, H. Glutathione participation in the prevention of cardiovascular diseases. Antioxidants 10, 1220 (2021).
Acknowledgements
Figure 1 was created using Canva.
Funding
A.K.R is supported by GIA Scheme of Department of Health Research (DHR), Indian Council of Medical Research (ICMR), Govt. of India (F. No. R.11013/12/2023-GIA/HR), National Heart, Lung, and Blood Institute (NHLBI) and Fogarty International Centre (FIC), NIH, USA grant D43TW009345 and IoE, 2022, FRP, University of Delhi.
Author information
Authors and Affiliations
Contributions
A.K.R. and M.Z.M. study concept and design, acquisition of data, analysis, and interpretation of data, drafting of the manuscript, statistical analysis and supervision. A.P. and M.Z.M acquisition of data, analysis, and drafting of the manuscript, statistical analysis, A.P., and T.A.T., interpretation of data and drafting manuscript, statistical analysis, Shalimar, R.C., A.K.S., R.T., P.M., and C.G., formulation of draft and critical revision of the manuscript for intellectual content. All authors contributed to the article and approved the submitted version.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ray, A.K., Priya, A., Malik, M.Z. et al. A bioinformatics approach to elucidate conserved genes and pathways in C. elegans as an animal model for cardiovascular research. Sci Rep 14, 7471 (2024). https://doi.org/10.1038/s41598-024-56562-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-56562-9
Keywords
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.