Abstract
The relationship between remnant cholesterol (RC) and homeostasis model assessment-insulin resistance (HOMA-IR) in the context of metabolic-associated fatty liver disease (MAFLD) remains an area of ambiguity. This investigation was designed to elucidate the potential association between RC and HOMA-IR in a cohort of American adults diagnosed with MAFLD. Data from 5533 participants were procured from the 2017–2018 US National Health and Nutrition Examination (NHANES) databases. A weighted linear regression model was employed to analyze the association between RC and HOMA-IR in the context of MAFLD. Preliminary analysis revealed that 44.67% of the participants were diagnosed with MAFLD, with a higher prevalence observed in individuals aged 50–64 years (31.84%, p < 0.0001) and in males compared to females (53.48% vs. 46.52%, p < 0.0001). A positive correlation was identified between RC and HOMA-IR in MAFLD patients. The threshold effect analysis model indicated a breakpoint at RC = 30 mg/dl, with a more pronounced positive correlation when RC < 30 mg/dl (β = 0.17, p < 0.001). Receiver operating characteristic analysis further demonstrated that among all lipid parameters, RC exhibited the largest area under the curve. The study findings suggest a positive correlation between RC and HOMA-IR in MAFLD patients, indicating that elevated RC may serve as an independent risk factor for MAFLD.
Similar content being viewed by others
Introduction
Non-alcoholic fatty liver disease (NAFLD) is a prevalent chronic liver condition with significant implications for patient prognosis, healthcare costs, and public health1,2. The recent shift in nomenclature to metabolic dysfunction-associated fatty liver disease (MAFLD) underscores its close ties with metabolic abnormalities3,4,5. MAFLD is diagnosed based on the presence of hepatic steatosis along with metabolic disorders like obesity, diabetes, or metabolic dysregulation in lean individuals, offering a broader diagnostic framework compared to NAFLD6,7,8.
Insulin resistance (IR), a condition where the body's cells exhibit a diminished response to the hormone insulin, plays a pivotal role in a host of metabolic disorders. It is a central feature in the development and progression of metabolic-associated liver diseases, including MAFLD. IR disrupts normal glucose and lipid metabolism, leading to increased fat synthesis and accumulation in the liver, a process known as hepatic steatosis9,10. This metabolic imbalance not only contributes to the buildup of fat in the liver but also exacerbates inflammation and fibrosis, which are key factors in the progression of liver disease11.
Dyslipidemia, characterized by abnormal lipid levels in the blood, plays a critical role in the pathogenesis of MAFLD. Notably, very low-density lipoproteins (VLDL) and their remnants are key contributors to triglyceride accumulation in metabolic liver disease12. Remnant cholesterol (RC), comprising cholesterol in VLDL, intermediate-density lipoproteins, and remnants of postprandial chylomicrons post-lipolysis, emerges as a significant factor in MAFLD. RC acts as a cholesterol-rich lipoprotein that promotes liver fat accumulation. Additionally, RC remnants can penetrate the hepatic endothelium and activate Kupffer cells, triggering local inflammation13. Through these pathways, RC may contribute to the development and progression of steatohepatitis. Thus, RC represents an emerging cardiometabolic risk factor that may be implicated directly in MAFLD pathogenesis14. This elevation in RC, combined with insulin resistance, which further dysregulates lipid metabolism, leads to an increase in RC and other lipotoxic species15. The accumulation of these metabolites in the liver can cause cellular stress and damage, potentially exacerbating MAFLD progression.
While previous research has investigated the roles of RC in NAFLD16,17, MAFLD presents a different clinical entity with distinct pathophysiological mechanisms. Unlike NAFLD, MAFLD is diagnosed based on metabolic dysfunctions, irrespective of alcohol consumption or other liver diseases. This distinction underscores the importance of examining the combined effects of RC and IR in MAFLD, particularly in the context of diverse populations. Most existing studies on NAFLD have focused on isolated risk factors or specific populations, leaving a critical need for research on the interplay of these factors in MAFLD. Therefore, this study aims to address this gap by exploring the association between RC and IR levels in a multi-ethnic cohort of MAFLD patients, utilizing data from the 2017–2018 National Health and Nutrition Examination Survey (NHANES).
Methods
Study design and participants
This study utilized data from the 2017–2018 cycle of the National Health and Nutrition Examination Survey (NHANES), a comprehensive cross-sectional survey executed by the National Center for Health Statistics of the Centers for Disease Control and Prevention in the United States. Notably, this cycle of NHANES introduced the assessment of liver function using ultrasound and vibration-controlled transient elastography (VCTE) for the first time. Liver steatosis was determined using a median Controlled Attenuation Parameter (CAP), while liver fibrosis was defined based on a median Liver Stiffness Measurement (LSM)18. The survey methodology included structured household interviews, which provided self-reported demographic data and medical records. Moreover, physical examinations, encompassing anthropometric measurements and blood sample collection, were conducted at mobile examination centers19,20.
Diagnostic criteria and definition of groups
Definition of MAFLD
The diagnosis of MAFLD was established based on the presence of hepatic steatosis, as determined by ultrasound, in conjunction with either excess weight/obesity, diabetes mellitus, or metabolic dysregulation3. Metabolic dysfunction was defined as the presence of at least two of the seven metabolic anomalies, as per international consensus: waist circumference ≥ 102 cm in men or 88 cm in women; blood pressure ≥ 130/85 mmHg or anti-hypertensive therapy; triglyceride level ≥ 150 mg/dL or specific medication; high-density lipoprotein cholesterol (HDL-C) level < 40 mg/dL for men and < 50 mg/dL for women; prediabetes was defined as fasting plasma glucose levels of 100–125 mg/dL, or HbA1c levels of 5.7% to 6.4%; HOMA-IR ≥ 2.5; and C-reactive protein (CRP) level > 2 mg/L.
Definition of liver steatosis and significant fibrosis
In this study, liver steatosis and clinically significant fibrosis (CSF; fibrosis stage ≥ 2) were defined by CAP and LSM cutoffs21. A CAP ≥ 285 dB/m (80% sensitivity and 77% specificity) was defined as hepatic steatosis, whereas LSM ≥ 8.6 kPa (66% sensitivity and 80% specificity) was defined as CSF.
Smoking and alcohol consumption
Smoking status was categorized as current, forlkmer, or never. Alcohol use was classified as never (< 12 drinks in a lifetime), former (≥ 12 drinks in a year and did not drink last year or did not drink last year but drank ≥ 12 drinks in a lifetime), current light/moderate (average of ≤ 1 drink per day for women and ≤ 2 drinks per day for men during the past year), and current heavy (average of > 1 drink per day for women or > 2 drinks per day for men over the past year) drinker22.
Demographic variables
The demographic data including age (18–34, 35–49, 50–64, ≥ 65 years), sex, race/ethnicity (White, Black, Hispanics/Latin Americans, Asian, other (non-Hispanic, including multiracial persons)), educational attainment (EA) (< high school, high school or General Education Development high school equivalency test (GED), ≥ college), poverty income ratio (PIR; < 1.30, 1.30–3.49, ≥ 3.50), and complication were self-reported in accordance with NHANES design. Body mass index (BMI) was calculated as weight (in kilograms) divided by the square of height (in meters). The Centers for Disease Control defined BMI status as normal weight (BMI ≤ 25.0), overweight (BMI 25.0–30.0), and obese (BMI ≥ 30.0)23. Also, ongoing variables, including systolic blood pressure, diastolic blood pressure, waist circumference, white blood cell count, hemoglobin, platelet count, hypersensitive-CRP, aspartate aminotransferase, alanine aminotransferase, gamma-glutamyl transferase, total cholesterol (TC), HDL-C, LDL-C, triglycerides (TG), albumin, plasma fasting glucose, fasting insulin, and HbA1c, were measured in the mobile testing centres using standard protocol . HOMA-IR was used to indicate IR by calculating fasting insulin (μU/mL) × fasting glucose (mmol/L)/22.5. RC = TC–HDL–C–LDL–C. Participants were divided into four groups, categorized into quartiles based on their RC levels. The quartile ranges were specifically defined as follows: Quartile 1 (Q1) (2–12 mg/dL), Quartile 2 (Q2) (13–18 mg/dL), Quartile 3 (Q3) (19–27 mg/dL), and Quartile 4 (Q4) (28- 99 mg/dL).
Statistical analysis
Given the complex design of NHANES, sampling weights were applied in all statistical analyses to ensure the representativeness of the results. For categorical variables such as age group, sex, race, education level, and comorbidities, percentages were calculated and analyzed using the chi-square test. Continuous variables, including various blood biochemical and physical measurements, were analyzed using t-tests.
To explore the correlation between RC and HOMA-IR within the MAFLD population, both univariate and multivariate linear regression analyses were employed. These analyses were adjusted for confounding variables, including age, race, education, BMI, smoking status, PIR, and alcohol intake status. The weighted linear regression model was specifically chosen to account for the NHANES survey's complex sampling structure, ensuring findings are reflective of the broader U.S. population.
Additionally, a threshold effect analysis model was utilized to determine whether there exists a specific point at which changes in RC levels begin to significantly impact HOMA-IR. This analysis helps in identifying potential nonlinear relationships and interaction effects, providing a deeper understanding of how RC levels influence insulin resistance in the context of MAFLD.
All analyses were conducted using R Studio software (version 4.2.0). A p-value less than 0.05 (two-tailed) was considered statistically significant.
Ethics approval and consent to participate
NHANES protocol approved by NCHS Research Ethics Review Board, and obtained informed consent from all participants.
Results
Prevalence
The prevalence of MAFLD in our study population was found to be 44.67%. Patients diagnosed with MAFLD were predominantly male and older in age. Detailed characteristics of the participants with MAFLD are outlined in Table 1. The highest proportion of MAFLD was observed in participants aged between 50 and 64 years (31.84%, p < 0.0001). In contrast, a larger proportion of individuals without MAFLD held a college degree or higher (32.29% vs. 26.57%, p < 0.01).
Comorbidities and laboratory and physical parameters
Table 2 presents the prevalence of comorbidities and the distribution of laboratory and physical parameters among the participants. Comorbidities were more prevalent in individuals with MAFLD compared to those without. Liver enzyme levels and metabolic indices were also slightly elevated in participants with MAFLD. The study observed a positive correlation between the levels of RC and HOMA-IR and the prevalence of MAFLD (p < 0.0001).
Relationships among RC, MAFLD, and HOMA-IR
Tables 3 and 4 display the baseline characteristics of the participants and the physical and laboratory parameters across RC quartiles, respectively. Significant differences were observed across RC quartiles in terms of age, ethnicity, educational level, BMI, waist circumference, blood pressure, liver enzymes, routine blood, and metabolic-related index distribution. RC levels were positively associated with the prevalence of MAFLD and HOMA-IR in a dose-dependent manner (p for trend = 0.0001, Fig. 1A,B). As shown in Table 5, there was a positive correlation between RC and HOMA-IR (odds ratio (OR) 1.09, 95% confidence interval (CI) 1.05–1.14, p < 0.0001), and RC also had a positive correlation with the risk of MAFLD (OR 1.47, 95% CI 1.14–1.89, p < 0.0001). However, an inverse correlation was observed between RC and high socioeconomic status.
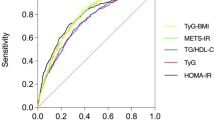
Receiver operating characteristic analysis
As shown in Fig. 2, the predictive value for MAFLD, which was highest for RC, followed by HOMA-IR, was better than TG, TC, LDL, and HDL.
ROC curve analysis of MAFLD-related lipid parameters and HOMA-IR. ROC, receiver operating characteristic; RC, remnant cholesterol; HOMA-IR, homeostasis model assessment-insulin resistance; HDL, high-density lipoprotein cholesterol; LDL, low-density lipoprotein cholesterol; TG, triglycerides; TC, total cholesterol.
Association between RC and HOMA-IR
We also used weighted generalized models and smoothing curve fitting to determine the association between RC and HOMA-IR (Fig. 3A,B). We further examined the threshold effect of RC on HOMA-IR using a nonlinear model (log-likelihood ratio = 0.003, Supplementary Table 1). For participants with MAFLD, when RC increased by 1 mg/dl, HOMA-IR of HOMA-IR increased by 0.17 (RC < 30 mg/dl). This result is consistent with the curve-fitting plots.
Relationship between RC and HOMA-IR. (A) Each black dot represents a sample. (B) Solid black line represents the smooth curve fit variables. The black dotted line shows a 95% confidence interval (CI) for the fit. They adjusted for age, sex, ethnicity, educational level, poverty income ratio, BMI, waist circumference, alcohol taking, smoking, systolic blood pressure, diastolic blood pressure.
Subgroups
Supplementary Fig. 1 reveals significant variations in MAFLD risk across RC levels, further stratified by HOMA-IR and BMI. This underscores the differential impact of RC, HOMA-IR, and BMI on MAFLD risk among participants. The higher the HOMA-IR level, the higher the risk of RC-related MAFLD (OR 1.95 per SD increase for the highest HOMA-IR level vs. OR 1.02 per SD increase for the lowest HOMA-IR level, P-interaction = 0.04). For BMI subgroups, the risk of RC-related MAFLD was higher in underweight people (OR 2.57 per SD increase with underweight people vs. OR 1.34 per SD increase with obesity, P-interaction = 0.001).
Association between RC and MAFLD vs NAFLD
As shown in Supplementary Table 2, the association was significantly stronger for increased levels of RC with MAFLD (OR 4.11, 95% CI 0.45–7.77, p < 0.0001) than NAFLD (OR 2.1, 95% CI − 0.52–4.71, p < 0.0001).
Discussion
The current study offers a comprehensive exploration of the prevalence, clinical characteristics, and the influence of RC on MAFLD within a multiethnic population in the United States. This investigation is the first to establish a positive correlation between RC and HOMA-IR in individuals with MAFLD. The prevalence of MAFLD was found to be approximately 44.7%, predominantly in middle-aged and older men. Compared to non-MAFLD patients, those with MAFLD exhibited higher levels of metabolic, inflammatory, lipid, and hepatic biomarkers and a higher prevalence of CSF. Among these, RC and HOMA-IR increased linearly with MAFLD prevalence.
The established role of LDL-C in cardiovascular disease is well-documented, with lower levels of LDL-C being associated with a reduced risk of cardiovascular events24. However, even with optimal control of LDL-C and management of other cardiovascular risk factors, a residual risk persists. Recent studies have highlighted that elevated levels of RC, found in triglyceride-rich lipoproteins, may contribute to this remaining cardiovascular risk25,26,27. RC has the capability to permeate arterial walls, potentially leading to atherosclerosis, foam cell formation, and low-grade inflammation. These conditions are exacerbated when RC is overexpressed in plasma due to factors such as excessive calorie consumption, obesity, diabetes, and genetic variations affecting lipoprotein metabolism28,29.
Recent research has shown that the redefined MAFLD is linked to increased probabilities of fatalities from all-cause and specific causes, indicating the urgent need for the early identification of high-risk individuals30. Furthermore, MAFLD development has been significantly influenced by disturbances in lipoprotein metabolism. Epidemiological studies have indicated that RC may serve as an independent predictor of long-term mortality in patients with MAFLD31. Our findings further reinforce this perspective, clearly demonstrating that higher levels of RC are associated with an increased risk of liver fibrosis in MAFLD patients. This association was consistent between patients with and without CSF, suggesting elevated RC levels may predict progression of liver fibrosis in MAFLD. The significant liner trend emphasizes the strength of the association between RC and liver fibrosis risk in MAFLD. Monitoring RC levels could aid fibrosis risk assessment in MAFLD patients. These findings point to a potential role of RC in the pathophysiology of liver fibrosis in MAFLD. Higher RC levels were associated with a higher risk of MAFLD, regardless of sex, age, and ethnicity. Notably, having a college degree or higher and having a PIR ≥ 3.5% of the poverty level were both negatively associated with RC for patients with MAFLD. A previous study revealed that increased obesity and socioeconomic status, as gauged by educational status, are causally associated with biological risk factors (lipids)32. This suggests that changing socioeconomic status is crucial for causing and controlling MAFLD.
Kessoku et al.33 examined 1365 biopsy-proven NAFLD cases included in the JSG-NAFLD database, revealing that HOMA-IR significantly increased with the stage of hepatic fibrosis. This underscores the progressive nature of IR in relation to liver disease severity. Similarly, Ballestri et al.34 demonstrated HOMA-IR as an independent predictor of progressive hepatic fibrosis in patients with biopsy-proven NAFLD, further substantiating the critical role of IR in the pathogenesis of liver fibrosis. Additionally, a HOMA-IR value of ≥ 2.90 was found to be an independent predictor of advanced fibrosis in nondiabetic NAFLD patients, suggesting a direct pathway where IR may activate hepatic stellate cells (HSCs)35. This finding is pivotal as it highlights the direct impact of metabolic dysfunction at the cellular level within the liver. Furthermore, hypertriglyceridemia, a key component of insulin resistance syndrome, has been linked to these hepatic changes. Ohnishi et al.36 observed a significant positive correlation between HOMA-IR and remnant-like particle cholesterol in Japanese individuals, aligning with the global understanding of dyslipidemia in metabolic diseases. This study’s results supported a positive correlation between higher RC and higher HOMA-IR in MAFLD patients, consistent with a previous study on RC. The study also observed that when the RC level increased by 1 mg/dl, HOMA-IR increased by 0.17 (RC < 30 mg/dl). These results underscore the role of IR in MAFLD. The positive correlation between HOMA-IR and RC levels in MAFLD patients supports the hypothesis that IR may exacerbate lipid dysregulation, contributing to MAFLD progression. This is in line with studies that have identified IR as a key driver of hepatic steatosis and inflammation in MAFLD.
This study highlights significant variations in the risk of MAFLD associated with RC across different BMI categories. Intriguingly, our findings indicate that individuals with a lower BMI may also be at a heightened risk of developing RC-related MAFLD. While it is well recognized that a higher BMI is commonly associated with an increased risk of cardiometabolic diseases, recent research has also identified a metabolically unhealthy, lean phenotype, albeit less prevalent. This underscores the complexity of metabolic disease risk, which is not solely confined to obesity37. Further exploration into the relationship between RC levels and MAFLD, particularly in varying BMI contexts, could open up new avenues for treatment strategies focusing on RC regulation. And in this study, the higher levels of RC could significantly increase the risk for MAFLD compared with NAFLD.
Despite these significant findings, this study has several limitations. Due to insufficient serial data on the relationship between RC and IR in the context of MAFLD, we couldn't evaluate their longitudinal variations. Moreover, while several covariates were controlled for, the potential for unaddressed and unmeasured confounding influences remains. Additionally, the representativeness of the study's sample across diverse ethnic and socioeconomic groups is limited, potentially affecting the generalizability of the results. Future research should focus on mechanistic studies to elucidate the underlying biochemical pathways of these associations. Interventional trials are also needed to assess the effectiveness of targeting RC and IR in managing MAFLD. Furthermore, exploring the impact of unaccounted confounders like genetic factors and lifestyle habits and conducting studies on dietary and pharmacological interventions could provide more targeted approaches for MAFLD management.
In conclusion, this study establishes a significant positive correlation between RC and the HOMA-IR in adults, shedding light on the identification and severity assessment of MAFLD. These findings suggest that RC can serve as an accessible, cost-effective, and reliable indicator for assessing MAFLD risk. Importantly, RC can aid clinicians in early identification of high-risk individuals, facilitating timely diagnostic confirmation through ultrasound and the initiation of early interventions, ultimately contributing to improved patient outcomes and potentially reducing MAFLD prevalence.
Data availability
Data can be downloaded from NHANES database (https://wwwn.cdc.gov/nchs/nhanes/continuousnhanes/default.aspx?BeginYear=2017).
References
Frey, S. et al. Prevalence of NASH/NAFLD in people with obesity who are currently classified as metabolically healthy. Surg. Obes. Relat. Dis. 16, 2050–2057 (2020).
Lonardo, A., Mantovani, A., Lugari, S. & Targher, G. NAFLD in some common endocrine diseases: Prevalence, pathophysiology, and principles of diagnosis and management. Int. J. Mol. Sci. 20, 2841 (2019).
Eslam, M. et al. A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. J. Hepatol. 73, 202–209 (2020).
Gofton, C., Upendran, Y., Zheng, M. H. & George, J. MAFLD: How is it different from NAFLD?. Clin. Mol. Hepatol. 29, S17–S31 (2023).
Eslam, M. et al. MAFLD: A consensus-driven proposed nomenclature for metabolic associated fatty liver disease. Gastroenterology. 158, 1999–2014 (2020).
Eslam, M. et al. The Asian Pacific Association for the Study of the Liver clinical practice guidelines for the diagnosis and management of metabolic associated fatty liver disease. Hepatol. Int. 14, 889–919 (2020).
Yamamura, S. et al. MAFLD identifies patients with significant hepatic fibrosis better than NAFLD. Liver Int. 40, 3018–3030 (2020).
Lin, S. et al. Comparison of MAFLD and NAFLD diagnostic criteria in real world. Liver Int. 40, 2082–2089 (2020).
Overi, D., Carpino, G., Franchitto, A., Onori, P. & Gaudio, E. Hepatocyte injury and hepatic stem cell niche in the progression of non-alcoholic steatohepatitis. Cells. 9, 590. https://doi.org/10.3390/cells9030590 (2020).
Lee, W. H., Najjar, S. M., Kahn, C. R. & Hinds, T. D. Jr. Hepatic insulin receptor: New views on the mechanisms of liver disease. Metabolism. 145, 155607. https://doi.org/10.1016/j.metabol.2023.155607 (2023).
Katsiki, N., Mikhailidis, D. P. & Mantzoros, C. S. Non-alcoholic fatty liver diseaseand dyslipidemia: An update. Metabolism. 65, 1109–1123 (2016).
Ginsberg, H. N. et al. Triglyceride-rich lipoproteins and their remnants: Metabolic insights, role in atherosclerotic cardiovascular disease, and emerging therapeutic strategies—a consensus statement from the European Atherosclerosis Society. Eur. Heart J. 42, 4791–4806 (2021).
Diehl, K. L. et al. Kupffer cells sense free fatty acids and regulate hepatic lipid metabolism in high-fat diet and inflammation. Cells. 9, 2258. https://doi.org/10.3390/cells9102258 (2020).
Wu, W. et al. Cumulative exposure to high remnant-cholesterol concentrations increases the risk of cardiovascular disease in patients with hypertension: A prospective cohort study. Cardiovasc. Diabetol. 22, 258. https://doi.org/10.1186/s12933-023-01984-4 (2023).
Jung, U. J. & Choi, M. S. Obesity and its metabolic complications: The role of adipokines and the relationship between obesity, inflammation, insulin resistance, dyslipidemia and nonalcoholic fatty liver disease. Int. J. Mol. Sci. 15, 6184–6223 (2014).
Huang, H. et al. Remnant cholesterol independently predicts the development of nonalcoholic fatty liver disease. J. Clin. Endocrinol. Metab. 108, 2907–2915 (2023).
Cheng, Y. et al. Remnant cholesterol, stronger than triglycerides, is associated with incident non-alcoholic fatty liver disease. Front Endocrinol. (Lausanne) 14, 1098078. https://doi.org/10.3389/fendo.2023.1098078 (2023).
Tian, T. et al. Dietary quality and relationships with metabolic dysfunction-associated fatty liver disease (MAFLD) among united states adults, results from NHANES 2017–2018. Nutrients. 14, 4505. https://doi.org/10.3390/nu14214505 (2022).
Centers for Disease Control and Prevention. NHANES 2017- 2018 overview.https://wwwn.cdc.gov/nchs/nhanes/continuousnhanes/overview.aspx?BeginYear=2017. Accessed August 1,2022.
Chen, T. C., Clark, J., Riddles, M. K., Mohadjer, L. K. & Fakhouri, T. H. I. National health and nutrition examination survey, 2015–2018: Sample design and estimation procedures. Vital Health Stat. 2, 1–35 (2020).
Siddiqui, M. S. et al. Vibration-controlled transient elastography to assess fibrosis and steatosis in patients with nonalcoholic fatty liver disease. Clin. Gastroenterol. Hepatol. 17, 156–163 (2019).
Hicks, C. W., Wang, D., Matsushita, K., Windham, B. G. & Selvin, E. Peripheral neuropathy and all-cause and cardiovascular mortality in U.S. adults : A prospective cohort study. Ann. Intern. Med. 174, 167–174 (2021).
Alberti, K. G., Zimmet, P. & Shaw, J. The metabolic syndrome–a new worldwide definition. Lancet (London, England) 366, 1059–1062 (2005).
Nordestgaard, B. G. Triglyceride-rich lipoproteins and atherosclerotic cardio- vascular disease: New insights from epidemiology, genetics, and biology. Circ. Res. 118, 547–563 (2016).
Joshi, P. H. et al. Remnant lipoprotein cholesterol and incident coronary heart disease: The Jackson heart and Framingham offspring cohort studies. J. Am. Heart Assoc. 5, e002765. https://doi.org/10.1161/JAHA.115.002765( (2016).
Li, K. et al. Associations between remnant lipoprotein cholesterol and central systolic blood pressure in a Chinese community-based population: A cross-sectional study. Lipids Health Dis. 20, 60. https://doi.org/10.1186/s12944-021-01490-0 (2021).
Balling, M. et al. VLDL Cholesterol accounts for one-half of the risk of myocardial infarction associated with apoB-containing lipoproteins. J. Am. Coll. Cardiol. 76, 2725–2735 (2020).
Carr, S. S., Hooper, A. J., Sullivan, D. R. & Burnett, J. R. Non-HDL-cholesterol and apolipoprotein B compared with LDL-cholesterol in atherosclerotic cardiovascular disease risk assessment. Pathology. 51, 148–154 (2019).
Ference, B. A. et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease: Evidence from genetic, epidemiologic, and clinical studies: A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur. Heart J. 38, 2459–2742 (2017).
Kim, D. et al. Metabolic dysfunction-associated fatty liver disease is associated with increased all-cause mortality in the United States. J. Hepatol. 75, 1284–1291 (2021).
Huang, H. et al. Remnant cholesterol predicts long-term mortality of patients with metabolic dysfunction-associated fatty liver disease. J. Clin. Endocrinol. Metab. 107, e3295–e3303 (2022).
Ezzati, M. et al. Rethinking the “diseases of affluence” paradigm: Global patterns of nutritional risks in relation to economic development. PLoS Med. 2, e133. https://doi.org/10.1371/journal.pmed.0020133 (2005).
Kessoku, T. et al. Simple scoring system for predicting cirrhosis in nonalcoholic fatty liver disease. World J. Gastroenterol. 20, 10108–10114 (2014).
Ballestri, S. et al. Nonalcoholic fatty liver disease is associated with an almost twofold increased risk of incident type 2 diabetes and metabolic syndrome: Evidence from a systematic review and meta-analysis. J. Gastroenterol. Hepatol. 31, 936–944 (2016).
Watt, M. J., Miotto, P. M., De Nardo, W. & Montgomery, M. K. The liver as an endocrine organ-linking NAFLD and insulin resistance. Endocr. Rev. 40, 1367–1393 (2019).
Ohnishi, H. et al. Relationship between insulin-resistance and remnant-like particle cholesterol. Atherosclerosis. 164, 167–170 (2002).
Sheng, G. et al. Waist-to-height ratio and non-alcoholic fatty liver disease in adults. BMC Gastroenterol. 21, 239. https://doi.org/10.1186/s12876-021-01824-3 (2021).
Author information
Authors and Affiliations
Contributions
S.W. and B.Q. were responsible for concept and design of study and final critical review of manuscript. S.W. was responsible for drafting manuscript, data collection, statistical analysis, and critical review of manuscript. S.W., Q.Z. were responsible for data collection and interpretation of data. All the authors have approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wang, S., Zhang, Q. & Qin, B. Association between remnant cholesterol and insulin resistance levels in patients with metabolic-associated fatty liver disease. Sci Rep 14, 4596 (2024). https://doi.org/10.1038/s41598-024-55282-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-55282-4
Keywords
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.